Introduction
Systemic lupus erythematosus (SLE) is a chronic
multisystemic autoimmune disease, characterized clinically by
multi-organ damage and the existence of elevated levels of
autoantibodies (1). Lupus
nephritis (LN) is one of the most severe organ manifestations of
SLE, occurring in 35 to 60% of SLE patients (2). Currently, end-stage renal disease
(ESRD) remains an inevitable outcome for patients with LN (3). Within 5 years of an initial lupus
nephritis diagnosis, 10-30% of patients with LN developed kidney
failure requiring kidney replacement therapy (4). Treatment of lupus nephritis typically
involves immunosuppressive and glucocorticoid therapy, although
these treatments may not be effective (5). Therefore, the management of LN has
been proven challenging over the past three decades, with limited
improvements observed in patients' outcomes.
B cells play a pivotal role in autoantibody
generation, cytokine secretion and antigen presentation in SLE
(6). Belimumab, a B cell-targeted
therapy approved for patients with SLE aged ≥5 years (7), has been used for the treatment of LN
in studies such as the international Belimumab in LN (Bliss-LN)
trial. The Bliss-LN trial revealed that belimumab, combined with
standard treatment, reduces the risks associated with LN (8).
B lymphocyte stimulator (BLyS) and a
proliferation-inducing ligand (APRIL) are members of the tumor
necrosis factor family and play significant roles in B cell
proliferation and differentiation (9). Telitacicept is the first dual
inhibitor of BLys/APRIL, serving as a new recombinant fusion
protein binding to both the ligand-binding domain of the TACI
receptor and the Fc component of human IgG (10). By binding and neutralizing BLys and
APRIL, telitacicept suppresses various stages of development,
thereby targeting plasma cells and mature B cells (11). Given its specific molecular
mechanisms, telitacicept is anticipated to effectively treat
autoimmune diseases (12). It was
approved for patients with SLE in China in March 2021(11) and has verified effectiveness in
both children (13) and adults
with SLE (14).
Although it is a promising drug for systemic lupus
erythematosus, there are few studies on telitacicept in patients
with LN in China, which limits its broad application across the
world. Therefore, based on specific molecular mechanisms and the
results of previous studies, the present study assessed the
effectiveness and safety of telitacicept in patients with LN. To
the best of our knowledge, the present trial was the first study
assessing the efficacy of telitacicept in patients with LN amidst
the COVID-19 pandemic.
Materials and methods
Patients
Following the diagnosis criteria for SLE established
by the American College of Rheumatology (15), the present study identified
patients with active LN, confirmed by kidney biopsy results at
Lishui Central Hospital (Lishui, China). Patients (14-85 years old
and unable to tolerate the side effects of immunosuppressive
medicines or glucocorticoids) receiving standard treatment in
conjunction with telitacicept were included in this trial, while
individuals allergic to human-sourced biological products, such as
telitacicept, or presenting with LN-induced organ damage and severe
or chronic infections, were excluded from this trial. Finally, 13
patients (14-85 years old; 11 female and 2 male) meeting the
inclusion criteria were enrolled from February 2022 to April 2023.
All patients whose data were not previously documented in any
publications provided written informed consent to utilize their
medical records. This trial was approved by the Ethics Committee of
Lishui Central Hospital and was also part of the Pioneer and
Leading Project of Zhejiang Province (project no. 2022C03172). In
addition, the present study was reviewed and approved by the
Ethical Committee of the Lishui Central Hospital (approval no.
2022-278).
Treatment protocol
Considering the disease progression, therapeutic
efficacy, patient tolerance and economic considerations, different
doses of telitacicept were administered to different patients. All
patients received standard therapy in addition to subcutaneous
injections of either 80 or 160 mg of telitacicept per week (median
duration of treatment, 36 weeks; range, 12-48 weeks), based on the
condition of each patient and their laboratory test results. As
disease control was achieved during the trial, the dosage of
glucocorticoids and immunosuppressive agents was gradually
tapered.
Primary outcome measures and
assessment
The systemic lupus erythematosus disease activity
index 2000 (SLEDAI-2K) score was considered the primary outcome to
evaluate the clinical remission status and categorize disease
activity (16). This index was
assessed at the beginning of the study and monthly following the
first dose of telitacicept. A reduction of more than four points in
the SLEDAI-2K score was considered satisfactory alleviation of the
disease activity Furthermore, changes in dosages of glucocorticoids
and immunosuppressive medicines, levels of complement proteins (C3
and C4), immunoglobulins (IgA, IgM and IgG), urinary protein (in
consideration of the economic burden on patients, we collected
urinary protein concentration data rather than 24-h urinary protein
measurements) and erythrocyte sedimentation rate were recorded
monthly as primary outcomes.
Secondary outcome measures and
assessment
Secondary outcome measures included serum creatinine
levels, estimated glomerular filtration rate (eGFR), plasma albumin
concentrations, platelet counts and treatment-emergent adverse
events, with data collected from medical histories and laboratory
test results. Although patients were enrolled at different time
points, all shared the same endpoint on April 30, 2023.
Statistical analysis
Statistical analyses were conducted using R (version
4.2.2) (17) and GraphPad Prism 9
(Dotmatics). Counting data are expressed as percentages [n (%)],
and measurement data are expressed as mean ± standard deviation or
median (range). The Shapiro-Wilk normality test was applied to
conduct normal tests on the data. Pre- and post-treatment data were
compared using paired students’ t-tests (in the presence of normal
distribution) or Wilcoxon signed-rank tests (in the absence of
abnormal distribution). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patients’ general information
Based on the inclusion and exclusion criteria, 13
patients with active LN were included in the present trial from
February 2022 to April 2023. The trial duration ranged from 12 to
48 weeks. No patients withdrew telitacicept treatment. There were
two males and 11 females (84.6%) in this study, with a median age
of 39.6±20.0 years. According to the ISN/RPS classification for LN,
six patients were classified as class IV+V, two as class III+V, two
as class IV, one as class III and two as class V. The median
duration of suffering from LN was 35 weeks (range, 8-212 weeks),
with a median SLEDAI-2K score of 13 (range, 8-35) at the baseline.
Before treatment with telitacicept, all patients received
glucocorticoid and immunosuppressive medicines, with eight patients
receiving more than two immunosuppressive agents (Table SI). These immunosuppressive
medicines included mycophenolate mofetil (n=7), hydroxychloroquine
(n=12), cyclosporine (n=1), amethopterin (n=1), tacrolimus (n=1)
and cyclophosphamide (n=1). For the 13 patients, telitacicept was
administered due to intolerance to side effects associated with
immunosuppressive medicines or glucocorticoid, or disease
recurrence (Table I). None of the
patients showed contraindications to the immunosuppressive
therapies.
 | Table IBaseline conditions of the 13 patients
with LN treated with telitacicept. |
Table I
Baseline conditions of the 13 patients
with LN treated with telitacicept.
| Characteristic | Value |
|---|
| Females, n (%) | 11 (84.6) |
| Mean age ± SD,
years | 39.6±20.0 |
| SLE duration,
monthsa | 36 (8, 212) |
| LN duration,
monthsa | 35 (8, 212) |
| Organ manifestation
before the first dose with telitacicept, n (%) | |
|
Erythema | 3 (23.1) |
|
Pneumonia | 1 (7.7) |
|
Thrombotic
microangiopathy | 1 (7.7) |
| Previous therapies
before telitacicept, n (%) | |
|
Hydroxychloroquine | 12 (92.3) |
|
Cyclophosphamide | 1 (7.7) |
|
Mycophenolate
mofetil | 7 (53.8) |
|
Biologics
used before | 0 (0.0) |
|
Multi-targeting
MMF + CsA/FK | 2 (15.4) |
|
Glucocorticoids
+ immunosuppressive medicines | 12 (92.3) |
| Reason for using
telitacicept, n (%) | |
|
Glucocorticoids
side effects | 11 (84.6) |
|
Immunosuppressive
medicines’ side effects | 13 (100.0) |
|
Non-remission/disease
recurrence | 7 (53.8) |
Efficacy and safety. Primary outcome
measures
All patients in the present trial received
telitacicept for a minimum of 12 weeks. At the endpoint, the median
duration of treatment with telitacicept was 36 weeks (range, 12-48
weeks) and the median SLEDAI-2K score was 6 (range, 2-10) (Table SII). Furthermore, compared with
the baseline, 11 patients (84.6%) experienced a reduction in their
SLEDAI-2K score by more than four points with telitacicept
treatment (P<0.0001; Fig. 1A;
Table SIII). The median dosage of
glucocorticoids decreased from 15 mg/d before treatment with
telitacicept to 2.5 mg/d after treatment (Fig. 1B). The daily glucocorticoid dosage
of all patients remained below 10 mg. The glucocorticoid dosage of
12 patients reduced by >25%, and six of these 12 patients
discontinued treatment with glucocorticoids. Before discontinuing
them, the conditions of the 6 patients were observed and blood test
outcomes, including C3, C4 and ESR levels, were monitored.
Additionally, the present study re-evaluated the SLEDAI-2K scores
of the six patients. Based on these assessments, the doctors made
decisions regarding discontinuation of glucocorticoids.
Prednisone dosage increased in 1 out of the 13
patients due to a relapse of LN during the trial (Table II). Meanwhile, the dosage of
immunosuppressive medicines decreased in six out of the 13
patients, requiring fewer types of immunosuppressive medicines. Two
patients discontinued their immunosuppressive drugs at the endpoint
(Fig. 1C; Table SII).
 | Table IIOutcomes of telitacicept in lupus
nephritis. |
Table II
Outcomes of telitacicept in lupus
nephritis.
| | SLEDAI-2K
(score) | eGFR, ml/min/1.73
m2 | |
|---|
| Case | Age, years | Pre-therapy | Post-therapy | Pre-therapy | Post-therapy | Glucocorticoid
reductiona | Plasma albumin
(change trend) | Urinary protein
(change trend)b |
|---|
| 1 | 31 | 11 | 8 | 45.6 | 26.8 | YES | Rise | NO/descend |
| 2 | 18 | 10 | 10 | 127.8 | 134.2 | NO/increase | Rise to normal | YES/descend to
normal |
| 3 | 14 | 35 | 10 | 10.1 | 9.5 | YES | Rise to normal | YES/descend |
| 4 | 23 | 20 | 2 | 95.1 | 123.1 |
YES/discontinued | Rise | YES/descend |
| 5 | 42 | 12 | 2 | 110.5 | 112.6 |
YES/discontinued | Rise | N |
| 6 | 24 | 20 | 6 | 120.3 | 118 |
YES/discontinued | Rise | YES/descend to
normal |
| 7 | 83 | 12 | 2 | 81.2 | 74.8 |
YES/discontinued | N | N |
| 8 | 47 | 12 | 3 | 97.3 | 79.8 |
YES/discontinued | N | N |
| 9 | 41 | 15 | 2 | 110.7 | 109.2 |
YES/discontinued | N | N |
| 10 | 72 | 16 | 6 | 61.5 | 67.3 | YES | Rise | YES/descend |
| 11 | 36 | 8 | 2 | 119.9 | 119 | YES | Rise | YES/descend to
normal |
| 12 | 45 | 13 | 6 | 116.8 | 113.9 | YES | Rise | N |
| 13 | 48 | 14 | 6 | 103 | 102.2 | YES | Rise to normal | N |
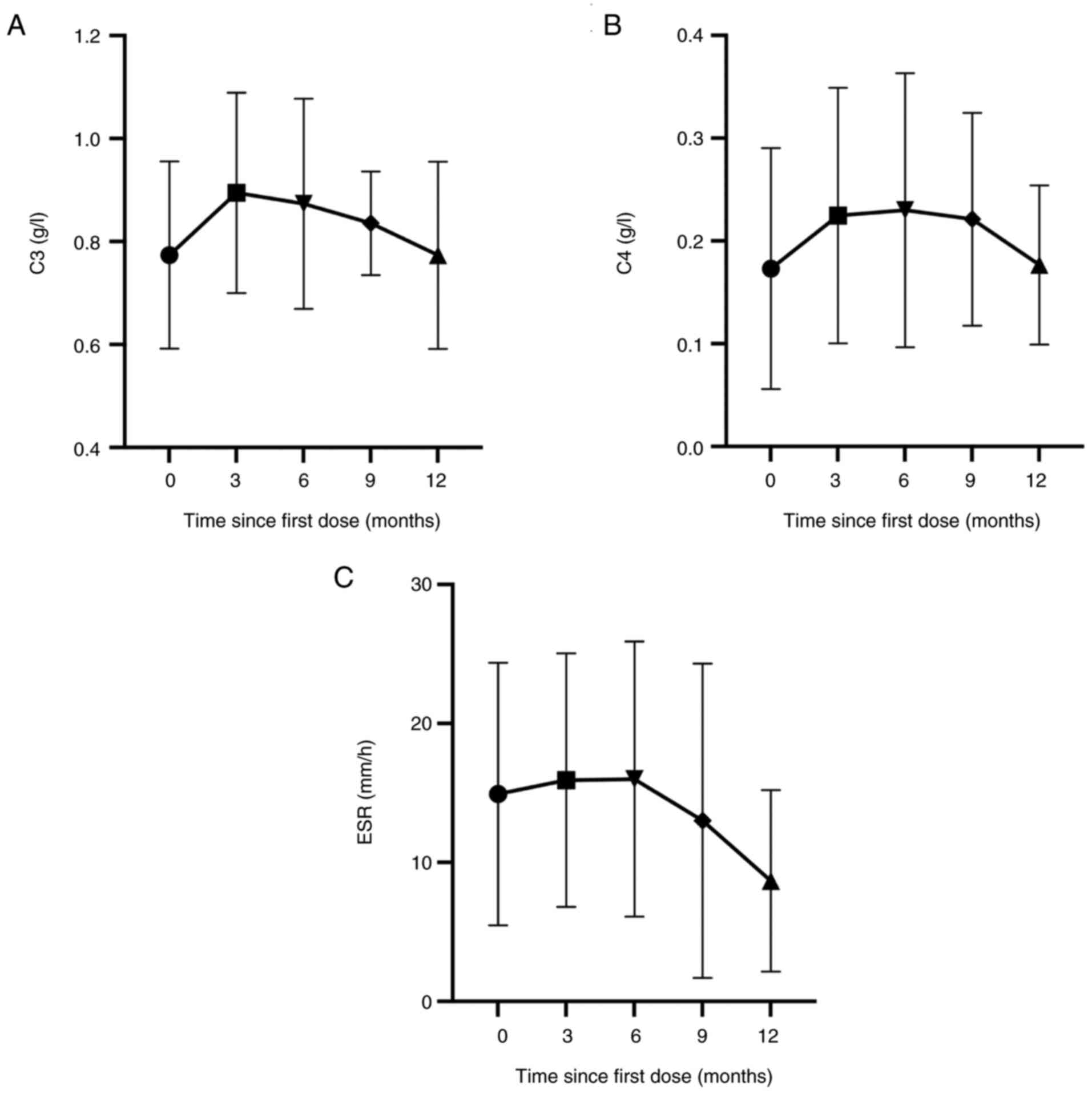
C3, C4 and erythrocyte sedimentation rate (ESR) were
reassessed for all patients at the endpoint, with all patients
showing a downward trend in ESR levels (Fig. 2). In addition, ten patients (76.9%)
showed a stable or increasing trend in C3 and C4 levels (Fig. 2). All patients had stable levels of
IgG, IgA and IgM, displaying a trend characterized by an initial
decline followed by subsequent elevation (Fig. 3). A total of 12 patients exhibited
a decrease in urinary protein at the endpoint compared to before
treatment with telitacicept. Seven patients exhibited a decline of
>0.5 g/l in urinary protein, and urinary protein levels were
normalized in three patients (Fig.
3 and Table II).
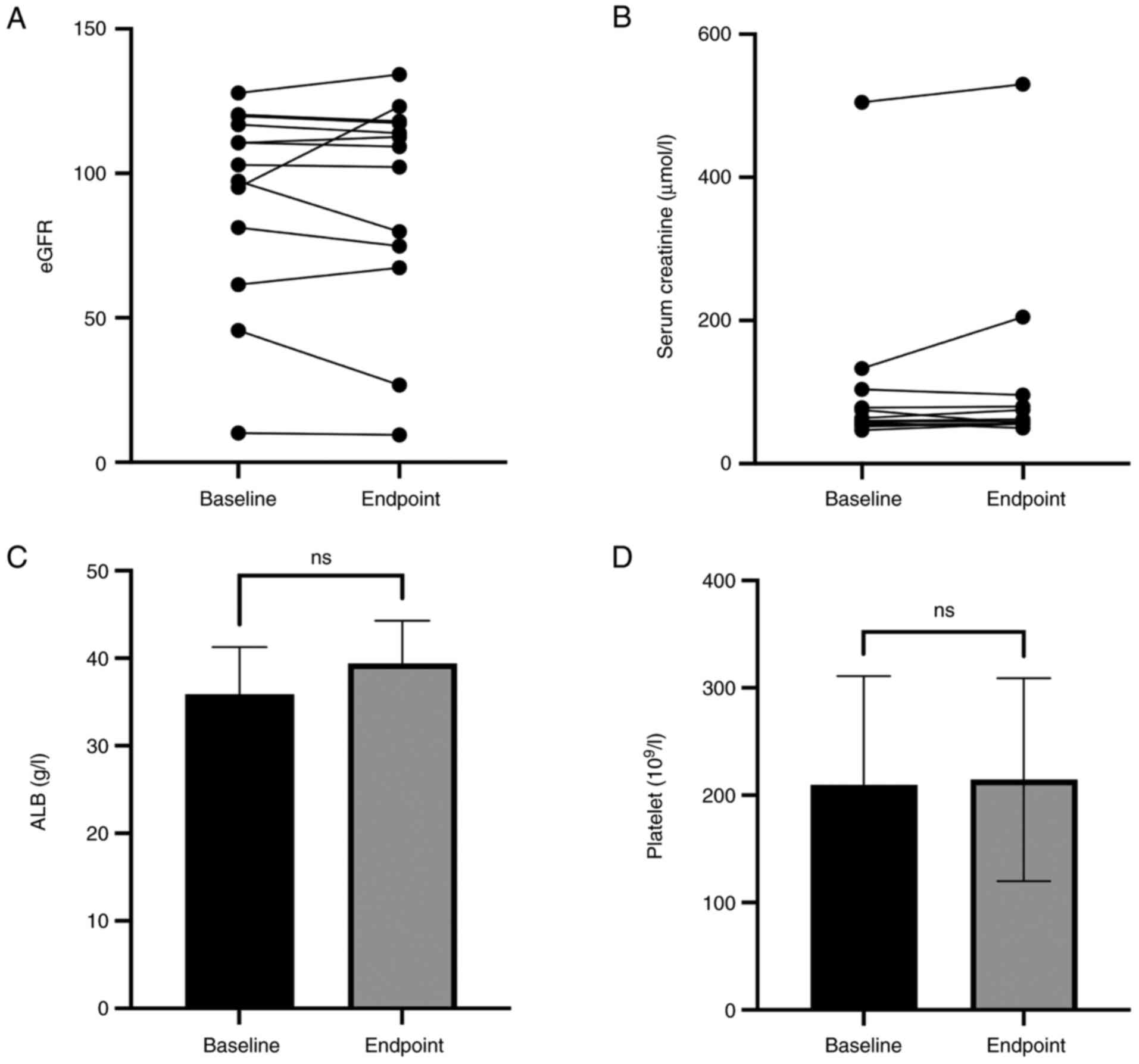
Secondary outcome measures
At the endpoint, few patients exhibited changes in
the eGFR (ml/min/1.73 m2). Notably, three patients
displayed improvement in renal function, and their eGFR increased
from 127.8 to 134.2, 95.1 to 123.1 and 61.5 to 67.3, respectively
(Fig. 4A and Table II). Renal function remained
largely unaffected in seven patients, while the eGFR of three
patients decreased during treatment with telitacicept (Table II). Serum creatinine levels of all
patients remained generally stable during the treatment period,
with slight elevation in three patients (Fig. 4A). After treatment with
telitacicept, plasma albumin levels remained normal in three
patients and increased in 10 patients. Plasma albumin levels were
normalized in three patients (Fig.
4C and Table II).
Additionally, all patients showed stable platelet levels during
treatment (Fig. 4D).
Drug adverse events
Telitacicept has been reported to cause adverse
events, such as infections and herpes zoster (12). However, none of the patients in the
present study experienced these symptoms. During treatment, seven
patients achieved disease activity control, and their telitacicept
dosage was reduced. One patient experienced a urinary tract
infection, and another suffered from a lower respiratory tract
infection. All these adverse events were mild to moderate and
relieved after treatment with systemic medications.
Effect of COVID-19 pandemic on
patients with LN
All the 13 patients were affected by COVID-19 during
the observation period. Among them, three patients developed fever,
and 10 patients remained asymptomatic (Table SIII).
Discussion
Systemic lupus erythematosus (SLE) is a chronic
multisystem autoimmune disorder that affects most organs,
especially the kidney (18,19).
LN is a significant complication of SLE, with the primary treatment
goal being the prevention of progressive renal damage and
subsequent renal failure (5,20).
The chronicity of LN and its recurrent flares correlate with
disease progression (4).
The prescribed immunosuppressive medicines typically
include mycophenolate mofetil, hydroxychloroquine, cyclosporine,
tacrolimus and cyclophosphamide, all of which lead to serious
adverse events (21,22). Hydroxychloroquine, for instance, is
excreted by the kidneys, which can increase renal burden and cause
pigment changes in the retinal macula, potentially leading to
vision loss in the absence of timely treatment (23). A number of recommendations suggest
that patients with LN and an eGFR <30 ml/min/1.7 m2
should receive lower doses of hydroxychloroquine to prevent toxic
adverse events (24,25). Cyclophosphamide leads to ovarian
failure, making it unsuitable for young women (26). Patients using mycophenolate mofetil
are highly likely to discontinue treatment due to gastrointestinal
toxicity (27). Although
glucocorticoids effectively control inflammation, their long-term
use is associated with chronic side effects, such as osteoporosis
and glaucoma (28). Complete
withdrawal from corticosteroids is the therapeutic objective for
all patients with a clinical response.
The patients in the present study received at least
one immunosuppressive medicine. Based on the profile of the
patients, the current study aimed to substitute these agents with
telitacicept at doses of 80 or 160 mg.
Telitacicept is the first ‘dual-target’ biological
agent that can effectively treat patients with SLE (14). A phase 3 study proved that it is
effective and well-tolerated by patients with SLE (29), even among pediatric patients
(13). It has been considered a
potential medicine for treating LN.
However, due to a supply chain shortage, all
patients in the trial discontinued treatment with telitacicept at
the endpoints. Nonetheless, the study continued to record and
analyze their medical data for 4 months after the endpoints.
During the 4-month follow-up period, three patients
receiving standard treatment needed a higher dose of mycophenolate
mofetil, hydroxychloroquine and cyclophosphamide. Follow-up data
revealed that the symptoms of the remaining patients [10 (76.9%)]
were under control, despite giving them the same doses of
immunosuppressive medicines and glucocorticoids. The combination of
telitacicept and standard treatment controlled their active LNs.
According to the guidelines, the 10 patients received their usual
doses of immunosuppressive medicines and glucocorticoids (30). However, dose escalation was
necessary for all 13 patients. Further studies may be needed to
provide a more detailed explanation for this discrepancy. No deaths
were recorded during this study. Two patients (15.4%) experienced
mild to moderate adverse events, all of whom recovered after
receiving systemic treatment.
All patients enrolled in this study were infected by
COVID-19 at different times, but none of them exhibited serious
complications, such as pneumonia. Further studies are needed to
unravel the potential effectiveness of telitacicept for respiratory
system-related diseases.
Compared with other studies on telitacicept
(31-33),
the present study did not include a large number of patients to
establish a control group. As previously mentioned, due to the
shortage of the supply chain, the duration of treatment with
telitacicept was not long.
Telitacicept, a novel biological agent, introduces a
new therapeutic paradigm, offering alternatives to glucocorticoid
therapy. Telitacicept, a new class of biological agents, was
effective in SLE. To the best of our knowledge, the present study
analyzed the efficacy and safety of telitacicept in Chinese
patients with LN during the COVID-19 pandemic for the first time.
Telitacicept may allow reducing the dosage of immunosuppressive
agents and glucocorticoids in patients with LN.
Supplementary Material
Treatment regimen, duration and dosage
of medication for patients with lupus nephritis before
enrollment.
Comparison of treatment regiments of
patients with lupus nephritis after telitacicept treatment.
Outcomes of telitacicept in lupus
nephritis.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Pioneer and Leading
Project of Zhejiang Province (grant no. 2022C03172).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors’ contributions
HZ made substantial contributions to the conception
of the study and drafted the manuscript. HQH and HLW collected
patient data. DXZ and HY analyzed the data. QKZ was responsible for
the interpretation of the data and ethical approval. LJ designed
the research. LJ and QKZ confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Ethical Committee of the Lishui Central Hospital (approval no.
2022-278). All patients whose data were not previously documented
in any publications provided written informed consent to utilize
their medical records.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mohamed A, Chen Y, Wu H, Liao J, Cheng B
and Lu Q: Therapeutic advances in the treatment of SLE. Int
Immunopharmacol. 72:218–223. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Parodis I, Tamirou F and Houssiau FA:
Prediction of prognosis and renal outcome in lupus nephritis. Lupus
Sci Med. 7(e000389)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen Y, Sun J, Zou K, Yang Y and Liu G:
Treatment for lupus nephritis: An overview of systematic reviews
and meta-analyses. Rheumatol Int. 37:1089–1099. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Parikh SV, Almaani S, Brodsky S and Rovin
BH: Update on lupus nephritis: Core curriculum 2020. Am J Kidney
Dis. 76:265–281. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Anders HJ, Saxena R, Zhao MH, Parodis I,
Salmon JE and Mohan C: Lupus nephritis. Nat Rev Dis Primers.
6(7)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shiraz AK, Panther EJ and Reilly CM:
Altered germinal-center metabolism in B cells in autoimmunity.
Metabolites. 12(40)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rovin BH, Furie R, Teng YKO, Contreras G,
Malvar A, Yu X, Ji B, Green Y, Gonzalez-Rivera T, Bass D, et al: A
secondary analysis of the Belimumab international study in lupus
nephritis trial examined effects of belimumab on kidney outcomes
and preservation of kidney function in patients with lupus
nephritis. Kidney Int. 101:403–413. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Onuora S: Adding belimumab improves lupus
nephritis. Nat Rev Rheumatol. 16(601)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Samy E, Wax S, Huard B, Hess H and
Schneider P: Targeting BAFF and APRIL in systemic lupus
erythematosus and other antibody-associated diseases. Int Rev
Immunol. 36:3–19. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shi F, Xue R, Zhou X, Shen P, Wang S and
Yang Y: Telitacicept as a BLyS/APRIL dual inhibitor for autoimmune
disease. Immunopharmacol Immunotoxicol. 43:666–673. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dhillon S: Telitacicept: First approval.
Drugs. 81:1671–1675. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fan Y, Gao D and Zhang Z: Telitacicept, a
novel humanized, recombinant TACI-Fc fusion protein, for the
treatment of systemic lupus erythematosus. Drugs Today (Barc).
58:23–32. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sun L, Shen Q, Gong Y, Li Y, Lv Q, Liu H,
Zhao F, Yu H, Qiu L, Li X, et al: Safety and efficacy of
telitacicept in refractory childhood-onset systemic lupus
erythematosus: A self-controlled before-after trial. Lupus.
31:998–1006. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen R, Fu R, Lin Z, Huang C and Huang W:
The efficacy and safety of telitacicept for the treatment of
systemic lupus erythematosus: A real life observational study.
Lupus. 32:94–100. 2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Aringer M and Petri M: New classification
criteria for systemic lupus erythematosus. Curr Opin Rheumatol.
32:590–596. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gladman DD, Ibañez D and Urowitz MB:
Systemic lupus erythematosus disease activity index 2000. J
Rheumatol. 29:288–291. 2022.PubMed/NCBI
|
|
17
|
Computing VARFFS. R: A language and
environment for statistical computing. 2020.
|
|
18
|
Kaul A, Gordon C, Crow MK, Touma Z,
Urowitz MB, van Vollenhoven R, Ruiz-Irastorza G and Hughes G: .
Systemic lupus erythematosus. Nat Rev Dis Primers.
2(16039)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Golder V and Hoi A: Systemic lupus
erythematosus: An update. Med J Aust. 206:215–220. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Houssiau FA and Ginzler EM: Current
treatment of lupus nephritis. Lupus. 17:426–430. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yusuf IH, Sharma S, Luqmani R and Downes
SM: Hydroxychloroquine retinopathy. Eye (Lond). 31:828–845.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Surówka A, Prowans P, Żołnierczuk M,
Miśkiewicz M, Wawrowski T, Skodda M, Markowska M and
Kędzierska-Kapuza K: The effect of calcineurin inhibitors on MMPs
activity in heart and their side effects-a review of literature.
Int J Mol Sci. 24(10291)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Garrity ST, Jung JY, Zambrowski O, Pichi
F, Su D, Arya M, Waheed NK, Duker JS, Chetrit Y, Miserocchi E, et
al: Early hydroxychloroquine retinopathy: Optical coherence
tomography abnormalities preceding Humphrey visual field defects.
Br J Ophthalmol. 103:1600–1604. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kaye KS, Belley A, Barth P, Lahlou O,
Knechtle P, Motta P and Velicitat P: Effect of
cefepime/enmetazobactam vs. piperacillin/tazobactam on clinical
cure and microbiological eradication in patients with complicated
urinary tract infection or acute pyelonephritis: A randomized
clinical trial. JAMA. 328:1304–1314. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tunnicliffe DJ, Palmer SC, Henderson L,
Masson P, Craig JC, Tong A, Singh-Grewal D, Flanc RS, Roberts MA,
Webster AC and Strippoli GF: Immunosuppressive treatment for
proliferative lupus nephritis. Cochrane Database Syst Rev.
6(CD002922)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kado R and McCune WJ: Ovarian protection
with gonadotropin-releasing hormone agonists during
cyclophosphamide therapy in systemic lupus erythematosus. Best
Pract Res Clin Obstet Gynaecol. 64:97–106. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Galiwango PJ, Delgado DH, Yan R, Kozuszko
S, Smith R, Rao V and Ross HJ: Mycophenolate mofetil dose reduction
for gastrointestinal intolerance is associated with increased rates
of rejection in heart transplant patients. J Heart Lung Transplant.
27:72–77. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ugarte A, Danza A and Ruiz-Irastorza G:
Glucocorticoids and antimalarials in systemic lupus erythematosus:
An update and future directions. Curr Opin Rheumatol. 30:482–489.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang L, Li J, Xu D, Fang J, Van
Vollenhoven R and Zhang F: OP0137 Efficacy And Safety Of
Telitacicept, A Novel Blys/April Dual Inhibitor, In Patients With
Systemic Lupus Erythematosus: A phase 3, randomized,
placebo-controlled 52-week study. Ann Rheumatic Dis. 82:90–91.
2023.
|
|
30
|
Avasare R, Drexler Y, Caster DJ,
Mitrofanova A and Jefferson JA: Management of lupus nephritis: New
treatments and updated guidelines. Kidney360. 4:1503–1511.
2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lv J, Liu L, Hao C, Li G, Fu P, Xing G,
Zheng H, Chen N, Wang C, Luo P, et al: Randomized phase 2 trial of
telitacicept in patients with IgA nephropathy with persistent
proteinuria. Kidney Int Rep. 8:499–506. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wu D, Li J, Xu D, Merrill JT, van
Vollenhoven RF, Liu Y, Hu J, Li Y, Li F, Huang C, et al:
Telitacicept in patients with active systemic lupus erythematosus:
Results of a phase 2b, randomised, double-blind, placebo-controlled
trial. Ann Rheum Dis. 83:475–487. 2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ji L, Geng Y, Zhang X, Deng X, Song Z, Tan
M, Tan Y, Qu C and Zhang Z: B cell pathway dual inhibition for
systemic lupus erythematosus: A prospective single-arm cohort study
of telitacicept. MedComm. 5(e515)2024.PubMed/NCBI View
Article : Google Scholar
|