Introduction
A member of the Netrin family, Netrin-4 (NTN4), is
an essential secreted protein present in the vascular endothelium,
and is involved in tumor metastasis and brain development (1). NTN4 is present in neural stem cells
and contributes to neurite growth in olfactory bulb explants
(2). NTN4 mRNA levels in invasive
carcinoma of the breast are lower compared with those in
surrounding tissues (3).
Conversely, increased levels of NTN4 in melanoma are linked to
metastasis (4). High NTN4
expression in endothelial cells (ECs) participates in inhibiting
endothelial cell migration, promoting EC survival and vascular
system formation and is essential for vascular health and stability
(5). NTN4 impedes the motility and
organization of human microvascular ECs in a controlled laboratory
environment (6). NTN4 also fosters
blood vessel formation in zebrafish models, with its knockdown
leading to notable vascular system defects (7). In addition, exogenous NTN4 stimulates
vascular smooth muscle cell adhesion and migration, and exhibits a
pro-survival effect (8).
Vascular endothelial (VE)-cadherin, a specialized
adhesion protein, resides specifically at the intercellular
junctions of ECs (9). VE-cadherin
is essential to maintaining vascular integrity, mediating cell-cell
adhesion and facilitating signal transduction for angiogenesis and
inflammatory responses (10).
VE-cadherin maintains endothelial cell morphology and junctions,
preserving vascular barrier function through its interaction with
β/α-catenin proteins (11).
Previous studies have reported the abnormal expression of
VE-cadherin in certain aggressive tumors, such as invasive glioma,
melanoma and breast cancer (12-14).
In addition, suppressing VE-cadherin function inhibits tubule
formation in ECs (15). Mice
lacking VE-cadherin die mid-gestation due to serious vascular
defects (16). As aforementioned,
NTN4 also inhibits cell migration, promotes vascular system
development and enhances cell survival in ECs. Nevertheless, the
regulatory pathway between VE-cadherin and NTN4 has not been
investigated.
Several signaling pathways control the expression of
VE-cadherin, one of which is the NF-κB pathway (17). NF-κB is a vital transcription
factor that plays a key role in cellular processes, inflammation
and immune responses (18). IκB-α
is a protein that inhibits NF-κB and keeps NF-κB inactive in the
cytoplasm (19). Upon receiving
activation signals, such as lipopolysaccharide, cytokines, viral
protein and oxygen free radicals, IκB-α is degraded, allowing NF-κB
to translocate into the cell nucleus and regulate gene
transcription (20). NF-κB is
involved in the expression of adhesion molecules (such as vascular
cell adhesion molecule-1), chemotactic factors and pro-inflammatory
cytokines (such as IL-6 and IL-8) in vascular ECs (21). NF-κB binding sites are located in
the promoter regions of a number of transcriptional regulatory
genes that are expressed in response to inflammatory mediators,
such as lipopolysaccharides, IL-1 or TNF-α (22). ECs with suppressed NF-κB activity
exhibit reduced VE-cadherin expression and suffer from compromised
endothelial barriers (23). This
indicates that NF-κB has a multifaceted function in ECs, primarily
through regulating VE-cadherin expression.
The present study aimed to investigate whether NTN4
overexpression in ECs could influence cell function and VE-cadherin
expression, and whether the NF-κB signaling pathway is involved in
this process.
Materials and methods
Cell culture and treatment
HUVECs (CRL-1730) were purchased from the American
Type Culture Collection and cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.), supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin. The cells were
kept at a temperature of 37˚C in a humid environment containing 5%
CO2. PDTC powder (Beyotime Institute of Biotechnology)
was dissolved in dimethyl sulfoxide (DMSO; Beijing Solarbio Science
& Technology Co., Ltd.) at a concentration of 10 mM. For
inhibitory experiments, HUVECs were treated with PDTC at a final
concentration of 10 µM for 2 h at 37˚C. The control groups were
treated with an equal amount of DMSO solution.
Cell transfection
The full length coding sequence of human NTN4
(NM_021229) was synthesized and cloned into GV657 expression vector
by GeneChem, Inc. The corresponding empty GV657 plasmid was used as
negative control. HUVECs were seeded in 6-well plates and allowed
to grow to 50-60% confluency. For each well, 2.5 µg plasmid DNA was
incubated at room temperature for 15 min and transfected using
Lipofectamine™ 3000 reagent (cat. no. L3000015; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Following 6 h incubation at 37˚C, the culture medium was replaced
with fresh media. The cells were harvested for experiments 24 h
after transfection.
Cell Counting Kit-8 (CCK-8) assay
Cell viability was measured using the CCK-8 assay
kit (cat. no. CA1210; Beijing Solarbio Science & Technology
Co., Ltd.), according to the manufacturer's instructions. In brief,
cells were seeded in a 96-well plate at a density of 5x10³ per
well. On the second day, 10 µl CCK-8 solution was added to each
well. After 4 h of incubation, the absorbance was measured on a
Multiskan GO spectrophotometer (Thermo Fisher Scientific, Inc.) at
450 nm.
Wound healing assay
HUVECs were seeded in 6-well plates and allowed to
grow to 100% confluency. Cells were serum starved for 2 h at 37˚C
with 2% FBS medium. The cell monolayer was scratched using a
sterile pipette nozzle and washed with PBS to remove debris. Images
of six arbitrary fields were captured during the scratch assay at 0
and 24 h in order to measure the scratch area and determine the
migration area under a light microscope (Olympus Corporation).
Wound healing was analyzed by ImageJ software 1.4.3 (National
Institutes of Health). The migration rate was calculated as
follows: Migration rate (%)=[(scratch width at 0 h-scratch width at
24 h)/scratch width at 0 h] x100% .
Transwell migration assay
The experiment was carried out using an 8-µm pore
Transwell chamber system (Corning, Inc.) in a 24-well plate. HUVECs
(5x103 cells/well) were seeded in the upper inserts in
200 µl serum-free DMEM. The lower chamber was filled with 600 µl
DMEM containing 5% FBS as a chemoattract to direct cell migration.
After 24 h of incubation at 37˚C, the cell inserts were washed with
PBS and fixed with 4% formaldehyde solution (1 ml per well) for 30
min at 37˚C. The cells were then stained with 0.1% crystal violet
(1 ml per well) at 37˚C for 30 min. The upper chamber was wiped
using swabs to remove non-migrated cells and washed with PBS three
times. Ultimately, images were captured using an inverted
microscope (DMi8; Leica Microsystems, Inc.) at a magnification of
x400, and migrated HUVECs were counted manually.
FITC-dextran Transwell assay
The experiment was carried out using a 0.4-µm pore
Transwell chamber system (Corning, Inc.) in a 24-well plate. HUVECs
(2x104 cells/well) were seeded in the upper inserts in
200 µl DMEM . The lower chamber was filled with 600 µl DMEM
supplemented with 10% FBS. When cells grew to 100% confluence, the
upper chamber was supplemented with 3 µl 70 kDa FITC-dextran (20
mg/ml; cat. no. FD250S; Sigma-Aldrich; Merck KGaA). After 4 h of
incubation at 37˚C, the medium from the lower chamber was
transferred into a 96-well plate and measured using a fluorometer
(Multiskan GO), at an emission wavelength of 520 nm and an
excitation wavelength 485 nm.
Western blotting
HUVECs were rinsed three times with ice-cold PBS and
then lysed in RIPA lysis buffer containing 1% protease inhibitor
(Beyotime Institute of Biotechnology) for 30 min on ice. The
lysates were centrifuged at 15,000 x g at 4˚C for 15 min and the
supernatants were collected and used for quantification of total
proteins by a BCA protein assay kit (Thermo Fisher Scientific,
Inc.). Following heat denaturation at 95˚C for 15 min, the protein
samples (15 µg) were separated by SDS-PAGE on 10% polyacrylamide
gels and then transferred onto PVDF membranes. Next, 5% non-fat
milk was used to block the membrane (Santa Cruz Biotechnology,
Inc.) for 2 h at room temperature. Antibodies were diluted in
primary antibody dilution buffer (Beyotime Institute of
Biotechnology) and incubated overnight at 4˚C. The following
primary antibodies were used: Rabbit anti-NF-κB p65 (1:1,000, cat.
no. 8242; Cell Signaling Technology, Inc.), mouse anti-NTN4
(1:1,000, cat. no. MAB1254; R&D Systems, Inc.), rabbit
anti-VE-cadherin (1:1,000, cat. no. ab33168; Abcam) and mouse
anti-IκB-α (1:1,000, cat. no. 4814; Cell Signaling Technology,
Inc.). β-actin (1:10,000, 20536-1-AP, Proteintech Group, Inc.) was
used as the loading control. The membranes were incubated with
HRP-conjugated secondary antibodies: Goat Anti-Rabbit IgG
(111-035-003, Jackson ImmunoResearch Laboratories, Inc) and Goat
anti-Mouse IgG (both 1:10,000, 115-035-003, Jackson ImmunoResearch
Laboratories, Inc) at room temperature for 2 h. Immunoblots were
visualized using Immobilon Western Chemiluminescent HRP substrate
(MilliporeSigma) on a Bio-Rad imaging system (Bio-Rad Laboratories,
Inc.). Densitometry was performed using ImageJ version 1.8.
Reverse transcription-quantitative PCR
(RT-qPCR)
RNA was isolated from HUVECs using
TRIzol® reagent from Invitrogen (Thermo Fisher
Scientific, Inc.). Subsequently, cDNA synthesis and amplification
were performed using the HiScript® III RT Super Mix
(cat. no. R323-01; Vazyme Biotech, Co., Ltd.). The RT procedure
was: 2 min at 42˚C, 15 min at 37˚C, 5 sec at 85˚C and the 30 min at
4˚C. For RT-qPCR analysis, ChamQ Universal SYBR qPCR Master Mix
(cat. no. Q711-02; Vazyme Biotech, Co., Ltd.) was used. The
thermocycling conditions were as follows: 95˚C for 30 sec, 40
cycles at 95˚C for 10 sec and 60˚C for 30 sec. The internal
reference gene used was GAPDH. The relative expression levels of
the target genes were measured using the 2-ΔΔCq method
(24). The following primer
sequences (5'-3') were used: GAPDH forward,
GGAGCGAGATCCCTCCAAAAT and reverse, GGCTGTTGTCATACTTCGCATGG;
NTN4 forward, ACACTCAGGTAAATGCGAATGT and reverse,
ACCTTTTTAATCTTCACATTGACCT; VE-cadherin forward,
GCGACTACCAGGACGCTTTCA and reverse, CATGTATCGGAGGTCGATGGTG.
Statistical analysis
GraphPad Prism 5.0 (GraphPad; Dotmatics) was used
for statistical analysis. All data are presented as the mean ± SD,
unless otherwise stated. Each experiment was performed at least
three times independently, and statistical significance was
calculated using an unpaired Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
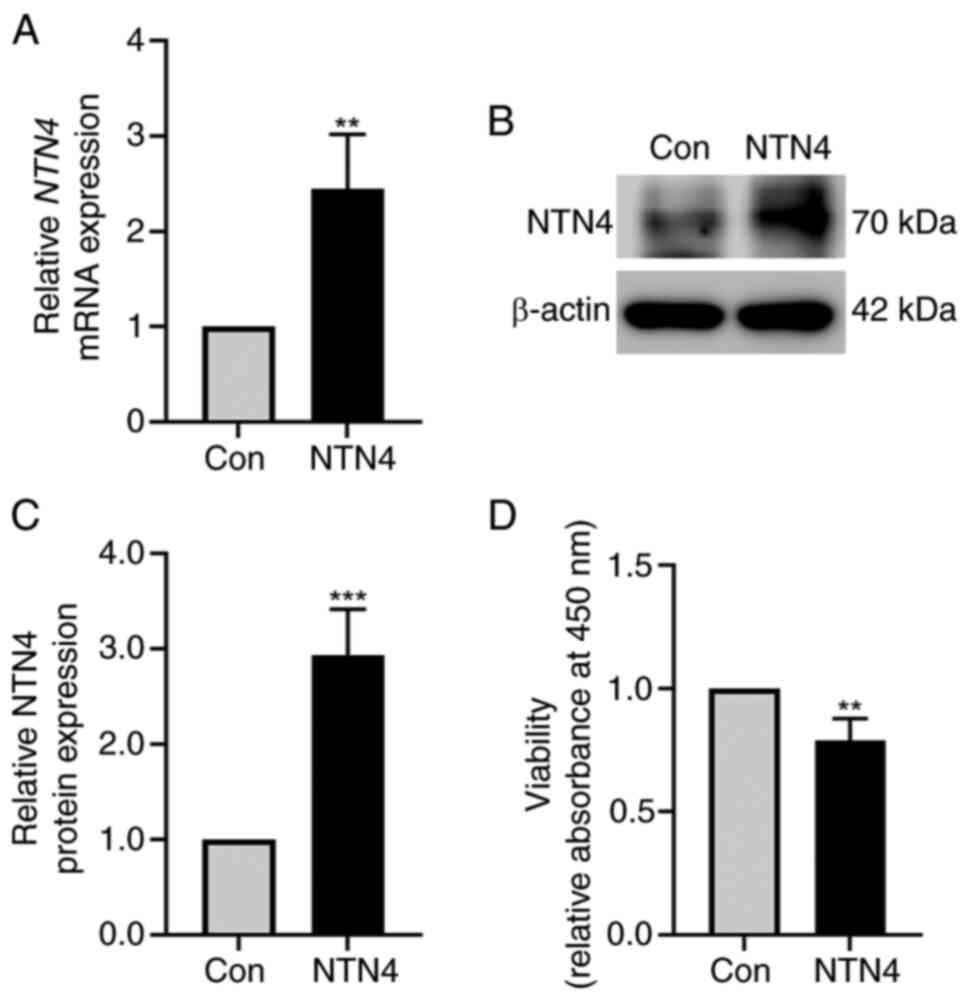
NTN4 overexpression reduces HUVEC
viability
HUVECs were transfected with control and
NTN4-overexpressing plasmid. NTN4 expression was validated using
western blotting and RT-qPCR. NTN4 mRNA expression was
significantly increased in NTN4-overexpressing HUVECs (Fig. 1A). NTN4 protein levels were also
markedly higher in NTN4-overexpressing HUVECs (Fig. 1B). In addition, densitometry of
protein bands showed a significant increase in NTN4 expression in
NTN4-overexpressing HUVECs compared with the control group
(Fig. 1C). To test the role of
NTN4 in HUVEC viability, cells were subjected to a CCK-8 assay
following transfection with control or NTN4-overexpressing
plasmids. The findings showed that cell viability was significantly
lower in NTN4-overexpressing HUVECs compared with that in the
control group (Fig. 1D).
Therefore, NTN4 overexpression was shown to inhibit HUVEC cell
viability.
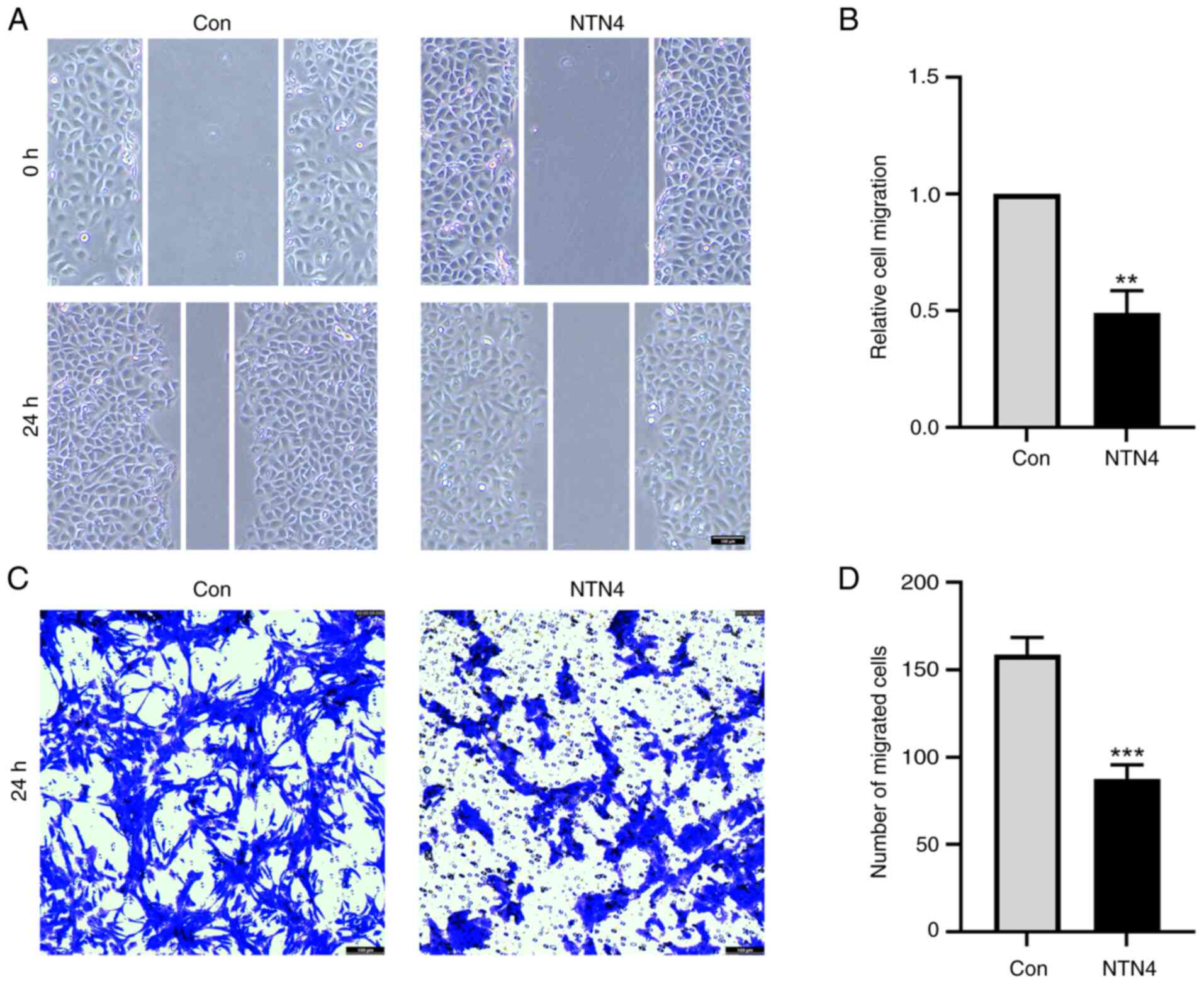
NTN4 overexpression reduces HUVEC
migration
To assess the effect of NTN4 overexpression on HUVEC
migration, wound healing and Transwell assays were performed.
Relative cell migration of NTN4-overexpressing cells was
significantly reduced at ~50% of that in the control group
(Fig. 2A and B). In the Transwell assay, the number of
HUVECs that migrated from the top to the lower chamber was
significantly lower in the NTN4-overexpressing group compared with
that in the control group (Fig. 2C
and D). Overall, NTN4
overexpression in HUVECs resulted in impaired migration in both
wound healing experiments and Transwell assays.
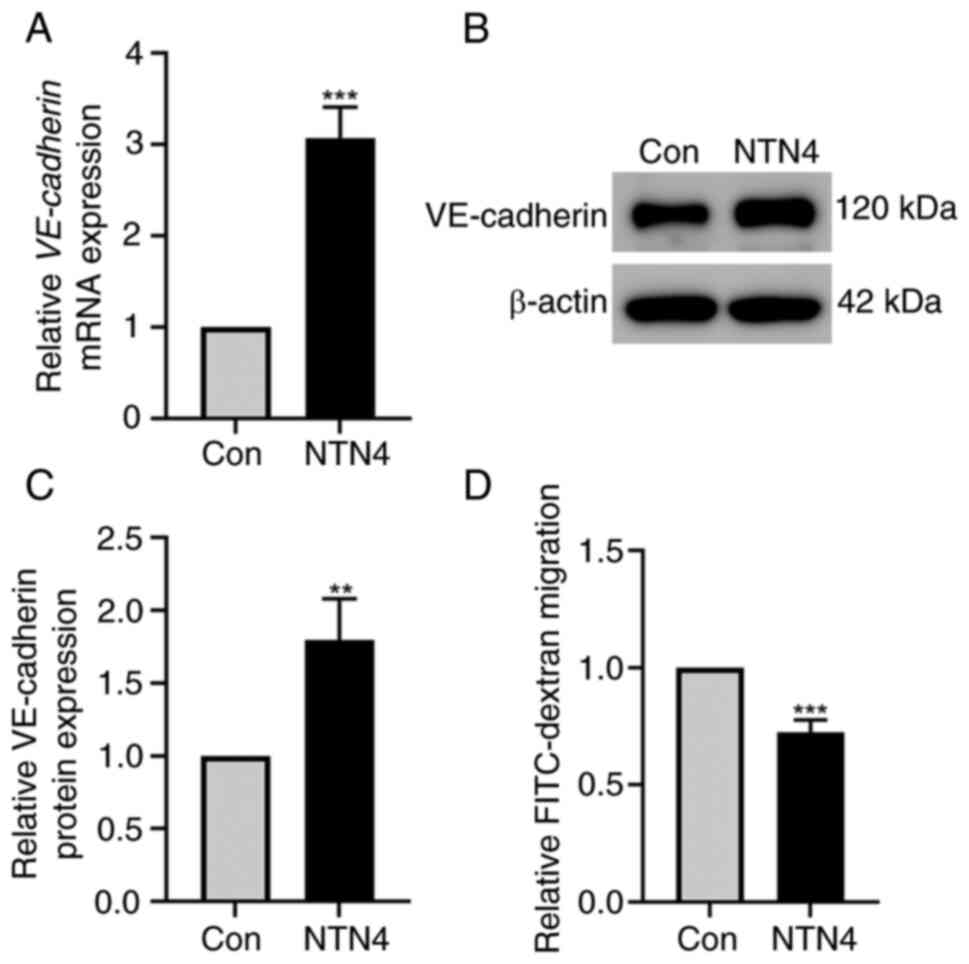
NTN4 overexpression increases the
expression of VE-cadherin in HUVECs and reduces the permeability of
HUVECs
HUVECs were transfected with control and
NTN4-overexpressing plasmids to assess the impact of NTN4
overexpression on VE-cadherin expression level and permeability.
RT-qPCR results demonstrated a significant increase in VE-cadherin
mRNA levels in HUVECs transfected with the NTN4-overexpressing
plasmid compared with that in the control group (Fig. 3A). The western blotting results
revealed an increase in the VE-cadherin protein expression level in
HUVECs after NTN4 overexpression (Fig.
3B). In addition, densitometry of protein bands revealed a
significant difference in VE-cadherin expression between the two
groups (Fig. 3C). VE-cadherin
controls the adhesion of vascular ECs, which helps to preserve the
integrity and permeability of the vascular endothelial layer
(25). Therefore, the present
study measured the permeability of cell monolayers using a
FITC-dextran Transwell assay. The FITC-dextran migration was
significantly reduced in cells with NTN4 overexpression compared
with the control (Fig. 3D). As a
result, overall, NTN4 overexpression increased VE-cadherin
expression levels and decreased HUVEC permeability.
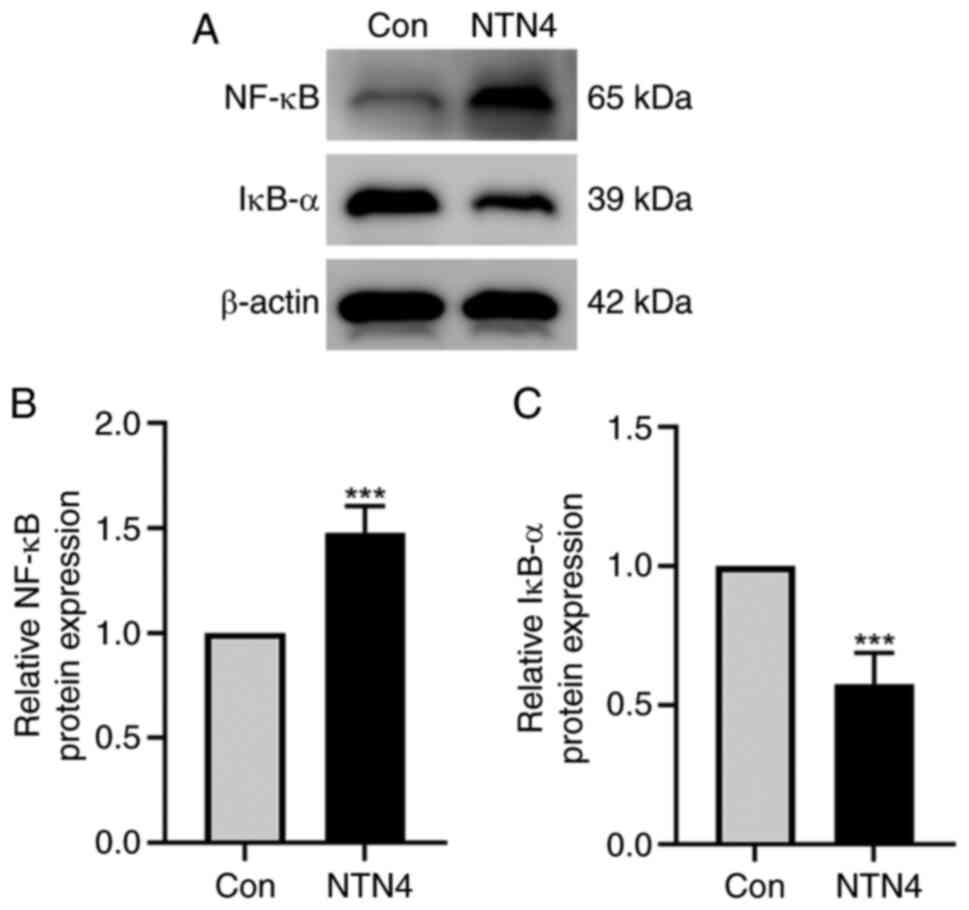
NTN4 overexpression increases NF-κB
and decreases IκB-α protein expression in HUVECs
HUVECs were transfected with control and
NTN4-overexpressing plasmids, and the protein expression of NF-κB
and IκB-α was assessed using western blotting. The findings
indicated a reduction in IκB-α protein expression and an increase
in NF-κB protein expression in the NTN4-overepxression group
(Fig. 4A). Densitometry of protein
bands showed significant difference in both NF-κB and IκB-α
expression between the two groups (Fig. 4B and C). Thus, in HUVECs, NTN4 overexpression
caused a decrease in IκB-α protein expression and an increase in
NF-κB protein expression.
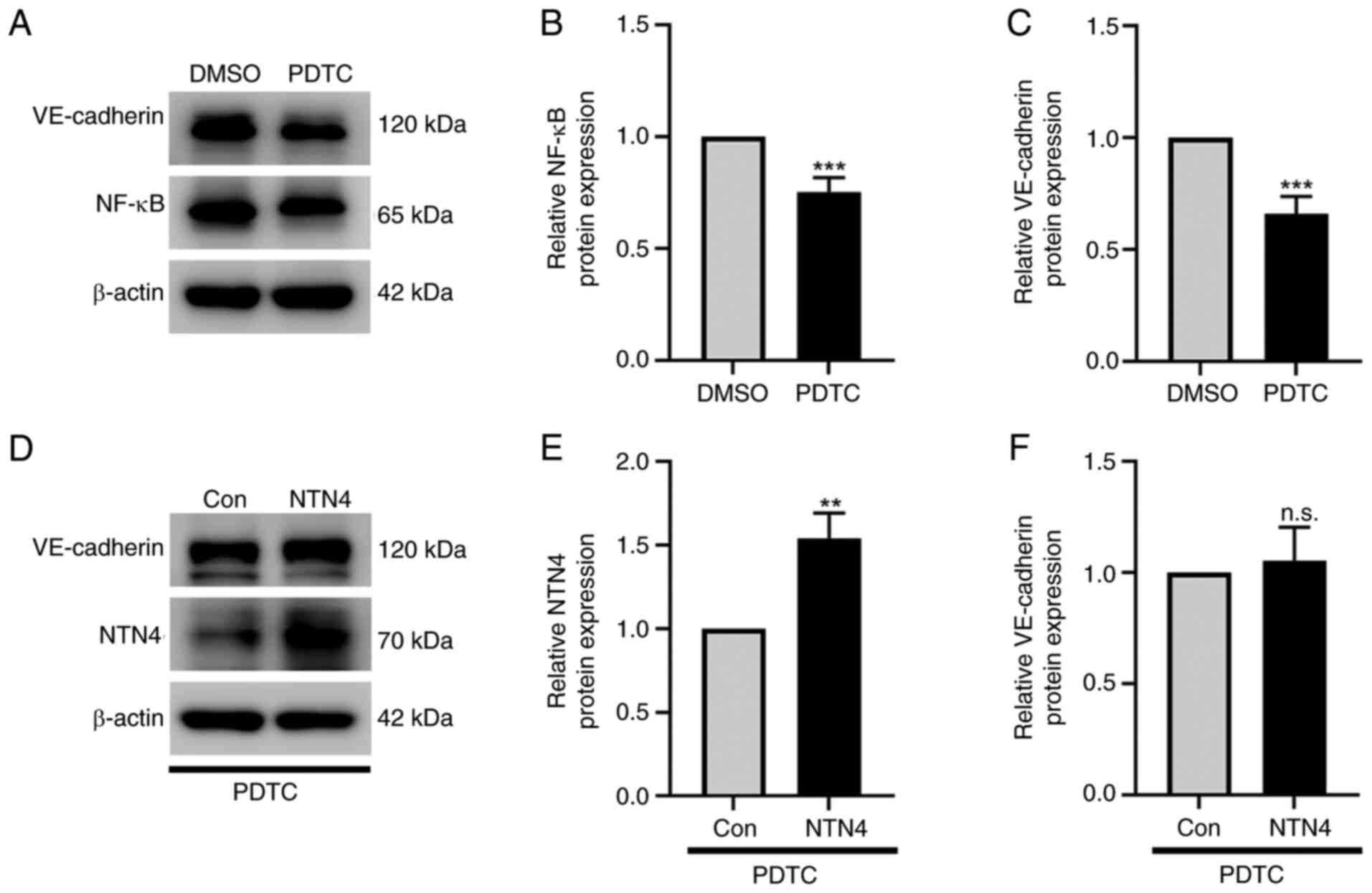
In HUVECs treated with NF-κB inhibitor
PDTC, NTN4 overexpression does not alter VE-cadherin
expression
The protein expression of VE-cadherin, NTN4 and
NF-κB was determined in HUVECs treated with PDTC using western
blotting. PDTC significantly suppressed NF-κB (Fig. 5A and B) and VE-cadherin expression (Fig. 5A and C) in normal HUVECs at a dose of 10 µM
compared with the DMSO-treated control. The intensity of protein
bands was evaluated by normalizing them to β-actin (Fig. 5B and C). However, when NTN4 was significantly
overexpressed in HUVECs treated with the NF-κB inhibitor PDTC
(Fig. 5D and E), VE-cadherin protein expression was not
significantly different compared with the control group (Fig. 5D and F). The protein band intensities were
measured in relation to β-actin to evaluate the expression levels
of VE-cadherin and NTN4 (Fig. 5E
and F). According to these
findings, NTN4 overexpression stimulated the NF-κB signaling
pathway, which in turn induced the expression of VE-cadherin in
HUVECs.
Discussion
In the present study, an NTN4-overexpression plasmid
was transfected into HUVECs to induce NTN4 overexpression. HUVEC
migration and cell viability were inhibited by NTN4 overexpression.
Furthermore, overexpression of NTN4 resulted in decreased HUVEC
permeability and increased VE-cadherin expression. Subsequent
investigations showed that NTN4 overexpression increased NF-κB
protein expression and decreased IκB-α protein expression in
HUVECs. In HUVECs treated with NF-κB inhibitor PDTC, NTN4
overexpression did not cause a change in the expression of
VE-cadherin.
The Netrin family member NTN4, which is highly
conserved, is essential for healthy vascular function, tumor growth
and neural development (1). For
instance, a previous study showed that NTN4 overexpression prevents
clear cell renal cell carcinoma growth (26). In breast cancer cells, NTN4
overexpression leads to reduced migration and invasion rates
(27). In addition, diminishing
NTN4 reduces EC permeability (5).
In line with the aforementioned findings, the present study
revealed that NTN4 overexpression inhibited the migration and
viability of HUVECs and reduced their permeability.
VE-cadherin maintains intercellular adhesion and
structural integrity between vascular ECs. In addition, VE-cadherin
regulates cellular dynamics including migration, proliferation and
permeability (28). The
phosphorylation of VE-cadherin results in VE-cadherin
internalization into clathrin-coated vesicles and the consequent
disassembly from intercellular junctions. This is another route for
VE-cadherin to regulate endothelial permeability (25). VE-cadherin is regulated by vascular
endothelial growth factor (VEGF). VEGF induces VE-cadherin to
internalize fast, endangering the integrity of the endothelial
barrier (25). Furthermore, in
human cells, the VE-cadherin promoter is actively suppressed by the
transcription factor Slug (29).
In addition, bone morphogenetic protein 6 controls the
internalization of VE-cadherin, which increases the permeability of
human ECs (30). Furthermore, the
NF-κB signaling pathway, which is necessary for the inhibition of
apoptosis and the promotion of cell survival, modulates VE-cadherin
(23,31). IκB-α functions as a protein that
inhibits the NF-κB signaling pathway (19). The speed of endothelial barrier
collapse increases when VE-cadherin levels are significantly
reduced, which is associated with the blocking of NF-κB (23). However, the relationship between
NTN4 and VE-cadherin has not yet been studied. The present study
revealed that NTN4 overexpression increased VE-cadherin expression
in HUVECs, suggesting a novel way to modulate VE-cadherin.
NF-κB triggers the transcription of multiple genes
linked to inflammation, resulting in the control of cell adhesion
and survival (32). Concurrently,
the PI3K/AKT pathway is activated by the interaction of NTN4 with
integrin β4(33). The NF-κB
signaling pathway is triggered by the AKT pathway, which increases
cell survival (34). A previous
study has indicated that NTN4 improves endothelial cell survival in
a way that is dependent on time and dosage (35). These results suggest a synergistic
interaction among NTN4, the PI3K/AKT pathway and NF-κB signaling.
Furthermore, NTN4 and NF-κB serve roles in preserving endothelial
permeability. NF-κB increases the expression of adhesion molecules,
enhancing the adhesiveness and permeability of ECs (36). Through integrins α2β1 and α3β1,
endothelium-derived NTN4 promotes pancreatic epithelial cell
adhesion (37). The present study
showed that overexpressing NTN4 in the HUVECs led to significantly
increased levels of NF-κB and significantly decreased levels of
IκB-α, suggesting the activation of the NF-κB signaling pathway.
Moreover, VE-cadherin expression was not induced by NTN4
overexpression in HUVECs treated with NF-κB inhibitors. Therefore,
the present study demonstrated that NTN4 overexpression increased
VE-cadherin expression levels in a NF-κB signaling-dependent
manner.
In conclusion, the present study provided evidence
that the NTN4 overexpression decreased endothelial cell viability
and migration. Furthermore, the present study revealed a novel role
of NTN4 in the regulation of VE-cadherin expression and related
mechanism, as well as the protection of endothelial barrier
integrity by NTN4. These findings provided a novel regulatory
mechanism of VE-cadherin expression, as well as a direction for
future studies to investigate the role of NTN4 in endothelial
barrier-related diseases. Nevertheless, nuclear NF-κB data and
in vivo data are lacking due to time constraints. These
limitations should be further addressed in future
investigations.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Natural Science
Foundation of Shandong Province (grant no. ZR2020MH181) and
National Natural Science Foundation of China (grant no.
82171318).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
DZ contributed to the experimental design,
acquisition of data and data analysis. ZZ and KW contributed to the
writing and editing of the manuscript, as well as the analysis and
interpretation of the data. SZ and JL contributed to the
conception, experimental design, acquisition of data, data
analysis, and the writing and editing of the manuscript. DZ, SZ and
JL confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dong F, Liu Y, Yan W, Meng Q, Song X,
Cheng B and Yao R: Netrin-4: Focus on its role in axon guidance,
tissue stability, angiogenesis and tumors. Cell Mol Neurobiol.
43:1663–1683. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Koch M, Murrell JR, Hunter DD, Olson PF,
Jin W, Keene DR, Brunken WJ and Burgeson RE: A novel member of the
netrin family, β-Netrin, shares homology with the β chain of
laminin: Identification, expression, and functional
characterization. J Cell Biol. 151:221–234. 2000.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yi L, Lei Y, Yuan F, Tian C, Chai J and Gu
M: NTN4 as a prognostic marker and a hallmark for immune
infiltration in breast cancer. Sci Rep. 12(10567)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bruikman CS, Zhang H, Kemper AM and van
Gils JM: Netrin family: Role for protein isoforms in Cancer. J
Nucleic Acids. 2019(3947123)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang H, Vreeken D, Leuning DG, Bruikman
CS, Junaid A, Stam W, de Bruin RG, Sol WMPJ, Rabelink TJ, van den
Berg BM, et al: Netrin-4 expression by human endothelial cells
inhibits endothelial inflammation and senescence. Int J Biochem
Cell Biol. 134(105960)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nacht M, St Martin TB, Byrne A, Klinger
KW, Teicher BA, Madden SL and Jiang Y: Netrin-4 regulates
angiogenic responses and tumor cell growth. Exp Cell Res.
315:784–794. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lambert E, Coissieux MM, Laudet V and
Mehlen P: Netrin-4 acts as a pro-angiogenic factor during zebrafish
development. J Biol Chem. 287:3987–3999. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lejmi E, Bouras I, Camelo S, Roumieux M,
Minet N, Leré-Déan C, Merkulova-Rainon T, Autret G, Vayssettes C
and Clement O: Netrin-4 promotes mural cell adhesion and
recruitment to endothelial cells. Vasc Cell. 6(1)2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Aman J and Margadant C: Integrin-dependent
cell-matrix adhesion in endothelial health and disease. Circ Res.
132:355–378. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lampugnani MG, Dejana E and Giampietro C:
Vascular endothelial (VE)-cadherin, endothelial adherens junctions,
and vascular disease. Cold Spring Harb Perspect Biol.
10(a029322)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Vestweber D: VE-cadherin: The major
endothelial adhesion molecule controlling cellular junctions and
blood vessel formation. Arterioscler Thromb Vasc Biol. 28:223–232.
2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Boda-Heggemann J, Régnier-Vigouroux A and
Franke WW: Beyond vessels: Occurrence and regional clustering of
vascular endothelial (VE-)cadherin-containing junctions in
non-endothelial cells. Cell Tissue Res. 335:49–65. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hendrix MJC, Seftor EA, Meltzer PS,
Gardner LM, Hess AR, Kirschmann DA, Schatteman GC and Seftor RE:
Expression and functional significance of VE-cadherin in aggressive
human melanoma cells: Role in vasculogenic mimicry. Proc Natl Acad
Sci USA. 98:8018–8023. 2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Martin TA, Watkins G, Lane J and Jiang WG:
Assessing microvessels and angiogenesis in human breast cancer,
using VE-cadherin. Histopathology. 46:422–430. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Navaratna D, Maestas J, McGuire PG and Das
A: Suppression of retinal neovascularization with an antagonist to
vascular endothelial cadherin. Arch Ophthalmol. 126:1082–1088.
2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gory-Fauré S, Prandini MH, Pointu H,
Roullot V, Pignot-Paintrand I, Vernet M and Huber P: Role of
vascular endothelial-cadherin in vascular morphogenesis.
Development. 126:2093–2102. 1999.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hou Y, Li F, Karin M and Ostrowski MC:
Analysis of the IKKbeta/NF-kappaB signaling pathway during
embryonic angiogenesis. Dev Dyn. 237:2926–2935. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB
signaling in inflammation. Signal Transduct Target Ther.
2(17023)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sun Z and Andersson R: NF-kappaB
activation and inhibition: A review. Shock. 18:99–106.
2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang X, Peng H, Huang Y, Kong W, Cui Q, Du
J and Jin H: Post-translational modifications of IκBα: The state of
the art. Front Cell Dev Biol. 8(574706)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sprague AH and Khalil RA: Inflammatory
cytokines in vascular dysfunction and vascular disease. Biochem
Pharmacol. 78:539–552. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Martin Rd, Hoeth M, Hofer-Warbinek R and
Schmid JA: The transcription factor NF-κB and the regulation of
vascular cell function. Arterioscler Thromb Vasc Biol. 20:E83–E88.
2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Colás-Algora N, García Weber D,
Cacho-Navas C, Barroso S, Caballero A, Ribas C, Correas I and
Millán J: Compensatory increase of VE-cadherin expression through
ETS1 regulates endothelial barrier function in response to TNFα.
Cell Mol Life Sci. 77:2125–2140. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Giannotta M, Trani M and Dejana E:
VE-cadherin and endothelial adherens junctions: Active guardians of
vascular integrity. Dev Cell. 26:441–454. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ke S and Guo J, Wang Q, Shao H, He M, Li
T, Qiu T and Guo J: Netrin family genes as prognostic markers and
therapeutic targets for clear cell renal cell carcinoma: Netrin-4
acts through the Wnt/β-catenin signaling pathway. Cancers (Basel).
15(2816)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yang H, Ting X, Geng YH, Xie Y, Nierenberg
JL, Huo YF, Zhou YT, Huang Y, Yu YQ, Yu XY, et al: The risk variant
rs11836367 contributes to breast cancer onset and metastasis by
attenuating Wnt signaling via regulating NTN4 expression. Sci Adv.
8(eabn3509)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gavard J and Gutkind JS: VEGF controls
endothelial-cell permeability by promoting the
beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol.
8:1223–1234. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Hultgren NW, Fang JS, Ziegler ME, Ramirez
RN, Phan DTT, Hatch MMS, Welch-Reardon KM, Paniagua AE, Kim LS,
Shon NN, et al: Slug regulates the Dll4-Notch-VEGFR2 axis to
control endothelial cell activation and angiogenesis. Nat Commun.
11(5400)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Benn A, Bredow C, Casanova I, Vukičević S
and Knaus P: VE-cadherin facilitates BMP-induced endothelial cell
permeability and signaling. J Cell Sci. 129:206–218.
2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ma B and Hottiger MO: Crosstalk between
Wnt/β-Catenin and NF-κB signaling pathway during Inflammation.
Front Immunol. 7(378)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang T, Ma C, Zhang Z, Zhang H and Hu H:
NF-κB signaling in inflammation and cancer. MedComm (2020).
2:618–653. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Hu Y, Ylivinkka I, Li L, Chen P,
Hautaniemi S, Nyman TA, Keski-Oja JKO and Hyytiäinen M: Abstract
3348: Netrin-4/Integrin beta-4 interaction promotes glioblastoma
cell proliferation and protects from temozolomide induced cellular
senescence via activating PI3K/AKT pathway. Cancer Res. 74:3348.
2014.
|
|
34
|
Kane LP, Shapiro VS, Stokoe D and Weiss A:
Induction of NF-κB by the Akt/PKB kinase. Curr Biol. 9:601–604.
1999.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Larrieu-Lahargue F, Welm AL, Thomas KR and
Li DY: Netrin-4 induces lymphangiogenesis in vivo. Blood.
115:5418–5426. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Singh V, Kaur R, Kumari P, Pasricha C and
Singh R: ICAM-1 and VCAM-1: Gatekeepers in various inflammatory and
cardiovascular disorders. Clin Chim Acta. 1(117487)2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yebra M, Diaferia GR, Montgomery AM, Kaido
T, Brunken WJ, Koch M, Hardiman G, Crisa L and Cirulli V:
Endothelium-derived Netrin-4 supports pancreatic epithelial cell
adhesion and differentiation through integrins α2β1 and α3β1. PLoS
One. 6(e22750)2011.PubMed/NCBI View Article : Google Scholar
|