Introduction
Hepatocellular carcinoma (HCC) is one of the most
aggressive types of cancer, which is the fourth most common cancer
worldwide, with 840,000 cases in 2018, but there is currently no
effective treatment for HCC (1).
Lenvatinib is a small molecule inhibitor drug that can act on
multiple receptor tyrosine kinases and can suppress vascular
endothelial growth factor (VEGF) receptor, fibroblast growth factor
receptor and platelet-derived growth factor receptor (2,3). The
REFLECT trial reported that the overall survival rate of patients
with advanced HCC (aHCC) who were treated with lenvatinib was no
lower than that of patients treated with sorafenib, with the
patient group treated with lenvatinib demonstrating better
indicators in all secondary efficacy endpoints (4). Although lenvatinib is promising as a
treatment for patients with HCC, lenvatinib resistance has
gradually emerged as a challenge. Lenalidomide, an immunomodulatory
thalidomide derivative, has been approved for the treatment of
multiple myeloma and myelodysplastic syndromes (5). Furthermore, lenalidomide can inhibit
angiogenesis by decreasing VEGF expression (6).
A previous study reported that
lenalidomide-stimulated T cells are partially activated by T-cell
receptors, increased production of interleukin-2 and interferon-γ
and increased the cytotoxic effects of natural killer and other T
cells (7). Research has also
reported that lenalidomide can inhibit tumor angiogenesis and is
considered to inhibit the immunosuppression (8). A number of studies have reported that
lenalidomide combined with sorafenib can improve HCC response
rates, both in vitro and in vivo (9,10).
Our previous studies have also preliminarily demonstrated that
lenalidomide can inhibit the proliferation of HCC cell lines in
vitro; however, the specific mechanism requires further study.
As lenvatinib resistance becomes increasingly common (11), numerous patients with aHCC have
begun to require second-line drug treatments. Therefore, the choice
of second-line treatment is critical. For patients with aHCC, poor
treatment efficacy can result in patients missing the optimal
treatment window and can lead to an irreversible situation.
Lenalidomide has begun to be used to treat HCC; however, to the
best of our knowledge, its efficacy in patients with
lenvatinib-resistant HCC has not yet been reported on in the
literature.
In the present study, a case of a patient with
lenvatinib-resistant aHCC who responded well to lenalidomide
treatment is reported. This case suggests that lenalidomide is
effective for patients with aHCC who develop resistance to
lenvatinib. However, large-scale clinical studies are warranted to
support the hypothesis that lenalidomide can reverse lenvatinib
resistance in patients with aHCC. Moreover, the mechanism of
lenalidomide in the treatment of lenvatinib-resistant HCC requires
further study.
Case report
A 47-year-old man presented to a local hospital with
new-onset right upper abdominal pain. Abdominal computed tomography
(CT) and Doppler color ultrasonography revealed a tumor located
near the main hepatic portal vein and segment VIII of the right
liver.
The patient was transferred to Henan Provincial
People's Hospital (Zhengzhou, China) in November 2020 for further
examination and treatment. The patient had been infected with
hepatitis B virus a number of years prior and was not receiving any
antiviral treatment at the time. The patient was diagnosed with HCC
accompanied by intrahepatic metastasis and liver cirrhosis.
Hepatitis B virus DNA level was 4.14x101 IU/ml, initial
α-fetoprotein (AFP, reference value: 0-7 ng/ml) level was 9.86
ng/ml and their carbohydrate antigen 19-9 (CA199, reference value:
0-35 U/ml) level was 36.53 U/ml. CT performed at our hospital
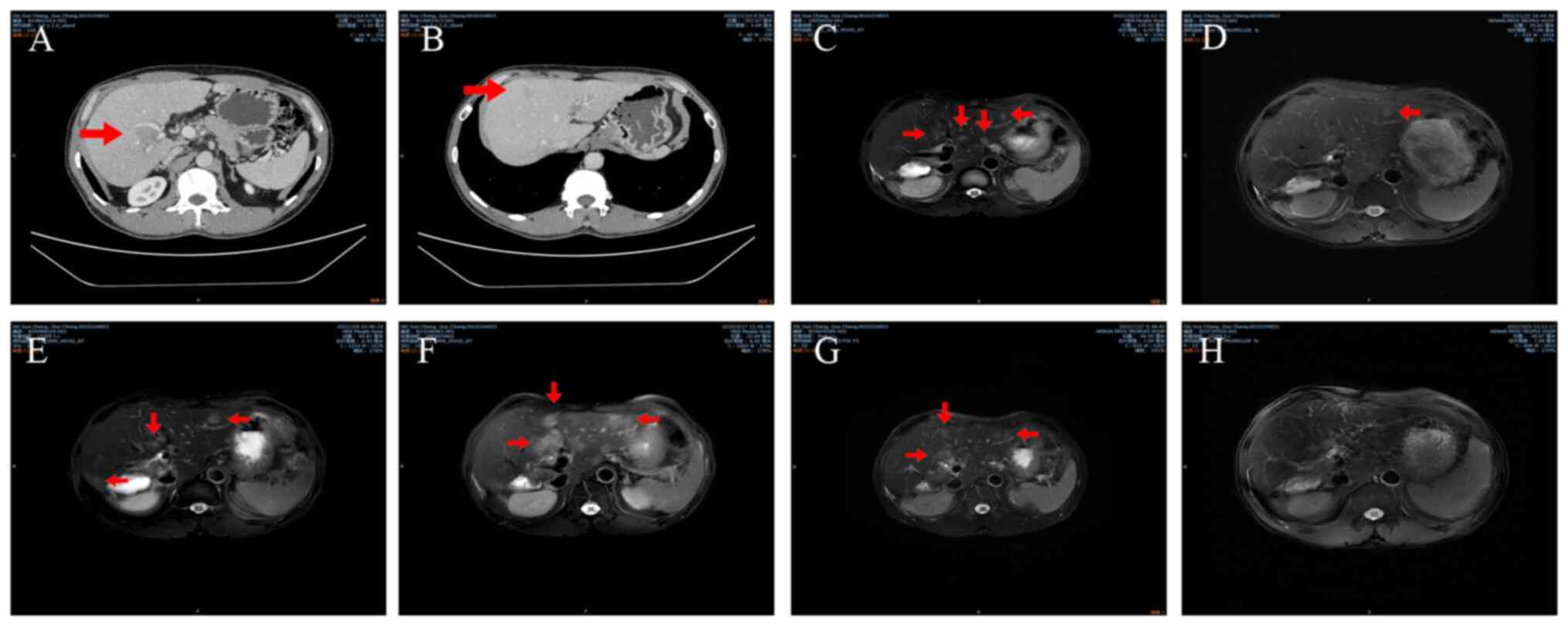
revealed neoplasms located in the right hepatic lobe and segment
VIII (Fig. 1A and B). The performance status score of the
patient was 0 and their Child-Pugh score was 5 (12,13).
The tumor sizes were 2.1x2.2 and 1.8x1.9 cm. The tumor was defined
as stage B according to the Barcelona Clinic Liver Cancer criteria
and as stage IIA according to the China Liver Cancer Staging
criteria (14,15).
The patient underwent an extended right
hemihepatectomy and eighth-segment hepatectomy procedure in
November 2020. Pathological report of the resected tissues
indicated that the liver neoplasms were tumors of right lobe
origin. The microvascular invasion status was classified as M1. The
section VIII tumor was also classified as HCC with necrosis at
Edmondson-Steiner grade II (16),
with a thick beam shape and a diameter of 1.5 cm, with no definite
vascular tumor thrombi or nerve invasion. Lenvatinib treatment (8
mg once a day for 11 months.) was started 1 month after the
surgery. The patient was regularly followed-up with, according to
the standard protocol for HCC treatment (17).
A total of 11 months after the surgery in October
2021, magnetic resonance imaging (MRI) demonstrated multiple
intrahepatic masses that were considered to represent recurrences
(Fig. 1C). There was no notable
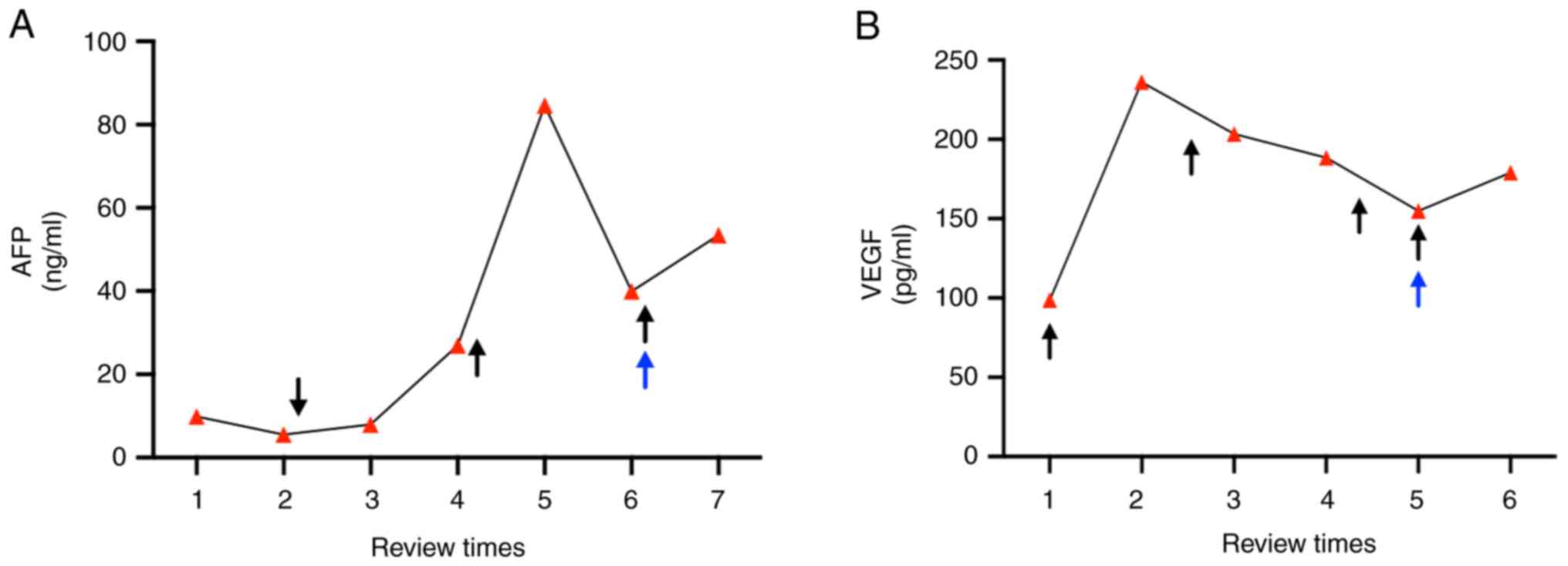
increase in the AFP level (5.51 ng/ml) of the patient.
Transarterial chemoembolization (TACE) was performed 3 days after
MRI and treatment with anti-PD-1 antibody sintilimab (200 mg every
21 days.) injection was initiated 13 days after MRI. Dynamic
contrast-enhanced perfusion MRI (2021-11) demonstrated that the
disease had not progressed and the length of tumor (8.21 mm) had
shrunk slightly based on Response Evaluation Criteria in Solid
Tumors (RECIST). Therapy with lenvatinib plus anti-PD-1 was
continued. A subsequent MRI scan in February 2022, demonstrated
that the disease had progressed in terms of both size (6.74 mm) and
quantity (3 distinct tumors) based on RECIST, as the tumor was
larger compared with on the previous MRI scan (Fig. 1E). Because of this, lenvatinib was
discontinued and regorafenib (Take 160 mg once a day.) combined
with anti-PD-1 (200 mg every 21 days.) was administered instead,
with TACE performed 2 days later in February 2022. Another MRI scan
in May 2022 demonstrated that disease progression had occurred once
again, in terms of both size and quantity, as the tumor was larger
(15.56 mm) and there were more lesions (More than 6 distinct
tumors) compared with the previous scan based on RECIST (Fig. 1F). The AFP level (reference value:
0-7 ng/ml) of the patient had also increased to 84.59 ng/ml and
their VEGF level (reference value: 0-160 pg/ml) had increased to
201.46 pg/ml. A second TACE procedure was performed in May 2022.
Imaging and laboratory results demonstrated that the disease was
resistant to regorafenib and anti PD-1; however, the regimen
remained unchanged. Another MRI scan in July 2022 demonstrated that
the mass had not notably changed since the previous scan (Fig. 1G). Although the AFP (39.97 ng/ml)
and VEGF (154.82 pg/ml) levels of the patient had slightly
decreased and the tumors appeared smaller than before, their
numbers had increased (More than 8 distinct tumors) notably. It was
therefore considered that the disease was resistant to regorafenib
combined with anti-PD-1. The patient was then administered
lenalidomide (Take 25 mg once a day.) and lenvatinib (Take 8 mg
once a day.). An MRI scan performed after this in October 2022,
demonstrated that the size of the mass had notably decreased
(Fig. 1H), indicating that the
patient had achieved a partial response. By January 2023, the AFP
levels of the patient fell to 53.43 ng/ml and their VEGF level
increased to 236.14 pg/ml compared with result in July 2022, and
the patient reported no particular discomfort. The patient refused
a follow-up MRI; however, according to their AFP levels and
clinical manifestations the patient had been in a stable disease
state for nearly 6 months (Last follow up: January 2023). The
treatment timeline of the patient is summarized in Fig. 2.
Discussion
HCC is a major cause of cancer-related mortality
(18). With recent developments in
medical research, breakthroughs have been made in the treatment of
HCC and its treatment is no longer limited to traditional
approaches, such as surgical resection (19). Treatment efficacy in patients with
early-stage HCC is gradually increasing; however, the treatment of
aHCC still faces major challenges. In recent years, although
targeted therapy and immunotherapy have improved the outcomes for
patients with aHCC, the 5-year survival rate of these patients
remains at ~20% (20). Sorafenib
was the first targeted drug approved by the United States Food and
Drug Administration for the treatment of aHCC. Compared with
sorafenib, lenvatinib has been reported to be no worse in terms of
overall survival and achieve statistically significant improvements
in progression-free survival, time to progress, and objective
response rate (21). Thus, the
discovery and application of lenvatinib for the treatment of aHCC
represents a notable milestone. However, with the increasing
clinical application of lenvatinib for this purpose, the problem of
lenvatinib resistance in aHCC has become increasingly prominent.
Therefore, alternative treatments for patients with
lenvatinib-resistant aHCC merit further exploration.
The present case report describes the case of a
patient with aHCC who underwent hepatectomy and was treated with
lenvatinib postoperatively, based on histopathological findings; 11
months after the surgery, the patient was confirmed to have tumor
recurrence and was subsequently treated with lenvatinib plus
anti-PD-1 therapy. After 3 months of standard treatment, the tumor
progressed further and regorafenib plus anti-PD-1 was administered.
The patient underwent three TACE sessions and their AFP and VEGF
levels both decreased, although imaging findings indicated
continued disease progression. The patient was then switched to
lenalidomide plus lenvatinib and achieved partial remission after 3
months of this treatment.
In a phase II clinical trial of patients with aHCC
who had previously received sorafenib, 15% demonstrated a partial
effective response (22).
Therefore, it has been reported that a combination of lenalidomide
and sorafenib can produce enhanced antitumor effects. Perhaps due
to the simultaneous application of two drugs with different
mechanisms of action, effective therapeutic effects have been
achieved. In the present case, the high VEGF levels of the patient
following surgery indicated that their tumor may have been
sensitive to anti-angiogenic drugs, which suggested the possibility
of using lenalidomide. Whether lenalidomide can reverse the
resistance of aHCC to lenvatinib remains unclear.
The present case report suggested that lenalidomide
may help to overcome lenvatinib resistance in aHCC treatment. To
the best of our knowledge, this is the first reported case in the
literature of a lenalidomide-induced response to lenvatinib
resistance following HCC disease progression. Further clinical
investigations into the efficacy of lenalidomide for the treatment
of lenvatinib-resistant HCC are therefore warranted.
Acknowledgements
The authors would like to thank Dr Shuai Zhou
(Translational Medicine Center, University of Zhengzhou, Zhengzhou,
China) and Dr Jiali Zhang (Translational Medicine Center,
University of Zhengzhou, Zhengzhou, China) for providing an office
space to discuss this article.
Funding
Funding: The present research was funded by the Henan Provincial
Science and Technology Project (grant no. 202102310151) and the
Henan Provincial Medical Science and Technology Research Program
Project (grant no. SBGJ202102027).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
XZ conceived and designed the study. PL, QKL and QF
collected the data and performed the literature search. TQ analyzed
and interpreted the data. PFY, JYC and YZW were responsible for
acquisition of data. XZ and TQ confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
This case report has been informed and agreed to be
published by the patient and the attending doctor, and an informed
consent form has been signed.
Patient consent for publication
The patient agreed to publication and signed an
informed consent form.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen Q and Yang XF: Research progress in
targeted therapy of hepatocellular carcinoma. Chin Med Sci J.
36:57–65. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Wang T, Zhang Q, Wang N, Liu Z, Zhang B
and Zhao Y: Research progresses of targeted therapy and
immunotherapy for hepatocellular carcinoma. Curr Med Chem.
28:3107–3146. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hatanaka T, Naganuma A and Kakizaki S:
Lenvatinib for hepatocellular carcinoma: A literature review.
Pharmaceuticals (Basel). 14(36)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shen YC, Lin ZZ, Hsu CH, Hsu C, Shao YY
and Cheng AL: Clinical trials in hepatocellular carcinoma: An
update. Liver Cancer. 2:345–364. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Davies F and Baz R: Lenalidomide mode of
action: Linking bench and clinical findings. Blood Rev. 24 (Suppl
1):S13–S19. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yokouchi H, Nagasato D, Mitamura Y, Egawa
M, Tabuchi H, Misawa S, Kuwabara S and Baba T: Alterations in
choroidal vascular structures due to serum levels of vascular
endothelial growth factor in patients with POEMS syndrome. Sci Rep.
13(10650)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Motz GT and Coukos G: The parallel lives
of angiogenesis and immunosuppression: Cancer and other tales. Nat
Rev Immunol. 11:702–711. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Jin H, Shi Y, Lv Y, Yuan S, Ramirez CFA,
Lieftink C, Wang L, Wang S, Wang C, Dias MH, et al: EGFR activation
limits the response of liver cancer to lenvatinib. Nature.
595:730–734. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ou DL, Chang CJ, Jeng YM, Lin YJ, Lin ZZ,
Gandhi AK, Liao SC, Huang ZM, Hsu C and Cheng AL: Potential
synergistic anti-tumor activity between lenalidomide and sorafenib
in hepatocellular carcinoma. J Gastroenterol Hepatol. 29:2021–2031.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shahda S, Loehrer PJ, Clark RS, Spittler
AJ, Althouse SK and Chiorean EG: Phase I study of lenalidomide and
sorafenib in patients with advanced hepatocellular carcinoma.
Oncologist. 21:664–665. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Qin Y, Han S, Yu Y, Qi D, Ran M, Yang M,
Liu Y and Li Y, Lu L, Liu Y and Li Y: Lenvatinib in hepatocellular
carcinoma: Resistance mechanisms and strategies for improved
efficacy. Liver Int: May 3, 2024 (Epub ahead of print).
|
|
13
|
Tsoris A and Marlar CA: Use of the child
pugh score in liver disease. In: StatPearls [Internet]. Treasure
Island (FL): StatPearls Publishing, 2024.
|
|
14
|
Gmür A, Kolly P, Knöpfli M and Dufour JF:
FACT-Hep increases the accuracy of survival prediction in HCC
patients when added to ECOG Performance Status. Liver Int.
38:1468–1474. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Moazzam Z, Alaimo L, Endo Y, Lima HA,
Shaikh CF, Ratti F, Marques HP, Cauchy F, Lam V, Poultsides GA, et
al: Variations in textbook oncologic outcomes after curative-intent
resection: Early versus intermediate hepatocellular carcinoma based
on Barcelona clinic liver cancer criteria and child-pugh
classification. Ann Surg Oncol. 30:750–759. 2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhong BY, Jiang JQ, Sun JH, Huang JT, Wang
WD, Wang Q, Ding WB, Zhu XL and Ni CF: Prognostic performance of
the china liver cancer staging system in hepatocellular carcinoma
following transarterial chemoembolization. J Clin Transl Hepatol.
11:1321–1328. 2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang Y, Yuan D, Sun H, Pan X, Lu F, Li H,
Huang Y and Tang S: Non-invasive preoperative prediction of
Edmondson-Steiner grade of hepatocellular carcinoma based on
contrast-enhanced ultrasound using ensemble learning. Front Oncol.
13(1116129)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Koulouris A, Tsagkaris C, Spyrou V, Pappa
E, Troullinou A and Nikolaou M: Hepatocellular carcinoma: An
overview of the changing landscape of treatment options. J
Hepatocell Carcinoma. 8:387–401. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
National Health Commission of the People's
Republic of China Medical Administration and Hospital
Administration. Standardization for diagnosis and treatment of
hepatocellular carcinoma (2022 edition). Chin J Dig Surg.
21:143–168. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Safran H, Charpentier K, Dubel G, Soares
G, Berz D, Shipley JL, Faricy-Anderson KE, Plette AM, Bishop K and
Espat NJ: Lenalidomide for advanced hepatocellular cancer (HCC) in
patients progressing on or intolerant to sorafenib. J Clin Oncol.
28 (15 Suppl)(S4159)2010.
|
|
21
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cheng AL, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron AD, Park JW, Han G, Jassem J, et al: Phase III
trial of lenvatinib (LEN) vs sorafenib (SOR) in first-line
treatment of patients (pts) with unresectable hepatocellular
carcinoma (uHCC). J Clin Oncol. 35 (15 Suppl)(S4001)2017.
|