Introduction
Cardiovascular diseases (CVDs) are responsible for
~17 million fatalities annually across the globe, constituting ~1/3
of all global deaths (1).
Atherosclerosis is essentially the leading factor behind
occurrences of CVDs-associated incidents, such as stroke and
myocardial infarction. Therefore, the study of the pathogenesis of
atherosclerosis has profound significance for the prevention and
treatment of CVDs and the protection of human health. Mitochondria,
serving as organelles with multiple functions, are known for their
versatility (2). While their role
in generating cellular energy has been extensively researched, they
also play a part in various cellular processes such as maintaining
calcium levels (3), producing
steroids (4) and mediating redox
signaling (5). Reactive oxygen
species (ROS) are mainly produced by mitochondria and are necessary
for the cell as secondary signaling agents and regulated by
numerous antioxidant molecules and proteins (6). Mitochondria overproduction of ROS
will lead to its functional disorder, resulting in the decrease of
oxidative phosphorylation level, endothelial peroxidation damage,
calcium homeostasis disorder, loss of mitochondrial membrane
potential (ΔΨM) and release of cytochrome c and eventually
induce apoptosis of cells (7). The
apoptosis of vascular endothelial cells (VECs) causes damage to the
inner wall of the blood vessels and oxidized low-density
lipoprotein is transported to the space under the arterial wall,
leading to its deposition in vascular smooth muscle cells and
macrophages and the occurrence of atherosclerosis (8,9).
Additionally, high levels of ROS can cause damage to mitochondrial
DNA (mtDNA) (10). Due to the
robust replication ability but limited repair capacity of mtDNA,
the accumulation of mtDNA damage exacerbates mitochondrial
dysfunction (10). Therefore,
mitochondrial dysfunction caused by excessive ROS is the main cause
of atherosclerosis. By improving mitochondrial respiratory
function, the progression of atherosclerosis can be attenuated.
The Mongolian gerbil (Meriones unguiculatus),
is a rodent belonging to the hamster family (Muridae) within the
gerbil subfamily and gerbil genus (Meriones). The wild
gerbil is mainly distributed in Inner Mongolia and its adjacent
arid and semi-arid areas (11).
The Mongolian gerbil is a special experimental animal in China,
with distinctive biological characteristics and has important
research and application value in the field of biomedicine
(12). A recent study has
demonstrated that the hypercholesterolemic model in gerbils could
be established by feeding a high-fat diet for 1 month, but
atherosclerosis did not develop in gerbils for up to 6 months
(13). Due to this biological
property, gerbils serve as an excellent animal model for
investigating atherosclerosis. However, there is currently a lack
of research investigating the underlying mechanisms responsible for
atherosclerosis resistance in Mongolian gerbils both domestically
and internationally.
The present study conducted a comparative analysis
of mitochondrial function-related indicators in VECs from Mongolian
gerbils, Sprague-Dawley (SD) rats and humans. These findings
elucidated the intrinsic relationship between mitochondrial
function of Mongolian gerbil VECs and atherosclerosis, which may
provide a theoretical foundation for the development of novel
approaches towards the prevention and treatment of
atherosclerosis.
Materials and methods
Isolation of VECs of Mongolian
gerbils
A total of 20 male Mongolian gerbils (6-8 weeks old,
weighing 50-70 g) were obtained from Key Laboratory of Experimental
Animal, Zhejiang Academy of Medical Sciences, China. They were
accommodated in cages of standard size, with four gerbils per cage
and a 12-h light/dark cycle. The air temperature was carefully
regulated to be within the range of 22±2˚C, and the humidity was
maintained at 60-70%.
The Mongolian gerbils were sacrificed with overdose
of pentobarbital sodium (200 mg/kg) via intraperitoneal injection.
The thoracic aorta was removed under strict aseptic precautions in
super-purgative working table and put into the petri dish filled
with RPMI-1640 medium (containing 10% FBS and 1%
penicillin/streptomycin; Gibco; Thermo Fisher Scientific, Inc.).
The connective tissue and adipose tissue outside the blood vessels
were stripped clean. The tissue was dissected into 2 mm3
pieces using a sterile cross-scalpel and washed twice in PBS after
low-speed centrifugation (210 x g, 1 min at 4˚C). The single-cell
suspension was obtained after incubation in collagenase solution,
enzymatic hydrolysis in DNase I solution, filtration through cell
filters, rinsing with PBS and trypsin digestion. All experimental
protocols involving gerbils were conducted in accordance with the
guidelines outlined by the NIH Guide for the Care and Use of
Laboratory Animals (8th edition; https://www.ncbi.nlm.nih.gov/books/NBK54050/).
Additionally, these procedures received ethical approval from the
ethics committee of Jinhua Polytechnic (approval no. 20190605).
Characterization of Mongolian gerbil
VECs
The morphology of Mongolian gerbil VECs was observed
under an optical microscope (Olympus BX53; Olympus Corporation;
magnification, x100).
For immunofluorescent tests, the isolated VECs were
washed by PBS, fixed with 4% paraformaldehyde for 10 min at room
temperature and then permeabilized with 0.1% TritonX-100 in PBS.
The VECs were blocked with goat serum (Gibco; Thermo Fisher
Scientific, Inc.), followed by incubation with primary antibody
CD31 (1:3,000; cat. no. ab222783; Abcam) overnight at 4˚C and then
incubation with secondary antibody (1:5,000; cat. no. ab7090;
Abcam) for 1 h at room temperature. After staining with DAPI for 2
min at 22˚C, images were captured with the aid of a fluorescence
microscope (Olympus Corporation).
Cell culture, cholesterol treatment
and cell viability measurement
Human primary VECs (cat. no. CP-H115) and SD rat
primary VECs (cat. no. CP-R100) were obtained from Procell Life
Science & Technology Co., Ltd. These cells obtained from the
commercial market are regarded as commodities, therefore their
sources, including relevant biological information from specific
patients or healthy individuals, have been removed from
identification. Therefore, ethics approval was waived for use of
primary human VECs by the ethics committee of Jinhua Polytechnic.
RPMI-1640 medium containing 10% FBS and 1% penicillin/streptomycin
was utilized for the cultivation of human VECs, rat VECs and
Mongolian gerbil VECs. The cultures were maintained at 5%
CO2 and 37˚C.
For the measurement of cell viability, the
aforementioned cells at a density of 3x105 were
cultivated into 96-well plates containing FBS-free RPMI-1640. The
cells were treated with different concentrations of cholesterol (0,
6.25, 12.5, 25, 50 and 100 mg/l) for 12 h. Subsequently, 10 µl of
CCK-8 solution (Dojindo Laboratories, Inc.) was added to the
respective wells. The duration of the reaction was 3 h. The
respective control groups were treated without cholesterol. Cell
viability was calculated using a BioTek microplate reader (BioTek;
Agilent Technologies, Inc.) at 450 nm.
Flow cytometry analysis of ROS,
Ca2+ concentration and ΔΨM
The level of ROS in the cells was assessed using
flow cytometry analysis, employing a ROS Detection Kit based on
DCFH-DA probe (Beyotime Institute of Biotechnology). A solution of
DCFH-DA at a final concentration of 10 µM was prepared by diluting
it with FBS-free medium. After harvesting and washing the cells
(1x106) twice with PBS, they were incubated with 500 µl
of 10 µM DCFH-DA for 20 min at 37˚C. Subsequently, the cells were
washed three times with FBS-free medium. Finally, detection and
analysis of cellular ROS levels were performed using a FACScan flow
cytometer with BD FACSDiva™ software v.6.0.1 (BD Biosciences)
equipped with an excitation wavelength of 488 nm (14).
For the measurement of intracellular Ca2+
concentration, the cells were rinsed with PBS three times and then
subjected to staining with 1 µM Fluo-3 AM (Beyotime Institute of
Biotechnology) for 30 min at a temperature of 37˚C in the dark.
Upon entering the cell, Fluo-3 AM can be enzymatically cleaved by
intracellular esterases, resulting in the formation of Fluo-3. The
binding of Ca2+ to Fluo-3 leads to emission of green
fluorescence. To determine the concentration of intracellular
Ca2+ concentration, flow cytometric analysis was
conducted using a FACScan flow cytometer with BD FACSDiva software
v.6.0.1 (BD Biosciences) (15).
Mitochondrial membrane potential was detected using
a mitochondrial membrane potential (ΔΨM) assay Kit with JC-1
(Beyotime Institute of Biotechnology). The cells were exposed to
JC-1 dye (2 µM) for 20 min at 37˚C in the dark, followed by two
washes with PBS. Flow cytometric analysis was performed using a
FACScan flow cytometer with BD FACSDiva software v.6.0.1 (BD
Biosciences) with excitation at 488 nm and emission filters set at
530 and 585 nm (16).
Mitochondria copy number test
The cells were subjected to TRIzol reagent (Thermo
Fisher Scientific, Inc.) for the isolation of total RNA, followed
by measurement of RNA concentration with NanoDrop2000
spectrophotometer (Thermo Fisher Scientific, Inc.). For cDNA
synthesis, 1 µg of RNA was reverse transcribed with Hifair II 1st
Strand cDNA Synthesis SuperMix (Shanghai Yeasen Biotechnology Co.,
Ltd.). PCR was performed using the GeneAmp XL PCR Kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) on a thermocycler (T
Gradient; Biometra GmbH). The conditions for PCR were: 95˚C for 5
min, followed by 40 cycles of 95˚C for 30 sec, 55˚C for 30 sec and
72˚C for 20 sec. Mitochondrial COX1 was served as a marker for
mtDNA, while GAPDH was used for normalization. The primers used are
listed in Table I. The
quantification of mtDNA content involved calculating the ratio
between mitochondrial COX1 and GAPDH.
 | Table IReverse transcription PCR
primers. |
Table I
Reverse transcription PCR
primers.
| Species | Gene | | Sequences | Accession
number |
|---|
| Mongolian
gerbil | COX1 | Forward |
5'-AGGAGCAGTCTTTGCCATCA-3' | NC_023263.1 |
| | | Reverse |
5'-GAGGGCAGCCATGTAGTCAT-3' | |
| | GAPDH | Forward |
5'-ACATGGCCTCCAAGGAGTAAGAA-3' | XM_021636934.2 |
| | | Reverse |
5'-TGCAGTGAGCTTTATTGATGGTATTC-3' | |
| Sprague-Dawley
rat | COX1 | Forward |
5'-ATTGGAGGCTTCGGGAACTG-3' | NC_001665.2 |
| | | Reverse |
5'-AGATAGAAGACACCCCGGCT-3' | |
| | GAPDH | Forward |
5'-CCGTATCGGACGCCTGGTT-3' | NM_017008.4 |
| | | Reverse |
5'-TCCTGGAAGATGGTGATGGGTT-3' | |
| Human | COX1 | Forward |
5'-CTCCCTCTCTCCTACTCCTGCTC-3' | NC_012920.1 |
| | | Reverse |
5'-GGCCCCTAAGATAGAGGAGAC-3' | |
| | GAPDH | Forward |
5'-AAGCCTGCCGGTGACTAAC-3' | NM_001256799.3 |
| | | Reverse |
5'-GCATCACCCGGAGGAGAAAT-3' | |
Detection of the levels of ATP,
NADH-CoQ reductase, superoxide dismutase (SOD), cytochrome c and
glutathione peroxidase (GSG-Px)
According to the instructions of ATP Assay Kit
(Nanjing Jiancheng Bioengineering Institute), NADH-CoQ reductase
Activity Assay Kit (Beijing Solarbio Science & Technology Co.,
Ltd.), Total Superoxide Dismutase Assay Kit (Nanjing Jiancheng
Bioengineering Institute), cytochrome c oxidase Assay Kit
(Nanjing Jiancheng Bioengineering Institute) and Glutathione
Peroxidase (GSH-PX) Assay Kit (Nanjing Jiancheng Bioengineering
Institute), the levels of ATP, NADH-CoQ reductase, SOD, cytochrome
c oxidase and GSH-Px were determined.
Statistical analysis
All experiments were conducted in triplicate in at
least three independent experiments. The data of cell viability was
analyzed by two-way ANOVA followed by Tukey's multiple comparisons
test. Statistical comparisons for other data in this study were
made only between control and model of one species, which were
assessed using the Student's t-test. Data analysis was conducted
using SPSS software version 22.0 (IBM Corp.). The data were
presented as mean ± standard deviation. P<0.05 was considered to
indicate a statistically significant difference.
Results
Identification of Mongolian gerbil
VECs
The morphology of primary isolated Mongolian gerbil
VECs was observed under a light microscope. It was found that the
isolated VECs showed irregular cell morphology (Fig. 1A). CD31 is a specific marker of
endothelial cells, so it was then further identified whether the
isolated cells were VECs. As illustrated in Fig. 1B, CD31-positive cells were observed
under a fluorescence microscope. These results indicated that the
Mongolian gerbil VECs were isolated successfully and could be used
in subsequent experiments. To establish cholesterol-induced
oxidative stress cell model, different concentrations of
cholesterol (0, 6.25, 12.5, 25, 50 and 100 mg/l) were used to
stimulate the VECs of Mongolian gerbils, SD rats and humans for 12
h. It was shown that the viability of the three types of VECs was
decreased by cholesterol treatment, with a concentration-dependent
manner (Fig. 1C). The inhibitory
effect of cholesterol treatment on the viability of Mongolian
gerbil VECs was markedly lower than the other two types of VECs at
the same concentration (P<0.01). From the cholesterol
concentration of 50 mg/l, the viability of three types of VECs was
markedly inhibited and with the increase of concentration, the cell
viability was basically no longer reduced. Therefore 50 mg/l of
cholesterol was selected for the succeeding experiments.
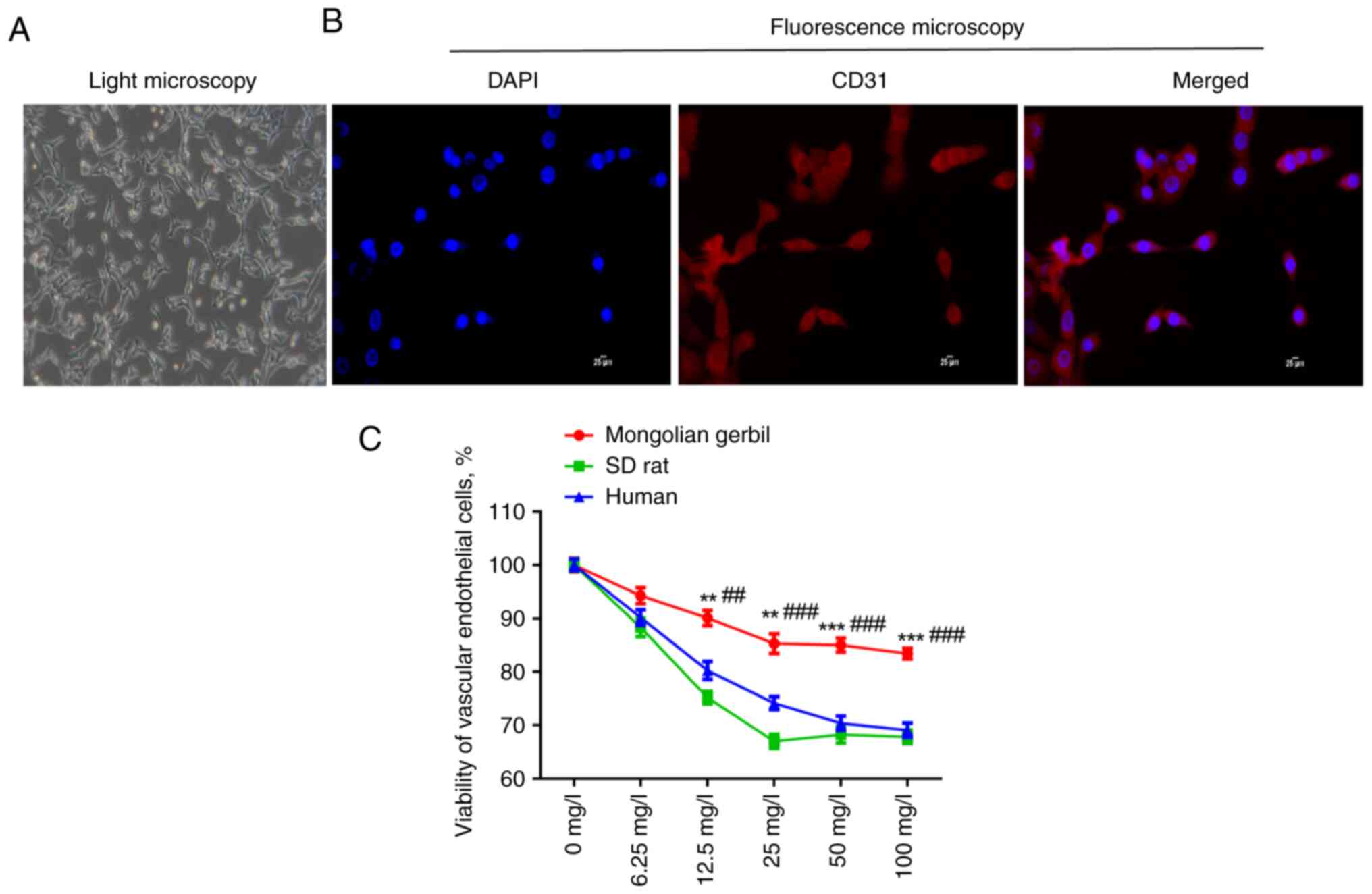 | Figure 1Identification of Mongolian gerbil
VECs. (A) The morphology of primary isolated Mongolian gerbil VECs
was observed under a light microscope. Magnification, x100. (B)
CD31-positive cells were observed under a fluorescence microscope.
Scale bar, 25 µm. (C) The viability of Mongolian gerbil VECs, SD
rat VECs and human VECs treated by different concentrations of
cholesterol (0, 6.25, 12.5, 25, 50 and 100 mg/l) was measured by
CCK-8 assay. **P<0.01, ***P<0.001 vs.
human; ##P<0.01, ###P<0.001 vs. SD rat.
VECs, vascular endothelial cells; SD, Sprague-Dawley. |
Effects of cholesterol on ROS
accumulation, Ca2+ concentration, ΔΨM and mtDNA copy
numbers in three types of VECs
Mitochondrial dysfunction is observed during the
progression of numerous CVDs, including atherosclerosis, while
normal mitochondrial function is associated with the balance
between ROS production, calcium homeostasis and ΔΨM. For this, 50
mg/l of cholesterol was used to treat VECs of Mongolian gerbils, SD
rats and humans for 12 h as the model group, while those VECs
without cholesterol treatment were served as the control group.
DCFH-DA staining assays indicated that compared with the respective
control group, there was a significant increase in ROS fluorescence
intensity in SD rat VECs model group (Fig. 2A; P<0.001) and human VECs model
group (P<0.001). Meanwhile, it was observed that Ca2+
overload was also occurred in SD rat VECs model group (Fig. 2B; P<0.001) and human VECs model
group (P<0.01). Further analysis demonstrated that the
impairment of ΔΨM was observed in the VECs model group of both SD
rats (Fig. 2C; P<0.01) and
humans (P<0.001), compared with the respective control group.
Additionally, the maintenance of mtDNA copy numbers has been
confirmed to be an essential factor for preservation of
mitochondrial function (17). As
shown in Fig. 2D, real-time PCR
revealed a significant reduction in mtDNA copy numbers in the VECs
model group of both SD rats (P<0.01) and humans (P<0.01).
Notably, it was found that compared with the control group of
Mongolian gerbil VECs, there were no significant differences in ROS
production, intracellular Ca2+ concentration, ΔΨM and
mtDNA copy numbers in Mongolian gerbil VECs model group (Fig. 2A-D).
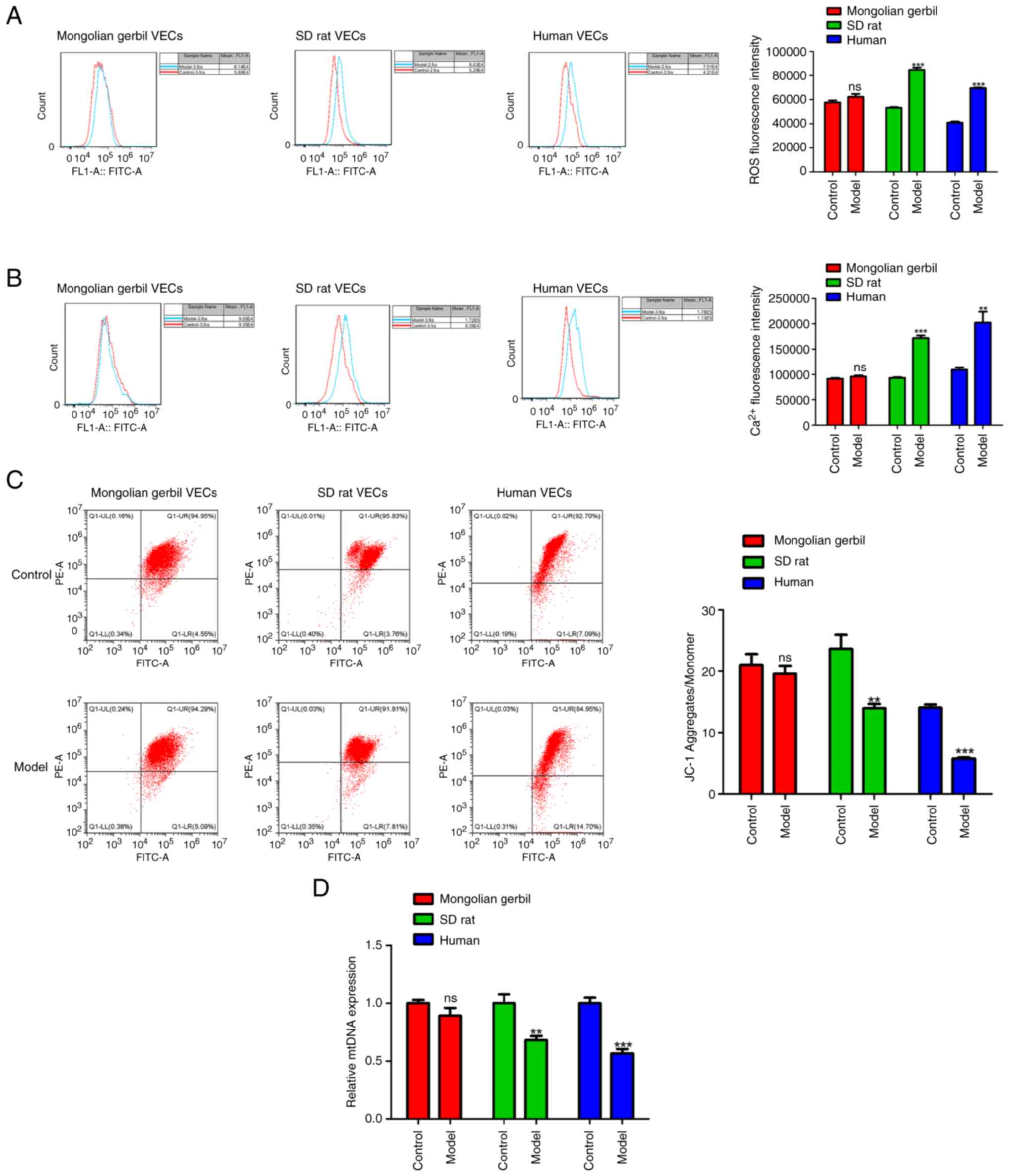 | Figure 2Effects of cholesterol on ROS
accumulation, Ca2+ concentration, ΔΨM and mtDNA copy
numbers in three types of VECs. (A) ROS generation was calculated
by DCFH-DA staining in cholesterol-induced Mongolian gerbil VECs,
SD rat VECs and human VECs. (B) Ca2+ concentration was
assessed by Fluo-3 AM staining in cholesterol-induced Mongolian
gerbil, SD rat and human VECs. (C) Changes of ΔΨM in
cholesterol-induced Mongolian gerbil, SD rat and human VECs were
shown by JC-1 staining. (D) mtDNA copy numbers detected by reverse
transcription PCR. **P<0.01,
***P<0.001, ns, no significance. ROS, reactive oxygen
species; ΔΨM, mitochondrial membrane potential; mtDNA,
mitochondrial DNA; VECs, vascular endothelial cells; SD,
Sprague-Dawley. |
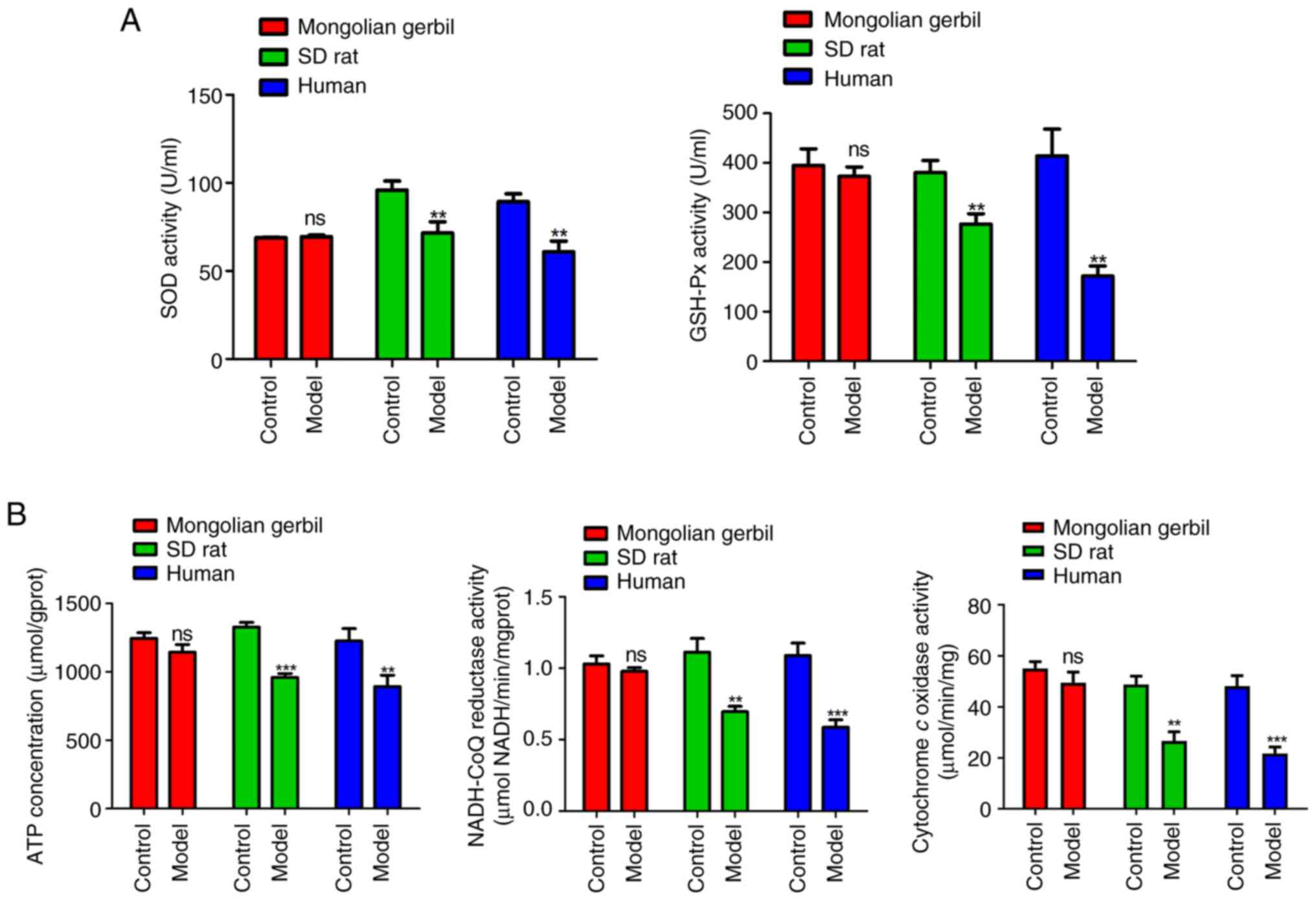
Effects of cholesterol on antioxidant
enzyme activities, ATP homeostasis and mitochondrial respiratory
chain complexes in three types of VECs
Mitochondrial dysfunction is also implicated in a
series of abnormal energy metabolism and enzymatic reactions. The
activities of antioxidant enzymes (SOD and GSH-Px) in three types
of VECs are shown in Fig. 3A.
Compared with the respective control groups, the activities of SOD
and GSH-Px were markedly decreased in the VECs model groups of both
SD rats (P<0.01) and humans (P<0.01). The production of ROS
in cells can be attributed to the mitochondrial respiratory chain.
ROS are generated as a result of electron leakage from mitochondria
during the ATP production process through electron-transport steps
(18). As illustrated in Fig. 3B, the results demonstrated that
compared with the respective control group, there was a significant
decrease in ATP production, mitochondrial respiratory chain
complexes I (NADH-CoQ reductase) activity and complexes IV
(cytochrome c oxidase) activity in the VECs model groups of
both SD rat (P<0.01) and humans (P<0.01). It was further
observed that there was no significant difference in SOD activity,
GSH-Px activity, ATP production, NADH-CoQ reductase activity and
cytochrome c oxidase activity in Mongolian gerbil VECs model
groups relative to its control group (Fig. 3A and B).
Discussion
The Mongolian gerbil has gained popularity as a
small animal model for studying lipid metabolism in researching
atherosclerosis due to its similarities to humans and its
resistance to atherosclerosis (19). While mice and rats have been
extensively used in biomedical research to gather data on CVDs,
there are significant differences between these animals and humans
when it comes to lipid metabolism, especially regarding high-fat
diets and changes in serum cholesterol levels caused by dietary
factors (20). Research has
indicated that gerbils exhibit blood cholesterol concentration
changes similar to those of humans in response to different types
and amounts of dietary fat (21).
Therefore, the present study conducted a comparative analysis of
mitochondrial function-related indicators in VECs from Mongolian
gerbils, SD rats and humans to uncover the intrinsic relationship
between mitochondrial function of Mongolian gerbil VECs and
atherosclerosis.
VECs function as a safeguarding shield within the
walls of blood vessels and has a vital role in upholding
physiological balance. Impaired functioning of VECs is considered a
significant risk factor for cardiovascular health and contributes
to the development of atherosclerosis (22). In the current study, three types of
cholesterol-induced oxidative damage cell models were established,
namely cholesterol-induced Mongolian gerbil VECs, SD rat VECs and
human VECs. There exists a wide range of research studies conducted
on animal models and humans, which provide substantial evidence to
support the idea that elevated levels of oxidative and
nitro-oxidative stress resulting from an augmented production of
ROS and diminished reserves of antioxidants play a significant role
in the development of atherosclerosis (23-27).
For example, Ekstrand et al (28) established a new imaging technique
to visualize and quantify intracellular and extracellular ROS
levels within intact mouse aortas. They demonstrated that both
intracellular and extracellular ROS levels were increased in
advanced lesions (28). Batty
et al (25) reported that
the production of ROS initiates several processes involved in
atherogenesis, including expression of adhesion molecules,
stimulation of vascular smooth muscle proliferation and migration,
apoptosis in the endothelium, oxidation of lipids, activation of
matrix metalloproteinases and altered vasomotor activity (25). These publicly available data
indicated that compared with healthy individuals, elevated ROS
levels are a risk factor for the occurrence of atherosclerosis.
The present study found that ROS amounts were
increased markedly in the VECs model groups of both SD rats and
humans, compared with the respective control groups, while there
seemed no significant differences between Mongolian gerbil VECs
model group and control group. These results to some extent implied
the anti-atherosclerotic properties of Mongolian gerbils. The
accumulation of calcium within the arterial wall is another
important characterization of atherosclerosis (29). In addition, studies have shown that
the mitochondrial Ca2+ levels play a pivotal role in the
generation of ROS (30,31). Ca2+ has the potential to
enhance the generation of ROS through both direct and indirect
mechanisms (32,33). Directly, they can stimulate enzymes
involved in ROS production, such as α-ketoglutarate dehydrogenase
and glycerol phosphate (32).
Indirectly, mitochondrial Ca2+ can activate nitric oxide
synthase, which leads to the formation of nitric oxide and the
blocking of complex IV, resulting in an excessive production of ROS
(33). As expected, high levels of
Ca2+ were observed in the VECs model groups of both SD
rats and humans, but no changes in Mongolian gerbil VECs model
group. These findings suggested that in cholesterol-induced SD rat
VECs and human VECs, the elevated Ca2+ levels surpassing
the physiological threshold result in Ca2+ overload,
leading to detrimental ROS production.
In addition, mitochondrial Ca2+ overload
can further compromise mitochondrial bioenergetics and functions,
resulting in diminished ATP production, subsequent dissipation of
ΔΨM and ultimately triggering cellular apoptosis (34,35).
Similarly, the present study, demonstrated a significant decrease
in ΔΨM and ATP production in cholesterol-induced SD rat and human
VECs, while no significant changes were observed in
cholesterol-induced Mongolian gerbil VECs, suggesting that
mitochondrial dysfunction occurs in the VECs model groups of both
SD rats and humans. This conclusion was further confirmed by the
decrease of mtDNA copy numbers in both SD rat and human VECs model
groups. According to a previous study, it has been found that mtDNA
is responsible for encoding several polypeptide subunits that make
up the mitochondrial respiratory chain complexes (I, III, IV), with
the exception of complexes II (36). Given that mutations in mtDNA can
result in the dysfunction of mitochondrial respiratory chain
complexes, excessive generation of ROS and synthesis of
pro-apoptotic proteins, eventually leading to cell apoptosis
(36) It was therefore
hypothesized that mitochondrial respiratory chain dysfunction
occurred in cholesterol-induced SD rat and human VECs. The results
that mitochondrial respiratory chain complexes I (NADH-CoQ
reductase) activity and complexes IV (cytochrome c oxidase)
activity were reduced in both SD rat and human VECs model groups
further validated this hypothesis. Excessive ROS production and
mitochondrial dysfunction are also implicated in a series of other
complex biological process such as redox imbalance (37). The enzymes SOD and GSH-Px play
crucial roles as antioxidant defense mechanisms within cells
(38). The present study found
that compared with the respective control groups, the activities of
SOD and GSH-Px were markedly decreased in SD rat and human VECs
model groups, but no changes were observed in cholesterol-induced
Mongolian gerbil VECs, which further indicated that oxidative
damage was obviously in cholesterol-induced SD rat VECs and human
VECs.
Some limitations of this study should not be
overlooked. First, it is imperative to measure the activities of
other mitochondrial respiratory chain complexes, such as complexes
II and III. Second, further investigation is required to assess the
activities of other enzymatic defenses in ROS scavenging systems,
including GSH-Px, catalase and malondialdehyde. Third, a more
comprehensive approach would involve delving deeper into the
underlying reasons behind the resistance of Mongolian gerbils to
atherosclerosis in animal models.
To summarize, the present study primarily compared
the effects of cholesterol treatment on VECs derived from Mongolian
gerbils, SD rats and humans. Notably, cholesterol treatment
markedly induced mitochondrial dysfunction in both SD rat and human
VECs; however, it exerted minimal effect on the normal
mitochondrial function of Mongolian gerbils. These findings provide
a theoretical basis for the development of innovative approaches
towards preventing and treating atherosclerosis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Zhejiang Province
Commonwealth Projects (project no. LGD20C040003).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MD and XS made substantial contributions to the
conception and design of the present study. XW, YD, HD, YL and YJ
made substantial contributions to the acquisition, analysis and
interpretation of the data. XW drafted the manuscript. MD, XS, XW,
YD, HD, YL and YJ confirm the authenticity of all the raw data. All
authors revised the manuscript critically for intellectual content.
All authors agreed to be accountable for all aspects of the work in
ensuring that questions related to the accuracy or integrity of any
part of the work are appropriately investigated and resolved. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were performed in
compliance with the guidelines outlined by the NIH Guide for the
Care and Use of Laboratory Animals (8th edition; https://www.ncbi.nlm.nih.gov/books/NBK54050/) and were
approved by the Ethics Committee of Jinhua Polytechnic (Jinhua,
China; approval no. 20190605).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Thomas H, Diamond J, Vieco A, Chaudhuri S,
Shinnar E, Cromer S, Perel P, Mensah GA, Narula J, Johnson CO, et
al: Global atlas of cardiovascular disease 2000-2016: The path to
prevention and control. Glob Heart. 13:143–163. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
McBride HM, Neuspiel M and Wasiak S:
Mitochondria: More than just a powerhouse. Curr Biol. 16:R551–R560.
2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bravo-Sagua R, Parra V, Lopez-Crisosto C,
Diaz P, Quest AF and Lavandero S: Calcium transport and signaling
in mitochondria. Compr Physiol. 7:623–634. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chien Y, Rosal K and Chung BC: Function of
CYP11A1 in the mitochondria. Mol Cell Endocrinol. 441:55–61.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Blajszczak C and Bonini MG: Mitochondria
targeting by environmental stressors: Implications for redox
cellular signaling. Toxicology. 391:84–89. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li R, Jia Z and Trush MA: Defining ROS in
biology and medicine. React Oxyg Species (Apex). 1:9–21.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Suarez-Rivero JM, Pastor-Maldonado CJ,
Povea-Cabello S, Álvarez-Córdoba M, Villalón-García I,
Talaverón-Rey M, Suárez-Carrillo A, Munuera-Cabeza M and
Sánchez-Alcázar JA: From mitochondria to atherosclerosis: The
inflammation path. Biomedicines. 9(285)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wu X, Zhang H, Qi W, Zhang Y, Li J, Li Z,
Lin Y, Bai X, Liu X, Chen X, et al: Nicotine promotes
atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis.
Cell Death Dis. 9(171)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
He X, Fan X, Bai B, Lu N, Zhang S and
Zhang L: Pyroptosis is a critical immune-inflammatory response
involved in atherosclerosis. Pharmacol Res.
165(105447)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wu H, Wang Y, Li W, Chen H, Du L, Liu D,
Wang X, Xu T, Liu L and Chen Q: Deficiency of mitophagy receptor
FUNDC1 impairs mitochondrial quality and aggravates dietary-induced
obesity and metabolic syndrome. Autophagy. 15:1882–1898.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xu LD, Zhang F, Chen C, Peng L, Luo WT,
Chen R, Xu P and Huang YW: Revisiting the mongolian gerbil model
for hepatitis E virus by reverse genetics. Microbiol Spectr.
10(e0219321)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang X, Wang C, He Y, Xing J, He Y, Huo
X, Fu R, Lu X, Liu X, Lv J, et al: Establishment of noninvasive
methods for the detection of Helicobacter pylori in
mongolian gerbils and application of main laboratory gerbil
populations in China. Biomed Res Int. 2022(6036457)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hong W, Zhang T, Yan J, Yu J, He B, Wu L,
Yao K, Mao W and Chen Z: Bioinformatics analysis of an animal model
of diet-induced nonalcoholic fatty liver disease with rapid
progression. Exp Biol Med (Maywood). 247:263–275. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jia F, Liu Y, Dou X, Du C, Mao T and Liu
X: Liensinine inhibits osteosarcoma growth by ROS-mediated
suppression of the JAK2/STAT3 signaling pathway. Oxid Med Cell
Longev. 2022(8245614)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kong X, Li M, Shao K, Yang Y, Wang Q and
Cai M: Progesterone induces cell apoptosis via the
CACNA2D3/Ca2+/p38 MAPK pathway in endometrial cancer.
Oncol Rep. 43:121–132. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yang H, Cui Y, Tang Y, Tang X, Yu X, Zhou
J, Yin Q and Shentu X: Cytoprotective role of humanin in lens
epithelial cell oxidative stress-induced injury. Mol Med Rep.
22:1467–1479. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jeng JY, Yeh TS, Lee JW, Lin SH, Fong TH
and Hsieh RH: Maintenance of mitochondrial DNA copy number and
expression are essential for preservation of mitochondrial function
and cell growth. J Cell Biochem. 103:347–357. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jeong EM, Chung J, Liu H, Go Y, Gladstein
S, Farzaneh-Far A, Lewandowski ED and Dudley SC Jr: Role of
mitochondrial oxidative stress in glucose tolerance, insulin
resistance and cardiac diastolic dysfunction. J Am Heart Assoc.
5(e003046)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wasan KM, Najafi S, Peteherych KD and
Pritchard PH: Effects of a novel hydrophilic phytostanol analog on
plasma lipid concentrations in gerbils. J Pharm Sci. 90:1795–1799.
2001.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Suckling KE and Jackson B: Animal models
of human lipid metabolism. Prog Lipid Res. 32:1–24. 1993.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ramachandran HD, Narasimhamurthy K and
Raina PL: Modulation of cholesterol induced hypercholesterolemia
through dietary factors in Indian desert gerbils (Meriones
hurrianae). Nutrition Res. 23:245–256. 2003.
|
|
22
|
Chen HI, Hu WS, Hung MY, Ou HC, Huang SH,
Hsu PT, Day CH, Lin KH, Viswanadha VP, Kuo WW and Huang CY:
Protective effects of luteolin against oxidative stress and
mitochondrial dysfunction in endothelial cells. Nutr Metab
Cardiovasc Dis. 30:1032–1043. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Marchio P, Guerra-Ojeda S, Vila JM,
Aldasoro M, Victor VM and Mauricio MD: Targeting early
atherosclerosis: A focus on oxidative stress and inflammation. Oxid
Med Cell Longev. 2019(8563845)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Victor VM, Apostolova N, Herance R,
Hernandez-Mijares A and Rocha M: Oxidative stress and mitochondrial
dysfunction in atherosclerosis: Mitochondria-targeted antioxidants
as potential therapy. Curr Med Chem. 16:4654–4667. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Batty M, Bennett MR and Yu E: The role of
oxidative stress in atherosclerosis. Cells. 11(3843)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ciccarelli G, Conte S, Cimmino G, Maiorano
P, Morrione A and Giordano A: Mitochondrial dysfunction: The hidden
player in the pathogenesis of atherosclerosis? Int J Mol Sci.
24(1086)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shaito A, Aramouni K, Assaf R, Parenti A,
Orekhov A, Yazbi AE, Pintus G and Eid AH: Oxidative Stress-induced
endothelial dysfunction in cardiovascular diseases. Front Biosci
(Landmark Ed). 27(105)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ekstrand M, Gustafsson Trajkovska M,
Perman-Sundelin J, Fogelstrand P, Adiels M, Johansson M,
Mattsson-Hultén L, Borén J and Levin M: Imaging of intracellular
and extracellular ROS levels in atherosclerotic mouse aortas ex
vivo: Effects of lipid lowering by diet or atorvastatin. PLoS One.
10(e0130898)2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mallat Z, Corbaz A, Scoazec A, Besnard S,
Lesèche G, Chvatchko Y and Tedgui A: Expression of interleukin-18
in human atherosclerotic plaques and relation to plaque
instability. Circulation. 104:1598–1603. 2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Feno S, Butera G, Reane DV, Rizzuto R and
Raffaello A: Crosstalk between calcium and ROS in
pathophysiological conditions. Oxid Med Cell Longev.
2019(9324018)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bou-Teen D, Kaludercic N, Weissman D,
Turan B, Maack C, Di Lisa F and Ruiz-Meana M: Mitochondrial ROS and
mitochondria-targeted antioxidants in the aged heart. Free Radic
Biol Med. 167:109–124. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Madreiter-Sokolowski CT, Thomas C and
Ristow M: Interrelation between ROS and Ca2+ in aging
and age-related diseases. Redox Biol. 36(101678)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gorlach A, Bertram K, Hudecova S and
Krizanova O: Calcium and ROS: A mutual interplay. Redox Biol.
6:260–271. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Di Lisa F and Bernardi P: A CaPful of
mechanisms regulating the mitochondrial permeability transition. J
Mol Cell Cardiol. 46:775–780. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Biasutto L, Azzolini M, Szabo I and
Zoratti M: The mitochondrial permeability transition pore in AD
2016: An update. Biochim Biophys Acta. 1863:2515–2530.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Moran M, Moreno-Lastres D, Marin-Buera L,
Arenas J, Martin MA and Ugalde C: Mitochondrial respiratory chain
dysfunction: implications in neurodegeneration. Free Radic Biol
Med. 53:595–609. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Nickel A, Kohlhaas M and Maack C:
Mitochondrial reactive oxygen species production and elimination. J
Mol Cell Cardiol. 73:26–33. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jena AB, Samal RR, Bhol NK and Duttaroy
AK: Cellular Red-Ox system in health and disease: The latest
update. Biomed Pharmacother. 162(114606)2023.PubMed/NCBI View Article : Google Scholar
|