Introduction
Hyperlipidemia is a prevalent medical condition
defined by increased lipid levels in the blood, especially
cholesterol and triglycerides, which is often connected with
cardiovascular and cerebrovascular diseases such as coronary heart
disease and cerebral atherosclerosis (1,2). One
of the most prevalent metabolic disorders amongst pilots is
hyperlipidemia (3). Previous
research found that long-term hyperlipidemia, which is a risk
factor for both pancreatitis and diabetes mellitus (1), might also increase the incidence of
cerebrovascular disease (4). As it
is closely related to the health of pilot, hyperlipidemia may
impact flight safety and shorten the flying career of pilots
(5), therefore it warrants further
investigation.
Several researchers have suggested that the
prevalence of dyslipidemia in pilots in China is substantially
higher than the overall population. Specifically, the prevalence of
dyslipidemia is reportedly 18.6% in the adult Chinese population
(6), but is 20.79% amongst pilots
in the Chinese civil aviation industry (7). This further increases to 22.7% in
military pilots and crew (8). Yu
et al (9) revealed that the
prevalence of hyperlipidemia in pilots has increased annually,
while the age of onset has decreased. For ~22 years, hyperlipidemia
has been one of the most prevalent disease amongst pilots (10) with Dong et al (11) reporting that hyperlipidemia is the
most common illness out of 270 other diseases in the pilot disease
spectrum. Another study found that hyperlipidemia ranks in the top
five in the spectrum of diseases among pilots and the incidence of
marginal elevation of blood lipids including total cholesterol,
triglyceride and low-density lipoprotein cholesterol (LDL-C) was
28% among 450 male flight personnel (7), which was significantly higher than
the prevalence of the general population of the same age.
Polymorphisms in the 8th intron of the LPL gene and
apolipoprotein E may affect the levels of blood lipids (12,13).
However, several studies have found no significant difference
between the genotypes of pilots and the general population
(14-16).
Therefore, it has been hypothesized that, instead of genetic
factors, hyperlipidemia in pilots might be more closely related to
environmental factors (17).
Specifically, pilots are routinely subjected to the high intensity
of flight training, physical exertion, frequent exposure to stress,
high-load flights, which requires pilots to put in more operational
skills and attention during the flight process, with increased
heart rate and muscle tensing (18), and high-calorie diets (19). All these environmental factors may
cause stress in the neural endocrine system and lead to metabolic
disorders (20). Furthermore,
researcher has shown that 98.9% of ~1,000 surveyed pilots have at
least one negative lifestyle habit, such as smoking and drinking
alcohol, which might also contribute to hyperlipidemia (21).
Although many studies have shown that the prevalence
of pilot hyperlipidemia is high, there is no detailed report on the
causes and characteristics of pilot hyperlipidemia. Previously, it
has been hypothesized that this increased prevalence is associated
with their unusual work environment (22), which involves high-intensity
training, often leading to an increased alcohol intake and a
high-fat diet. However, at present no complete supporting data or
quantitative risk estimations have been carried out. The aim of the
present study was to explore the association between physical
examination indicators and the incidence of hyperlipidemia in
pilots by using physical examination data.
Materials and methods
Data sources
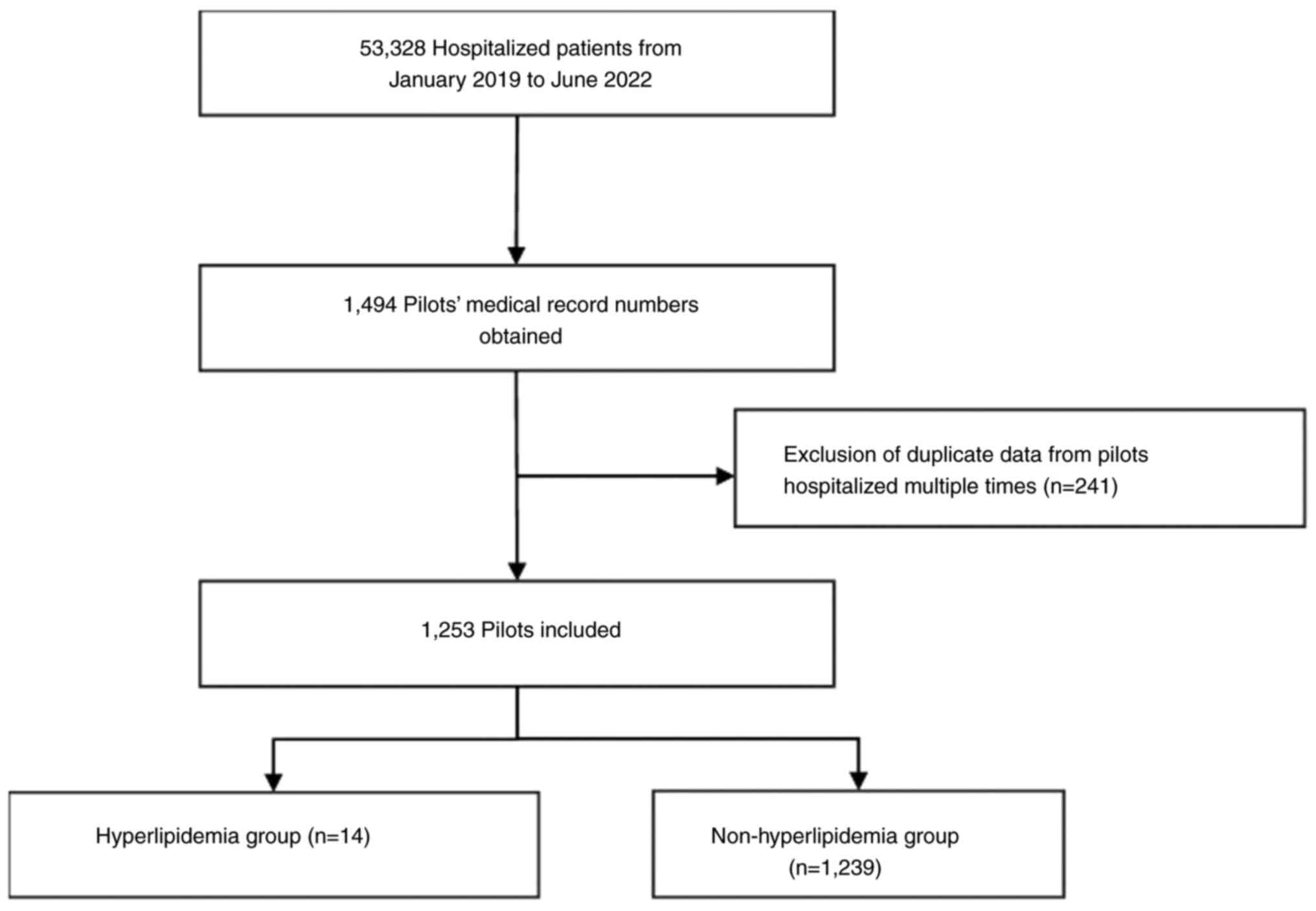
The present study was based on pilot physical
examination data, obtained from a sample of 1,253 pilots between
January 2019 and June 2022 at the Naval Medical Center of Naval
Medical University (Shanghai, China). The process of selection for
the present study is shown in Fig.
1. The inclusion criteria was as follows: i) Pilots that were
inpatients at the Naval Medical Center of Naval Medical University
(Shanghai, China) from January 2019 to June 2022; and ii) pilots
that underwent multiple physical examinations in the Naval Medical
Center of Naval Medical University. The exclusion criteria
included: i) Retired pilots; ii) pilots who had not undergone
various tests such as blood tests and had only received physical
therapy and traditional Chinese medicine treatment. The physical
examination data were described and evaluated after being cleaned,
and analysis results were presented in Table I, Table II and Table III. Based on diagnostic
information, 1,253 pilots were divided into two groups for the
present study: i) Pilots with hyperlipidemia (n=14) whose disease
diagnosis included hyperlipidemia [diagnosis code E78 in the
International Statistical Classification of Diseases 10 (ICD-10)
(23)]; and ii) pilots without
hyperlipidemia (n=1,239). The diagnosis of hyperlipidemia was total
cholesterol (TC) ≥6.2 mmol/l, triglyceride (TG) ≥2.3 mmol/l or
LDL-C ≥4.2 mmol/l (23).
 | Table ISummary of indicators for enumeration
data. |
Table I
Summary of indicators for enumeration
data.
| Indicators | n | Percentage |
|---|
| Marital status | | |
|
Unknown | 3 | 0.24 |
|
Other | 1 | 0.08 |
|
Widowed | 1 | 0.08 |
|
Unmarried | 855 | 68.24 |
|
Married | 393 | 31.36 |
|
Total | 1,253 | 100.00 |
| Ethnicity | | |
|
Han | 1,239 | 98.88 |
|
Manchu | 6 | 0.48 |
|
Mongol | 4 | 0.32 |
|
Tujia | 1 | 0.08 |
|
Unknown | 3 | 0.24 |
|
Total | 1,253 | 100.00 |
| Blood group
(ABO) | | |
|
A | 223 | 29.69 |
|
AB | 76 | 10.12 |
|
B | 198 | 26.36 |
|
O | 254 | 33.82 |
|
Total | 751 | 100.00 |
|
- | 6 | 0.80 |
|
+ | 742 | 99.20 |
|
Total | 748 | 100.00 |
| Allergy | | |
|
Penicillin
sodium for injection | 12 | 1.03 |
|
76% compound
meglumine diatrizoate injection | 1 | 0.09 |
|
Azithromycin | 2 | 0.17 |
|
Iodine | 1 | 0.09 |
|
Compound
sulfamethoxazole | 1 | 0.09 |
|
Sulfonamides | 5 | 0.41 |
|
Streptomycin | 1 | 0.09 |
|
Tetanus
antitoxin | 1 | 0.09 |
|
Penicillin | 16 | 1.37 |
|
Penicillin,
tetanus antitoxin | 1 | 0.09 |
|
Cephalosporin | 6 | 0.53 |
|
None | 1,119 | 95.97 |
|
Total | 1,166 | 100.00 |
 | Table IIT-test analysis of the risk factors
of hyperlipidemia. |
Table II
T-test analysis of the risk factors
of hyperlipidemia.
| | T-test | 95% confidence
interval |
|---|
| Indicators | P-value | Mean
difference | Standard error | The lower
limit | Ceiling |
|---|
| Age | <0.001 | -4.150 | 0.586 | -5.385 | -2.915 |
| Neutrophil
percentage | 0.097 | -3.492 | 2.104 | -7.620 | 0.635 |
| Apolipoprotein
B | 0.001 | 0.164 | 0.049 | 0.069 | 0.260 |
| Hepatitis B core
antibody | <0.001 | -0.719 | 0.066 | -0.849 | -0.588 |
| Hepatitis B E
antibody | 0.495 | -0.075 | 0.110 | -0.292 | 0.141 |
| Width of platelet
distribution | 0.124 | -0.233 | 0.152 | -0.531 | 0.064 |
| Platelets | 0.088 | 19.569 | 11.477 | -2.949 | 42.087 |
| Carbohydrate
antigen 50 | 0.151 | 1.035 | 0.721 | -0.380 | 2.450 |
| Carbohydrate
antigen 199 | 0.086 | 2.679 | 1.560 | -0.382 | 5.740 |
| Eosinophil
granulocyte | 0.301 | 0.052 | 0.049 | -0.053 | 0.158 |
| Mean platelet
volume | 0.074 | -0.540 | 0.302 | -1.132 | 0.053 |
| Thrombin time | <0.001 | 0.901 | 0.237 | 0.437 | 1.365 |
| Uric acid | 0.670 | 7.043 | 16.531 | -25.391 | 39.477 |
| Immunoglobulin
M | 0.207 | 33.391 | 26.384 | -18.522 | 85.303 |
| Immunoglobulin
G | 0.085 | 282.172 | 163.075 | -38.689 | 603.034 |
| Lymphocyte
count | 0.019 | 0.346 | 0.147 | 0.057 | 0.635 |
| Lymphocyte
percentage | 0.091 | 3.411 | 2.013 | -0.540 | 7.361 |
| Streptolysin | 0.151 | -35.402 | 24.663 | -83.799 | 12.995 |
|
Antithyroglobulin | 0.664 | -7.948 | 18.291 | -43.836 | 27.940 |
| Thyroid peroxidase
antibodies | 0.607 | -12.242 | 23.786 | -58.913 | 34.429 |
| α-fetoprotein | 0.095 | -0.564 | 0.337 | -1.224 | 0.097 |
| Red blood
cells | 0.077 | 1.489 | 0.841 | -0.161 | 3.140 |
| Aspartate
transaminase | 0.148 | -3.423 | 2.364 | -8.062 | 1.215 |
| Alanine
transaminase | 0.125 | -4.787 | 3.121 | -10.910 | 1.336 |
| Low-density
lipoprotein | <0.001 | 0.768 | 0.194 | 0.386 | 1.149 |
| Cholesterol | 0.019 | 0.482 | 0.205 | 0.080 | 0.885 |
| Large platelet
ratio | 0.086 | -3.592 | 2.087 | -7.686 | 0.503 |
| Complement C4 | 0.366 | -3.014 | 3.327 | -9.560 | 3.532 |
| Complement C3 | 0.179 | -15.401 | 11.423 | -37.877 | 7.076 |
| White blood
cells | 0.011 | 2.174 | 0.854 | 0.499 | 3.850 |
 | Table IIIChi-square test (or Fisher's exact
test) analysis of the risk factors of hyperlipidemia. |
Table III
Chi-square test (or Fisher's exact
test) analysis of the risk factors of hyperlipidemia.
| Indicators | Hyperlipidemia
group | Non-hyperlipidemia
group | P-value |
|---|
| Squamous cell
carcinoma antigen | | | 0.047 |
|
High | 11 | 1,167 | |
|
Normal (not
detected) | 3 | 72 | |
| Rheumatoid
factor | | | 0.018 |
|
High | 2 | 17 | |
|
Normal (not
detected) | 12 | 1,222 | |
| Low-density
lipoprotein | | | <0.001 |
|
Low | 0 | 584 | |
|
High | 11 | 306 | |
|
Normal | 3 | 349 | |
| High-density
lipoprotein | | | 0.091 |
|
Low | 1 | 267 | |
|
High | 1 | 298 | |
|
Normal (not
detected) | 12 | 674 | |
| Alkaline
phosphatase | | | 0.093 |
|
Low | 0 | 52 | |
|
High | 1 | 6 | |
|
Normal (not
detected) | 13 | 1,181 | |
| Globulin | | | 0.120 |
|
Low | 3 | 215 | |
|
High | 1 | 12 | |
|
Normal (not
detected) | 10 | 1,012 | |
| B27 human leukocyte
antigen | | | 0.002 |
|
Weak
positive | 0 | 1 | |
|
Positive | 1 | 22 | |
|
Negative | 13 | 703 | |
|
Not
tested | 0 | 513 | |
| Homocysteine | | | 0.001 |
|
High | 4 | 44 | |
|
Normal (not
detected) | 10 | 1,195 | |
| Apolipoprotein
AI | | | 0.049 |
|
High | 0 | 2 | |
|
Low | 3 | 666 | |
|
Normal (not
detected) | 11 | 571 | |
Variables
General conditions (such as height, weight, blood
pressure and heart rate), blood routine examination, urine routine
examination, blood biochemistry analysis, electrocardiogram and
ultrasound or X-ray of each organ were the primary components of
the physical examination.
There were 13 modules obtained from the electronic
medical records of the pilots, including the basic information,
admission record, first course of disease record, patient
notification, vital sign record, surgery information, inspection
information, examination information, diagnosis classification,
diagnosis information, nutritional risk screening score, discharge
record and discharge summary.
The basic information of patients, vital sign
records, surgery information, examination information, diagnosis
classification and diagnosis information are text-type variables or
structured data, but needed to be de-duplicated, standardized or
unified in dimensions. Therefore, in order to ensure the accuracy
of data analysis, the data format was defined, kept consistent,
duplicates were removed and variables were standardized. The
additional modules contain web-based text data or unstructured data
such as admission records, discharge summary, initial and daily
progress notes, discharge records and surgical records.
The data relating to the basic information of
patients was structured and cross-sectional and includes 44
variables, such as the number of hospitalizations, date of
hospitalization, sex and marital status. The two modules of
diagnosis information and diagnosis classification information are
the matching modules. The diagnosis name is displayed in Chinese,
and the associated ICD-10 code is provided for the diagnosis
categorization. Both modules are structured, longitudinal data.
The data relating to laboratory inspection
information is structured and longitudinal, and includes blood test
results (including biochemical tests, immunological tests, routine
blood tests and virology tests.) and clinical test results
(including urine and stool tests).
The data relating to routine physical examination
included the following detailed categories: Liver function, renal
function, electrolyte levels, hepatitis B test, hepatitis C
antibody test, treponema pallidum antibody test, human
immunodeficiency virus and antigen antibody detection, blood cell
analysis including whole blood C-reactive protein levels, urine
analysis including urine sediment levels, stool routine with occult
blood tests, prothrombin time measurement, prothrombin standard
ratio, activated partial thrombin time, prothrombin time,
fibrinogen concentration, levels of D-dimer, levels of fibrinogen
degradation product, liver and kidney function testing, blood lipid
levels and other tests such as gastrin testing, tumor markers
tests, thyroid tests, pepsin testing, ABO and Rh blood group
identification, and anti-rheumatoid lymphocyte testing.
The data relating to vital signs was structured and
longitudinal, including stool frequency, height, weight, systolic
blood pressure, diastolic blood pressure, respiration, heart rate
and body temperature.
The surgery information was semi-longitudinal data,
which included the type of surgery, the ICD code of the surgery,
the name of the surgeon, the name of the assistant and the time of
the surgery. However, it was challenging to extract and make use of
the other information, such as discharge summaries, which were
obtained from web page forms, which were in free text format, and
were unstructured data.
Statistical analysis
The data were analyzed with Statistics Analysis
System (version 9.4) and R language (version 4.2.2). For
enumeration data, chi-square test or Fisher's exact test was used
for comparison between groups, and for measurement data, two
independent samples (unpaired) t-test was used for comparison
between groups. In the single factor analysis, if P<0.1, the
factor was incorporated into the multi-factor model for further
modeling. All tests were two-sided and P<0.05 was considered to
indicate a statistically significant difference.
Logistic regression model, multilevel model and
boosting propensity score model were used to establish the
multivariate analysis model to find the risk factors of pilot
hyperlipidemia. The multilevel model, also termed the hierarchical
model, can deal with multilevel statistical methods with a
hierarchical structure. Some medical research data tend to be
hierarchical or clustered, and multilevel models can be used to
divide the residuals in the models to different levels (24). The individual random error is
relatively accurate, so it can be estimated more accurately than
using the traditional regression model (25). In the present study, the resident
region of pilots was used as a stratification factor.
Propensity score is a statistical method that has
been widely used in observational studies in recent years (26). It calculates the conditional
probability of an individual being assigned to an exposed group by
combining covariates from the exposed and non-exposed groups.
Individuals with similar values of exposed and non-exposed
components were then matched or adjusted, weighted or stratified to
estimate exposure effects. It uses the balanced distribution of
covariates data to achieve the objective of unbiased estimation of
exposure effects (27).
The boosting algorithm is a classification learning
algorithm, which can complete correct classification or recognition
of a dataset (28). As the
boosting algorithm can classify and predict individuals, it can
also be used to estimate propensity score (29). Compared with some traditional
regression and classification methods, the boosting algorithm has
the following advantages: i) It is a non-parametric classification
or recognition method, which does not need to satisfy the
assumption of log-linear parameters; ii) second, this method is a
strong learning algorithm, which can use other machine learning
algorithms, such as decision trees and support vector machines, as
the basis for its iterations, and produce more accurate
classification predictions and output propensity scores directly;
and iii) if there is interaction between various variables in the
data, or if the data is in a high-dimensional state, the method can
still be used to predict effectively and obtain relatively accurate
results (30).
Results
Demographic information
Among the 1,253 pilot participants, 393 were married
(31%), while 855 were unmarried (68.24%), as shown in Table I. In terms of permanent residence,
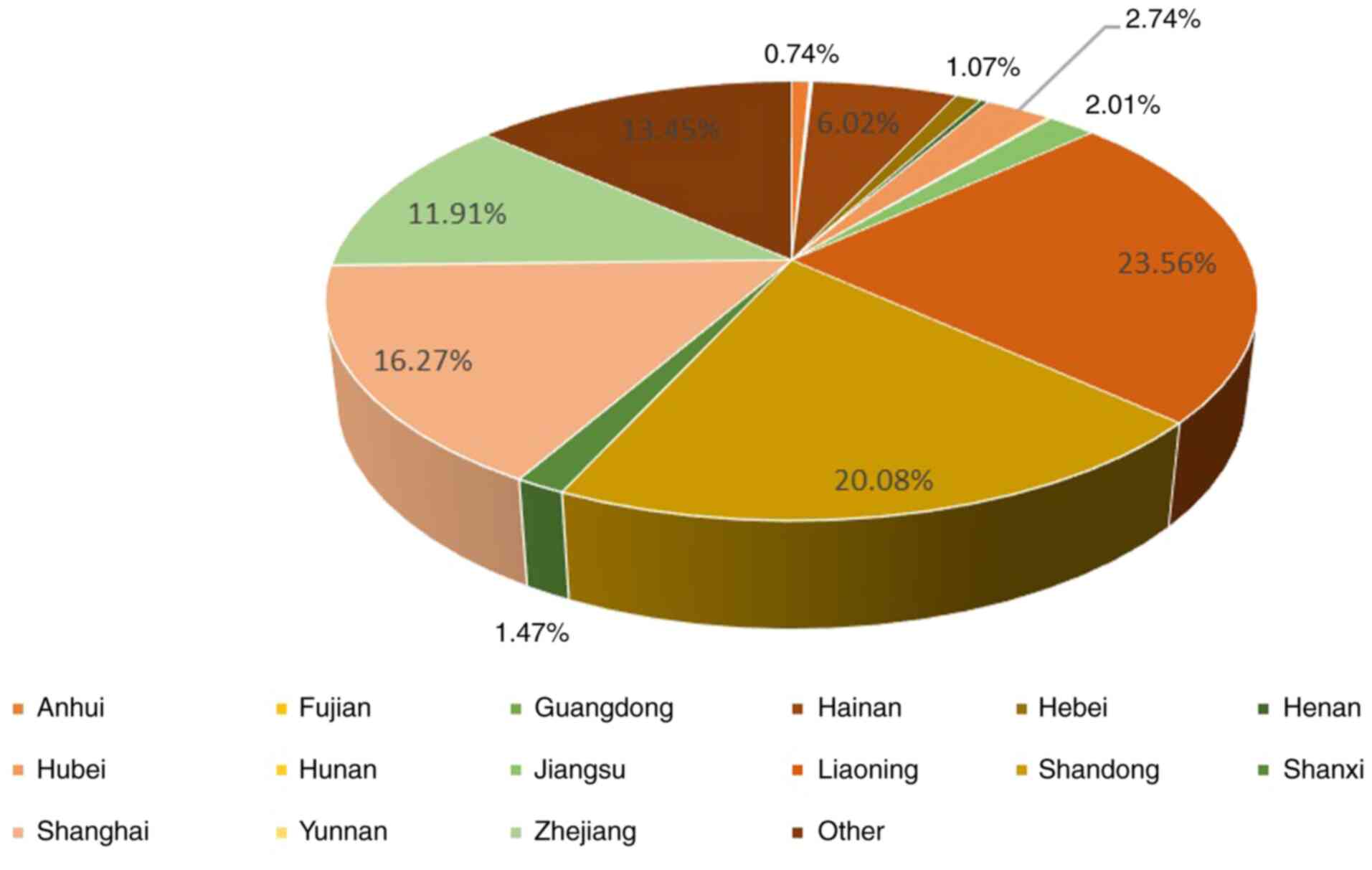
352 (23.56%) of the pilots lived in Liaoning followed by 251
(20.08%) in Shandong and 243 (16.27%) in Shanghai, as shown in
Fig. 2. Pilots were aged 19-57
years old, with a median age of 24.5 years old. The longest
hospital stay was 77 days, the shortest was 1 day and the median
hospital stay was 5 days.
Univariate analysis
Results of univariate analysis showed that age,
apolipoprotein B, hepatitis B core antibody, thrombin time,
lymphocyte count, cholesterol, squamous cell carcinoma antigen,
rheumatoid factor, low-density lipoprotein, B27 human leukocyte
antigen, homocysteine and apolipoprotein AI were different between
patients with hyperlipidemia and without hyperlipidemia (Tables II and III).
Multivariate analysis. Multivariate
logistic regression model
Multivariate logistic regression analysis was used
to analyze the data of 1,253 pilots. As there was a variation in
the physical examination items of each pilot, most of the
examination items were crossed but not overlapped, thus the sample
size for multiple factors analysis was small. A total of 947
patients had records including the data from the majority of the
tests. Of these patients, there were only 14 patients with
hyperlipidemia and 933 patients without hyperlipidemia. As shown in
Table IV, carbohydrate antigen
199, thrombin time and age might be the risk factors of
hyperlipidemia, which mean higher levels of these three markers
might increase the risk of diagnosis of hyperlipidemia. Low-density
lipoprotein and cholesterol are markers for the diagnosis of
hyperlipidemia, an increase in the levels of these markers also
represents an increased risk of hyperlipidemia. However, as a
routine blood test, white blood cell count, although statistically
significant, might not be used clinically as a marker for
hyperlipidemia due to the fact that white blood cell count is
usually used to determine the inflammatory response and could be
elevated in patients for a number of reasons including infection
(31).
 | Table IVResults of logistic regression
analysis on the risk factors of pilot hyperlipidemia. |
Table IV
Results of logistic regression
analysis on the risk factors of pilot hyperlipidemia.
| | 95% confidence
interval |
|---|
| Variable | β-value | Standard error | Wald | Degree of
freedom | P-value | Odds ratio | Lower limit | Upper limit |
|---|
| Carbohydrate
antigen 199 | 0.055 | 0.024 | 5.168 | 1 | 0.023 | 1.056 | 1.008 | 1.107 |
| Thrombin time | 1.442 | 0.353 | 16.639 | 1 | <0.001 | 4.228 | 2.115 | 8.451 |
| Squamous cell
carcinoma antigen | 0.095 | 0.072 | 1.772 | 1 | 0.183 | 1.100 | 0.956 | 1.266 |
| Low-density
lipoprotein | 5.213 | 1.417 | 13.534 | 1 | <0.001 | 183.591 | 11.422 | 2950.824 |
| Cholesterol | -3.970 | 1.397 | 8.073 | 1 | 0.004 | 0.019 | 0.001 | 0.292 |
| White blood
cells | 0.209 | 0.088 | 5.723 | 1 | 0.017 | 1.233 | 1.039 | 1.464 |
| Age | -0.195 | 0.081 | 5.778 | 1 | 0.016 | 0.822 | 0.701 | 0.965 |
A high-fat diet and age are widely recognized
factors related to hyperlipidemia (32). However in the present study, two
more relatively new risk factors, the carbohydrate antigen 199 and
thrombin time, were identified from the aforementioned multifactor
analysis. Although, whether these two relatively new risk factors
are causally related to the pathogenesis of hyperlipidemia needs to
be verified by further research.
Multilevel model analysis
The physical examination data of 1,253 pilots were
further analyzed using a multilevel model. The behavioral patterns
of the pilots may fit into certain residential clusters due to
different regional cultures and eating customs, thus the resident
region of each pilot was taken as a horizontal factor. As shown in
Figure 2, the majority of pilots
resided in the following provinces or regions, which were divided
into 8 categories based on their locations: i) Anhui, Henan and
Hubei (categorized as 1); ii) Hebei and Beijing (categorized as 2),
Hainan (categorized as 3); iv) Guangxi, Fujian and Guangdong
(categorized as 4); v) Jiangsu, Zhejiang and Shanghai (categorized
as 5); vi) Liaoning (categorized as 6); vii) Shandong and Shanxi
(categorized as 7); and viii) Yunnan and unknown regions
(categorized as 8).
The resident regions of the pilots were categorized
as aforementioned, and the spatial model was then applied to check
whether the data have a multilevel structure. The results showed
that the estimated intercept value of the second level was -5.444
and P=0.001. The results showed that the data of the present study
have obvious hierarchical structure. After which, the data were
analyzed by multilevel model analysis, and the results were shown
in Table V. After taking into
account the level factor of the pilot resident region, more
relevant factors were derived from the results of the
aforementioned multilevel model analysis for the diagnosis of
hyperlipidemia, including thrombin time, carbohydrate antigen 199,
lymphocyte count and rheumatoid factor. The results of thrombin
time and carbohydrate antigen 199 were consistent with the results
of the general logistic regression. However, lymphocyte count and
rheumatoid factor may require further exploring.
 | Table VResults of multilevel model analysis
on the risk factors of pilot hyperlipidemia. |
Table V
Results of multilevel model analysis
on the risk factors of pilot hyperlipidemia.
| Effect | Estimate | Standard error | Degree of
freedom | T-value | P-value |
|---|
| Constant term | -26.439 | 5.550 | 7 | -4.76 | 0.002 |
| Thrombin time | 0.979 | 0.274 | 1,482 | 3.57 | <0.001 |
| Carbohydrate
antigen 199 | 0.053 | 0.027 | 1,482 | 1.96 | 0.050 |
| Lymphocyte | 1.248 | 0.450 | 1,482 | 2.77 | 0.006 |
| Rheumatoid
factor | 3.037 | 0.940 | 6 | 3.23 | 0.018 |
Boosting propensity score model
After calculating the propensity score by the
boosting algorithm, the effect values of the covariates were
estimated by inverse probability weighting, and the results were
shown in Table VI. The results
were consistent with the results of the multilevel model analysis,
and more variable differences (lymphocyte count and rheumatoid
factor) could be obtained in comparison with the general logistic
regression model. The estimation of effect values was also more
precise and closer to the results of the multilevel model
analysis.
 | Table VIBoosting propensity score model
analysis of pilot hyperlipidemia risk factors. |
Table VI
Boosting propensity score model
analysis of pilot hyperlipidemia risk factors.
| | 95% confidence
interval |
|---|
| Parameter | Coefficient | Standard error | Wald card
square | P-value | Odds ratio | Lower limit | Upper limit |
|---|
| The constant
term | -38.896 | 1.926 | 407.868 | <0.001 | - | - | - |
| Thrombin time | 1.464 | 0.096 | 233.435 | <0.001 | 4.322 | 3.582 | 5.215 |
| Carbohydrate
antigen 199 | 0.040 | 0.017 | 5.459 | 0.020 | 1.041 | 1.007 | 1.077 |
| Lymphocyte | 1.514 | 0.175 | 75.025 | <0.001 | 4.544 | 3.226 | 6.400 |
| Rheumatoid
factor | 0.914 | 0.293 | 9.733 | 0.002 | 6.215 | 1.972 | 19.583 |
Discussion
Carbohydrate antigen 199 is widely distributed in
normal glandular epithelial cells, and exists as salivary mucin in
serum under physiological conditions (33). When inflammation and obstruction
occur in the glandular epithelial cells, the fluid in the lumen
cannot be released smoothly, and the vascular permeability increase
may lead to an increase in serum carbohydrate antigen 199 levels
(34). Previous studies have shown
that the increase of carbohydrate antigen 199 is related to
inflammation or metabolic diseases (35,36).
In addition, the decrease of metabolic clearance rate in blood may
also cause the increase of carbohydrate antigen 199 level (37). Secondary hyperlipidemia is often
induced by metabolic disorders (38), such as diabetes, obesity and
hypothyroidism. Therefore, there may be an interaction between
carbohydrate antigen 199 and hyperlipidemia.
Previous studies have shown that thrombin time is
closely related to the degree of cardiac cell damage (39). After the surface of the
arteriosclerosis plaque is damaged, platelets released from the
body will aggregate and adhere to the collagen and microfibers
under the intima of blood vessels in patients with cardiovascular
disease (CVD) (40). In severe
cases, persistent activation of the coagulation system and
hypercoagulation may lead to myocardial anoxia and metabolites may
not be effectively eliminated (41). As a chronic disease with abnormal
lipid metabolism, hyperlipidemia is closely related to the
occurrence of CVDs (42). Thus,
there might be some interactions between thrombin time and lipid
metabolism.
It has been noted that patients with active
rheumatoid arthritis may have higher serum lipid levels,
particularly cholesterol (43).
Rheumatic activity may influence lipid distribution and result in
lipid metabolism disorders in patient with rheumatoid arthritis
(44). Some researchers have
demonstrated that the serum TG and TC levels of patients with
rheumatoid arthritis were not higher than those of the general
population before they were diagnosed with rheumatoid arthritis
(45). Another study revealed that
the predominant dyslipidemia-related characteristics in patients
with rheumatoid arthritis were low TC, LDL-C and HDL-C (46). However, a case-control study
demonstrated that patients with rheumatoid arthritis have a greater
burden of coronary atherosclerosis at their first angiogram that is
independent of traditional cardiovascular risk factors (47). At the same time, hyperlipidemia
represented one of the major cardiovascular risk factors that
(47) affect children and
adolescents early in life (48).
Further studies and specific investigations are still necessary to
explore the potential relationship between blood lipids and
rheumatoid factors.
One possible explanation is that the increase of
inflammatory factors in serum may affect the function of adipose
tissue and liver (49). It was
also reported that the structure and function of vascular
endothelium changed noticeably during the active phase of
rheumatoid arthritis, and endothelial dysfunction may affect lipid
metabolism (50). It is also
speculated that disease activity in patients with rheumatoid
arthritis may be associated with lipid metabolism, and abnormal
lipid metabolism could aggravate inflammatory reaction (51,52).
Therefore, more clinical trials and cohort studies are needed to
clarify the causal relationship between rheumatoid factor and
hyperlipidemia.
Recently, hyperlipidemia has been identified as an
inflammation-related disease (53), and research has observed that
during the pathological development of atherosclerosis-related
CVDs, monocytes enter the endothelium after inflammation-induced
overexpression of endothelial cells (54). As already established, lymphocytes
are the protagonist of inflammatory and immune responses (55). A research study showed that
interleukin-38, mainly secreted by B lymphocyte, infiltrated
peripheral blood mononuclear cells or keratinocytes, has a
significant function in the development of hyperlipidemia and its
related CVDs (40). On the other
hand, the stimulation of interleukin-36, which is also a
lymphokine, could significantly inhibit the anti-hyperlipidemic
effects of the interleukin-36 receptor and affected the process of
hyperlipidemia and the related atherosclerosis (56). Therefore, the association between
inflammatory response with the development of hyperlipidemia and
CVDs is well established.
Using the platform of artificial intelligence data
analysis technology, the digitized data can be used for the
screening of relevant risk factors or causes of disease, and
formulate individualized health guidelines based on their vital
signs, behaviors, lifestyle and personal preference (57). Individual intervention could be
used to reduce the prevalence of hyperlipidemia, and reduce or
delay the occurrence and development of serious complications such
as cerebrovascular disease by altering health-related behaviors
(58).
Feasible approaches for lowering the prevalence of
hyperlipidemia include health promotion, health education and
targeted medical treatment (59).
Targeted health intervention may be achieved by gathering and
recording comprehensive data on the demographics, living habits,
lifestyle, occupational history, disease history, family history,
treatment history, clinical characteristics, laboratory tests,
imaging tests, functional tests, quality of life tests, health
status, genetic factors, physical fitness and life trajectory of
individuals (60).
The results of the present study indicate that in
order for pilots to keep blood lipid levels healthy, they should
undergo regular physical examinations to monitor the blood lipid
indicators, and measurements of carbohydrate antigen 199,
rheumatoid factor and thrombin time. Weight loss, smoking
cessation, healthy diet and maintaining physical and mental
well-being are also beneficial for controlling further
deterioration of blood lipid levels (61,62).
On the other hand, pilot management departments should implement
more health promotion and education programs to benefit pilots
(63).
It should be noted that in the present study, the
diagnostic criteria for hyperlipidemia were more stringent than
those in a previous study (64).
In the present study, if TC ≥6.2 mmol/l, TG ≥2.3 mmol/l or LDL-C
≥4.2 mmol/l, it was considered as hyperlipemia (65). However, most previous studies used
TC ≥5.2 mmol/l, TG ≥1.7 mmol/l or LDL-C ≥3.4 mmol/l as the
diagnostic criteria for hyperlipidemia, which might be considered
as dyslipidemia (63,66). This resulted in a relatively low
incidence of hyperlipidemia in the present study.
The main limitations of the present study were that
it was difficult to efficiently extract useful information from the
large amount of unstructured text in the electronic medical record
system, and required a significant amount of manpower and material
resources. The majority of the acquired variables had missing
values due to the varied contents of the physical examination,
which was not conducive to analysis. In addition, the resource data
of diagnoses was from the front page of the medical records, which
were mainly based on the knowledge of physicians and their ability
to diagnose all the conditions of the patients. If the doctors did
not notice the abnormal lipid parameters of the patients or did not
include all diagnoses on the front page of the medical record,
there might be bias of the data. However, this was considered
unlikely.
In conclusion, to the best of our knowledge, this is
the first time a large amount of data about pilots was structurally
categorized and thoroughly analyzed for the first, and multiple
advanced statistical models were carried out to obtain more
meaningful results. Several related risk factors of hyperlipidemia,
including carbohydrate antigen 199, thrombin time, lymphocytes and
rheumatoid factor were found in the present study, and might
provide the basis for further research.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are not
publicly available due to confidentiality restrictions, but may be
requested from the corresponding author.
Authors' contributions
FY designed the study, performed the data analysis,
interpreted the results, and wrote and revised the manuscript; YX
performed data collection and drew the plot. JY reviewed and
checked the references and tables, and performed the data collation
and validation. All authors read and approved the final version of
the manuscript. YX and JY confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The present study complied with the ethical
guidelines of the Declaration of Helsinki and was approved by the
Medical Research Ethics Committee of the Naval Medical Center
(Shanghai, China; approval no. 2019010101). Oral informed consent
for participation was obtained from the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ewald N, Hardt PD and Kloer HU: Severe
hypertriglyceridemia and pancreatitis: presentation and management.
Curr Opin Lipidol. 20:497–504. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Han Y, Jiang X, Qin Y, Zhao Y, Zhang G and
Liu C: Correction: A cross-sectional study exploring the
relationship between the dietary inflammatory index and
hyperlipidemia based on the national health and nutrition
examination survey (2005-2018). Lipids Health Dis.
22(161)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Malpica D: Metabolic syndrome,
hyperlipidemias, and associated clinical markers among military
airmen. Aerosp Med Hum Perform. 94:604–609. 2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nelson RH: Hyperlipidemia as a risk factor
for cardiovascular disease. Prim Care. 40:195–211. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Alaminos-Torres A, Martínez-Álvarez JR,
López-Ejeda N and Marrodán-Serrano MD: Atherogenic risk,
anthropometry, diet and physical activity in a sample of Spanish
commercial airline pilots. Int J Environ Res Public Health.
19(4128)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang D, Chen J, Cai W, Chen Y, Jiang C and
Yu X: Hyperlipidemia and intestinal microflora distributions among
flying personnel. Prev Med. 34:665–671. 2022.
|
|
7
|
Liu P, Bai S, Chen X, Mu H, Wang R, Li F
and Du P: Risk factors analysis and health management
countermeasures of dyslipidemia of flight personnel in 2020 annual
physical examination. In: Man-Machine-Environment System
Engineering: Proceedings of the 21st International Conference on
MMESE. Long S and Dhillon BS (eds). Springer, Singapore, pp88-93,
2022.
|
|
8
|
Maculewicz E, Pabin A, Kowalczuk K, Dziuda
Ł and Białek A: Endogenous risk factors of cardiovascular diseases
(CVDs) in military professionals with a special emphasis on
military pilots. J Clin Med. 11(4314)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yu L, Zhang Y and Wang L: Analysis of
hyperlipidemia in fighter pilots. Chin J Convalescent Med.
21:166–167. 2012.
|
|
10
|
Bin-Bin S, Xiao-Wei WU, Chun-Lei Z, et al:
Disease spectrum of hospitalized helicopter pilots. Mil Med J S
China, 2012.
|
|
11
|
Dong Y, Chen H, Ma J, et al: Investigation
and analysis of diseases of high-performance fighter pilots in
different regions. J Third Mil Med Univ. 22:2313–2314.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Smith RC, Segman RH, Golcer-Dubner T,
Pavlov V and Lerer B: Allelic variation in ApoC3, ApoA5 and LPL
genes and first and second generation antipsychotic effects on
serum lipids in patients with schizophrenia. Pharmacogenomics J.
8:228–236. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bays HE, Kirkpatrick C, Maki KC, Toth PP,
Morgan RT, Tondt J, Christensen SM, Dixon D and Jacobson TA:
Obesity, dyslipidemia, and cardiovascular disease: A joint expert
review from the obesity medicine association and the national lipid
association 2024. Obes Pillars. 10(100108)2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Huang H, Liu J, Feng Y and Chen W: The
distribution of apolipoprotein E gene polymorphism in Chinese civil
aircrews, and a possible risk factor to their overweight and
dyslipidemia is cumulative flight time. Clin Chim Acta. 416:36–40.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Qing C, Hong-Jin L and Tao C: Effect of
low density lipoprotein receptor gene nco site polymorphism on
blood lipid in pilots. J Prev Med Chin People's Liberation Army.
26:242–245. 2008.
|
|
16
|
Cai Q, Liu HJ, Chen T, et al: Relationship
between Hind III site polymorphism of lipoprotein lipase gene and
serum lipid level in pilots. Med J National Defending Forces S
China. 20:723–726. 2010.
|
|
17
|
Qing C, Hong-Jin L and Tao C: Relationship
between lipoprotein gene Ser447Ter site polymorphism and serum
lipid in pilots. Clin J Med Off. 39:209–211. 2011.
|
|
18
|
Jiang JY, Sun YC, Zhan X and Yan CQ: Study
on evaluating pilot workload based on muluti-source physiological
data fusion. Chin J Ergon. 29:2023.
|
|
19
|
Liu T, Qiu B, Zheng J, Zhang C, Qi Y, Fan
J, Li L and Gao J: Prevalence of and trends for dyslipidemia among
pilots from one airline in China. Int J Travel Med Glob Healt.
7:18–22. 2019.
|
|
20
|
Kong L, Zhao H, Wang F, Zhang R, Yao X,
Zuo R, Li J, Xu J, Qian Y, Kang Q and Fan C: Endocrine modulation
of brain-skeleton axis driven by neural stem cell-derived perilipin
5 in the lipid metabolism homeostasis for bone regeneration. Mol
Ther. 31:1293–1312. 2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Choi YY and Kim KY: Effects of physical
examination and diet consultation on serum cholesterol and
health-behavior in the Korean pilots employed in commercial
airline. Ind Health. 51:603–611. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xiao D, Li H, Wang X, Wang B, Yan Y and
Men K: Prevalence of disease spectrum and sick leave time
associated with illness in helicopter pilots. Aviat Space Environ
Med. 84:234–236. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
World Health Organization (WHO):
International statistical classification of diseases and related
health problems (ICD-10) in occupational health. WHO, Geneva, 1999.
https://iris.who.int/bitstream/handle/10665/66100/WHO_SDE_OEH_99.11.pdf?sequence=1.
Accessed May 26, 2024.
|
|
24
|
Atkins DC: Using multilevel models to
analyze couple and family treatment data: basic and advanced
issues. J Fam Psychol. 19:98–110. 2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Stawski RS: Multilevel analysis: An
introduction to basic and advanced multilevel modeling (2nd
edition). Struct Equ Model. 20:541–550. 2013.
|
|
26
|
Eilat-Tsanani S, Ernst P and Suissa S:
Real-world effectiveness of single-inhaler triple therapy for COPD:
Impact of diabetes comorbidity. COPD. 21(2327345)2024.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rosenbaum PR and Rubin DB: The central
role of the propoensity score in observational studies for causal
effects. Biometrika. 70:41–55. 1983.
|
|
28
|
Meir R and Rätsch G: An introduction to
boosting and leveraging. In: Advanced Lectures on Machine Learning.
Mendelson S and Smola A (eds). Springer, Berlin, Heidelberg,
pp119-184, 2003.
|
|
29
|
McCaffrey DF, Ridgeway G and Morral AR:
Propensity score estimation with boosted regression for evaluating
causal effects in observational studies. Psychol Methods.
9:403–425. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lee BK, Lessler J and Stuart EA: Improving
propensity score weighting using machine learning. Stat Med.
29:337–346. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
George EL and Panos A: Does a high WBC
count signal infection? Nursing. 35:20–2. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen JS, Xie PF and Feng H: The role of
exercise in improving hyperlipidemia-renal injuries induced by a
high-fat diet: A literature review. PeerJ.
11(e15435)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Pendry SD, Singhal N, Neo EL, Foreman D
and Winter JM: Elevation of the tumor marker CA19-9 in a pancreatic
cancer survivor with benign prostatic hyperplasia: A clinical case
report. Clin Case Rep. 12(e8929)2024.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu S and Yuan H: The further evaluation
of the clinical significance of serum CA199 and its diagnostic
value of hepatobiliary and pancreatic diseases. J Med Theor Prac
(Chin). 22:1417–1419. 2009.
|
|
35
|
Bao SQ, Li FB and Jiang X: Correlation
between carbohydrate antigen 199 and glycemic control in patients
with type 2 diabetes mellitus. Chin Med J (Engl). 132:984–986.
2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gul K, Nas S, Ozdemir D, Gumus M, Ersoy R
and Cakir B: CA 19-9 level in patients with type 2 diabetes
mellitus and its relation to the metabolic control and
microvascular complications. Am J Med Sci. 341:28–32.
2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Adachi M, Sekine T, Umemoto A, Tsukikawa
S, Imai K and Yachi A: Mechanism of clearance of circulating CA19-9
in rats. Tumour Biol. 11:51–58. 1990.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Al-Tonsi AA, Abdel-Gayoum AA and Saad M:
The secondary dyslipidemia and deranged serum phosphate
concentration in thyroid disorders. Exp Mol Pathol. 76:182–187.
2004.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Xu M, Li Y, Zhao W, Song X, Gan G, Li B
and Zhou X: Association between admission prothrombin time activity
and hospital readmission in heart failure: A retrospective study.
Clin Chim Acta. 548(117463)2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lai M, Peng H, Wu X, Chen X, Wang B and Su
X: IL-38 in modulating hyperlipidemia and its related
cardiovascular diseases. Int Immunopharmacol.
108(108876)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Xu DM, Li Q, Yi JX, Cai XJ, Xie L, Fang W,
Qiu JF, Xu CW, He CL, Xu XR, et al: Investigation of lymphocyte
subsets in peripheral blood of patients with dyslipidemia. Int J
Gen Med. 14:5573–5579. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Robinson GA, Peng J, Pineda-Torra I,
Ciurtin C and Jury EC: Metabolomics defines complex patterns of
dyslipidaemia in juvenile-SLE patients associated with inflammation
and potential cardiovascular disease risk. Metabolites.
12(3)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Georgiadis AN, Papavasiliou EC, Lourida
ES, Alamanos Y, Kostara C, Tselepis AD and Drosos AA: Atherogenic
lipid profile is a feature characteristic of patients with early
rheumatoid arthritis: Effect of early treatment-a prospective,
controlled study. Arthritis Res Ther. 8(R82)2006.PubMed/NCBI View
Article : Google Scholar
|
|
44
|
Yan J, Yang S, Han L, Ba X, Shen P, Lin W,
Li T, Zhang R, Huang Y, Huang Y, et al: Dyslipidemia in rheumatoid
arthritis: The possible mechanisms. Front Immunol.
14(1254753)2023.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Myasoedova E, Crowson CS, Kremers HM,
Fitz-Gibbon PD, Therneau TM and Gabriel SE: Total cholesterol and
LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis.
69:1310–1314. 2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Dursunoğlu D, Evrengül H, Polat B,
Tanrıverdi H, Çobankara V, Kaftan A and Kılıç M: Lp(a) lipoprotein
and lipids in patients with rheumatoid arthritis: Serum levels and
relationship to inflammation. Rheumatol Int. 25:241–245.
2005.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Warrington KJ, Kent PD, Frye RL, Lymp JF,
Kopecky SL, Goronzy JJ and Weyand CM: Rheumatoid arthritis is an
independent risk factor for multi-vessel coronary artery disease: A
case control study. Arthritis Res Ther. 7:R984–R991.
2005.PubMed/NCBI View
Article : Google Scholar
|
|
48
|
Mainieri F, La Bella S and Chiarelli F:
Hyperlipidemia and cardiovascular risk in children and adolescents.
Biomedicines. 11(809)2023.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wisse BE: The inflammatory syndrome: The
role of adipose tissue cytokines in metabolic disorders linked to
obesity. J Am Soc Nephrol. 15:2792–2800. 2004.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Peyronnel C, Totoson P, Martin H and
Demougeot C: Relevance of circulating markers of endothelial
activation for cardiovascular risk assessment in rheumatoid
arthritis: A narrative review. Life Sci. 314(121264)2023.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ferreira HB, Melo T, Paiva A and Domingues
MDR: Insights in the role of lipids, oxidative stress and
inflammation in rheumatoid arthritis unveiled by new trends in
lipidomic investigations. Antioxidants (Basel).
10(45)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Bonacina F, Pirillo A, Catapano AL and
Norata GD: HDL in immune-inflammatory responses: Implications
beyond cardiovascular diseases. Cells. 10(1061)2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kreiner FF, Kraaijenhof JM, von Herrath M,
Hovingh GKK and von Scholten BJ: Interleukin 6 in diabetes, chronic
kidney disease, and cardiovascular disease: Mechanisms and
therapeutic perspectives. Expert Rev Clin Immunol. 18:377–389.
2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Leclercq G, Servera LA, Danilin S,
Challier J, Steinhoff N, Bossen C, Odermatt A, Nicolini V, Umaña P,
Klein C, et al: Dissecting the mechanism of cytokine release
induced by T-cell engagers highlights the contribution of
neutrophils. Oncoimmunology. 11(2039432)2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Ou Q, Power R and Griffin MD: Revisiting
regulatory T cells as modulators of innate immune response and
inflammatory diseases. Front Immunol. 14(1287465)2023.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Bonfigli AR, Protic O, Olivieri F,
Montesanto A, Malatesta G, Di Pillo R and Antonicelli R: Effects of
a novel nutraceutical combination (BruMeChol™) in subjects with
mild hypercholesterolemia: Study protocol of a randomized,
double-blind, controlled trial. Trials. 21(616)2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Alanazi A: Clinicians' views on using
artificial intelligence in healthcare: Opportunities, challenges,
and beyond. Cureus. 15(e45255)2023.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lalo R, Zekja I and Kamberi F: Association
of cardiovascular disease risk and health-related behaviors in
stroke patients. Int J Environ Res Public Health.
20(3693)2023.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Michos ED, McEvoy JW and Blumenthal RS:
Lipid management for the prevention of atherosclerotic
cardiovascular disease. N Engl J Med. 381:1557–1567.
2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Damen JA, Hooft L, Schuit E, Debray TP,
Collins GS, Tzoulaki I, Lassale CM, Siontis GC, Chiocchia V,
Roberts C, et al: Prediction models for cardiovascular disease risk
in the general population: Systematic review. BMJ.
353(i2416)2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Ornish D, Scherwitz LW, Billings JH, Brown
SE, Gould KL, Merritt TA, Sparler S, Armstrong WT, Ports TA,
Kirkeeide RL, et al: Intensive lifestyle changes for reversal of
coronary heart disease. JAMA. 280:2001–2007. 1998.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Kreisberg RA and Oberman A: Medical
management of hyperlipidemia/dyslipidemia. J Clin Endocrinol Metab.
88:2445–2461. 2003.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Liu T, Qiu B, Zhang C, Deng M, Liang Z and
Qi Y: Health-related quality of life in pilots of a Chinese
commercial airline. Arch Environ Occup Health. 76:511–517.
2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Garg A and Radhakrishnan S: Pediatric
hyperlipidemia. Indian Heart J. 76 (Suppl 1):S104–S107.
2024.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Joint Committee on the Chinese Guidelines
for Lipid Management. Chinese guidelines for lipid management
(2023). Zhonghua Xin Xue Guan Bing Za Zhi. 51:221–255.
2023.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
66
|
Bernier AV, Sentner CP, Correia CE,
Theriaque DW, Shuster JJ, Smit GPA and Weinstein DA: Hyperlipidemia
in glycogen storage disease type III: Effect of age and metabolic
control. J Inherit Metab Dis. 31:729–732. 2008.PubMed/NCBI View Article : Google Scholar
|