Introduction
An intestinal fistula is a common and serious
complication of intestinal surgery (1), which can occur in patients of any
age. In a meta-analysis, Gefen et al (2) reported that 89.4%
of patients postoperatively developed an enterocutaneous fistula.
The most common fistula site was the small bowel, followed by the
colon. The underlying etiologies for enterocutaneous fistula
development were inflammatory bowel disease (IBD), malignancy and
radiation, and the incidence rate of postoperative intestinal
fistula in patients without major basic diseases was markedly
reduced (2). Regardless of the
surgical context, intestinal fistulas pose a considerable challenge
as a postoperative complication. The etiology of intestinal fistula
encompasses a range of factors, including abdominal trauma, cancer,
radiation, IBD and infectious diseases, and is often accompanied by
complications such as sepsis, malnutrition and electrolyte
disorders. Complex intestinal fistulas, accounting for ~28% of
cases, pose a significant challenge for surgeons (2,3).
Notably, successful management and early treatment
of intestinal fistulas are crucial to achieve good treatment
outcomes (2). When an intestinal
fistula occurs, patients may experience pain and severe skin
discomfort. If not handled appropriately, intestinal failure caused
by intestinal fistula and the series of complications it causes can
be fatal. The treatment of intestinal fistulas includes
conservative and surgical strategies, which usually have a
significant negative impact on the quality of life of patients.
Various treatment modalities have been employed to treat patients
with intestinal fistulas, including total parenteral nutrition.
Additionally, surgical intervention, such as the placement of
vacuum-assisted closure devices, fibrin glue application,
endoscopic procedures and repeated ostomy, may be utilized.
However, it is important to note that the cure rate and
effectiveness of these interventions can vary. Reoperation, mainly
open and laparoscopic, is occasionally necessary for severe and
complex intestinal fistulas; however, laparoscopic surgery is
technically difficult, and adhesions around the secondary surgery
are extensive, complicating the surgery. Open surgery causes
significant trauma, and patients may experience recurrent fistulas
or require colostomy, which reduces their quality of life.
Currently, there are limited effective and minimally invasive
treatment plans for patients with intestinal fistulas (4,5).
Intestinal fistula treatment can take several months
or even years, and is cumbersome and complex (6). The prolonged duration of treatment,
coupled with the nature of the treatment, can result in
psychological and physical trauma. Conservative approaches
typically prove ineffective and time-consuming. Additionally,
secondary surgical interventions for stoma reconstruction can
impose further physiological stress on patients. Moreover, there
exists considerable psychological pressure on patients with stomas,
with a number of patients expressing reluctance to accept such a
solution (7). Hence, opting for
minimally invasive treatment methods becomes crucial, aiming to
spare patients from undergoing additional stoma procedures, and
alleviating both physical and psychological burdens. Herein, the
present study describes the case of a patient with a postoperative
gastrointestinal fistula. Through the use of transurethral prostate
resection instrumentation, intestinal stent placement and drainage
was enabled. After multiple minimally invasive treatments spanning
6 months, the patient recovered and was discharged.
Case report
General information
A 51-year-old man with gastric torsion and
transverse colon redundancy underwent gastric reduction and
fixation, along with partial resection of the transverse and
descending colon, at a local hospital in June 2015 (Minle County
Traditional Chinese Medicine Hospital, Zhangye, China). On
postoperative day 5, discharge of fecal and foul purulent fluid
from the upper abdominal incision, abdominal distension and colonic
anastomotic leakage were observed. On the 7th post-operative day an
ileostomy was performed (Minle County Traditional Chinese Medicine
Hospital). After 2 weeks of hospitalization, the patient's
condition did not improve, and they were transferred to Hexi
University Affiliated Zhangye People's Hospital (Zhangye, China).
On admission, a physical examination was conducted and showed a
temperature of 38.8˚C (normal range, 36.1-37.0˚C), pulse rate of
118 beats/min (normal range, 60-100 beats/min), respiratory rate of
25 breaths/min (normal range, 16-20 breaths/min) and blood pressure
of 110/70 mmHg. The abdomen was slightly distended, with a 16-cm
longitudinal incision visible in the middle and upper abdomen, and
a 6-cm wound opening in the upper segment. Purulent exudation with
a foul odor was observed deep in the abdominal cavity. During
exploration using the fingers, the colon anastomosis opening was
reached, and contact was made with the titanium nail and an
overflow of a significant amount of purulent fluid. In the lower
abdomen, a longitudinal wound measuring 7 cm was observed,
requiring intermittent suture removal due to local infection, with
a visible drainage tube in the lower abdomen. Additionally, a small
intestinal stoma, measuring 4 cm in diameter, was noted in the
right lower abdomen. The skin around the stoma showed erosive
erythema and inflammation, with a diameter of ~8 cm. An abdominal
drainage tube was visible in the lower left abdomen, with a slight
discharge of purulent fluid. Auscultation revealed diminished bowel
sounds, while palpation indicated concave edema in both lower
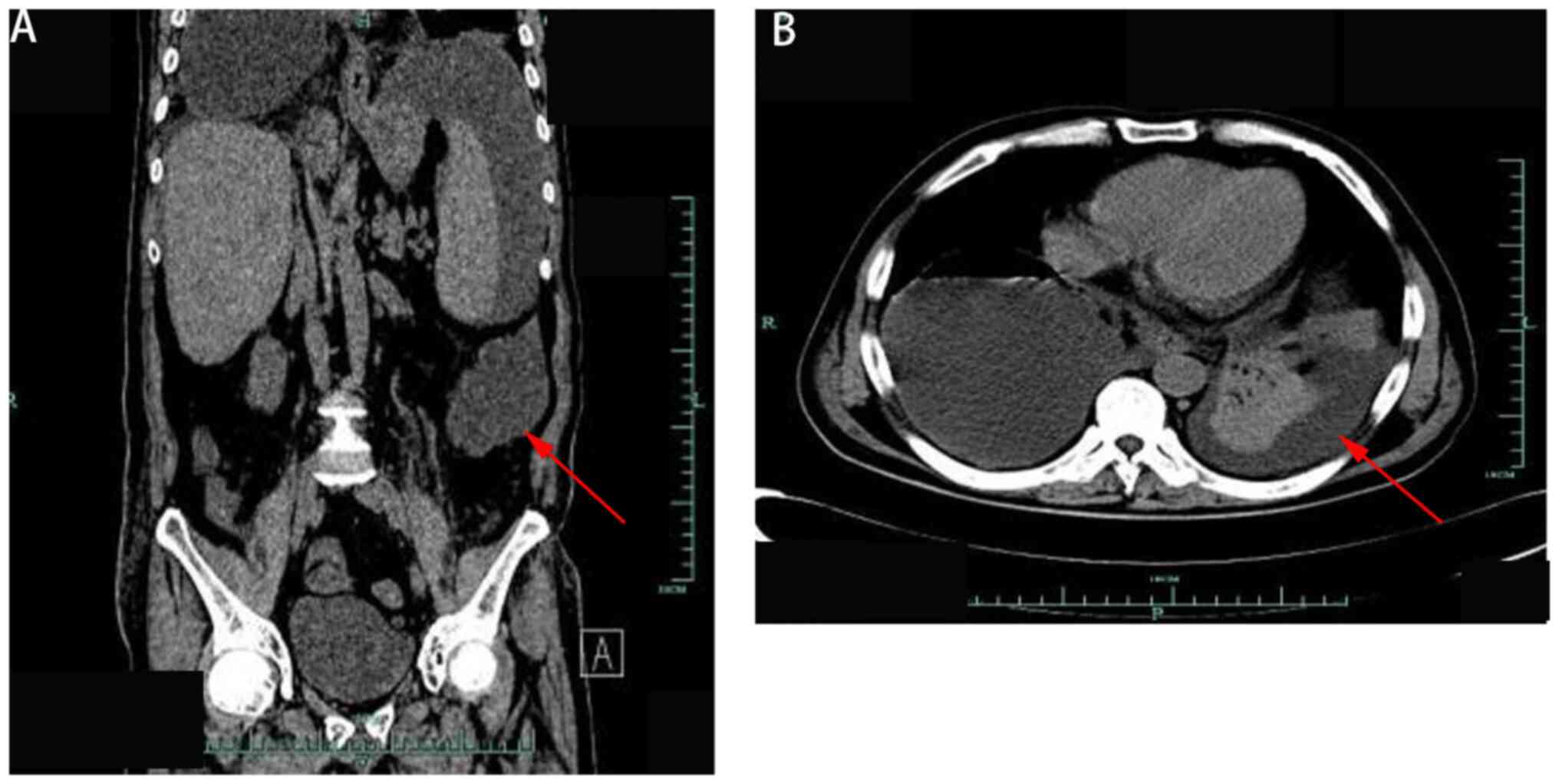
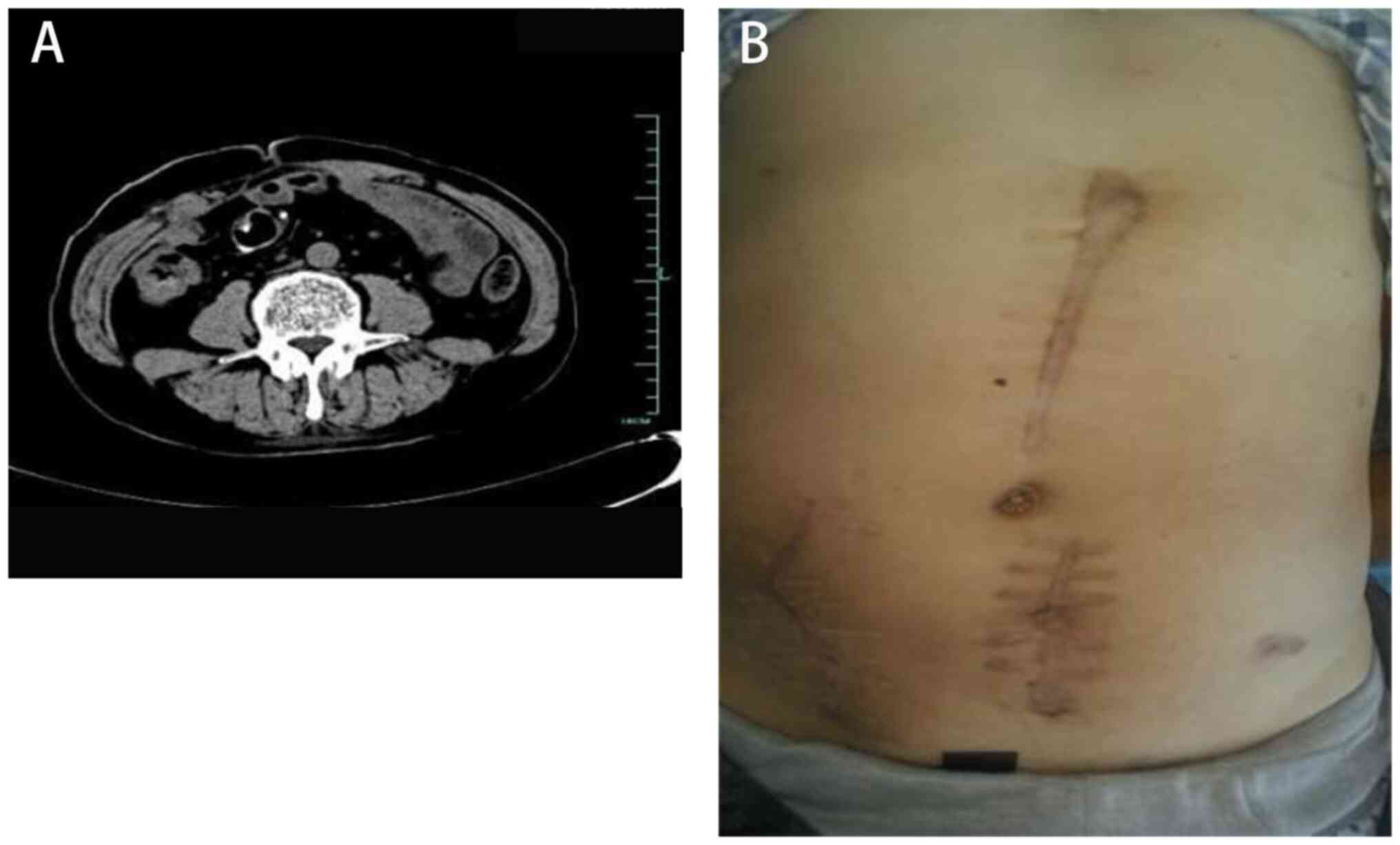
limbs. Computed tomography confirmed the presence of an abdominal
abscess (Fig. 1).
Final diagnosis
The patient was diagnosed with colonic anastomotic
fistula, abdominal abscess, localized peritonitis, incomplete
intestinal obstruction, bilateral pleural effusion and
hypoalbuminemia following small intestinal fistula surgery, and
gastric torsion reduction and fixation surgery.
Treatment
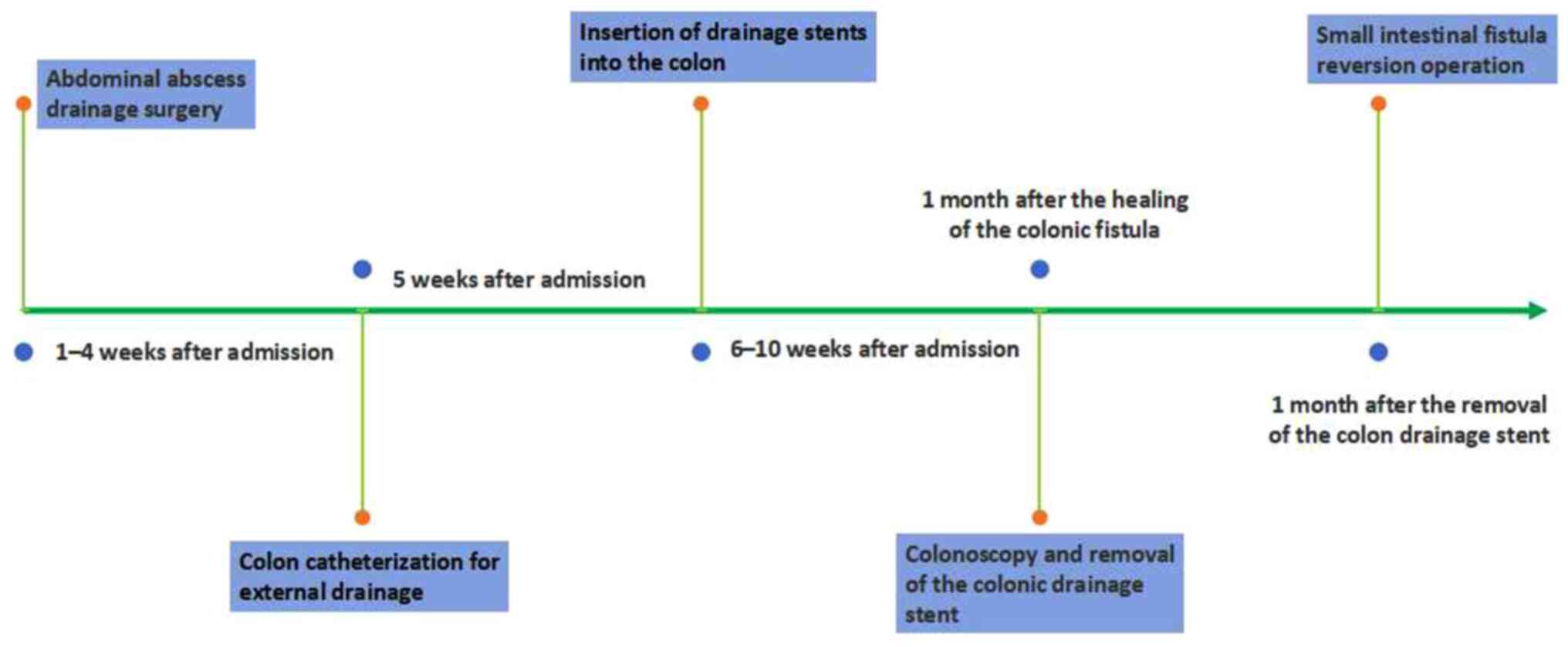
The first stage of treatment involved abdominal
abscess drainage surgery (1-4 weeks after admission): Thorough
opening, cleaning and flushing of the abdominal abscess was
performed under general anesthesia using sufentanil, propofol,
midazolam and rocuronium bromide. F24 rubber drainage tubes,
containing multiple side holes, were retained, and a tube was
placed in the right chest for drainage. Active nutritional support,
dressing changes for infected incisions and anti-infection
treatment with imipenem (intravenous infusion, 500 mg/6 h) were
simultaneously provided. The drainage tube was rinsed with diluted
iodine to maintain unobstructed drainage. After 3 weeks of
treatment, the condition of the patient stabilized and the
abdominal abscess gradually recovered. The pus drainage tubes were
removed individually and observation was continued for 1 week.
The second stage of treatment involved colon
catheterization for external drainage (5 weeks after admission).
The patient was placed in the supine position without anesthesia
and underwent an endoscopy through an upper abdominal incision
opening under direct vision. Use of the same transurethral prostate
resection instruments for visualization revealed residual titanium
nails and necrotic tissue at the colonic fistula site. The anterior
wall of the colon anastomosis had ruptured by ~1/3 of its
circumference. Proximal and distal ends of the colon were
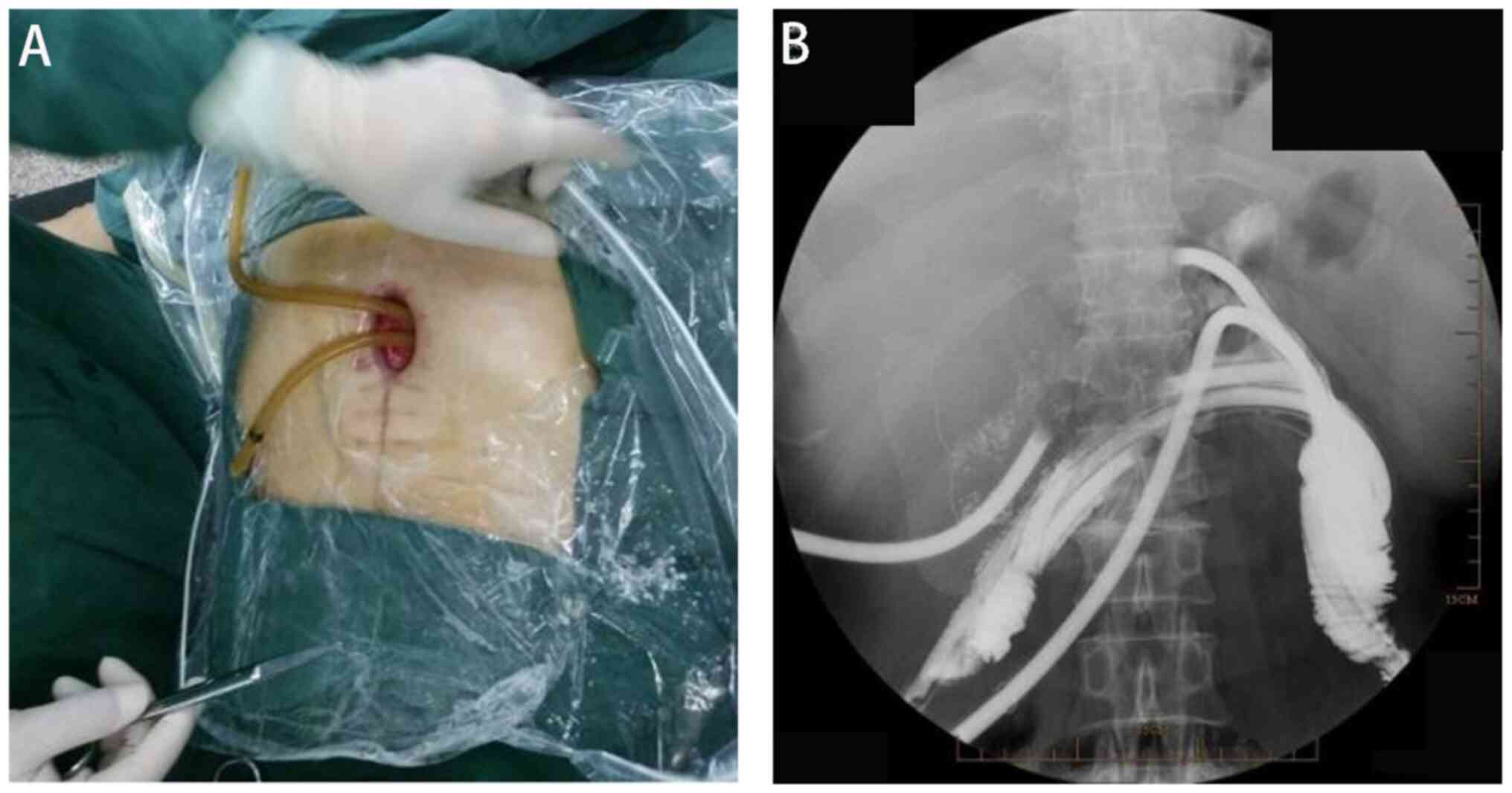
identified, and external drainage was established using an F24
porous rubber tube inserted with guidewire guidance (Fig. 2). The tubes were rinsed daily with
physiological saline solution. After 1 week, the intestinal
flushing solution was clear, and the granulation tissue at the
abdominal opening was red and moist, without purulent
secretions.
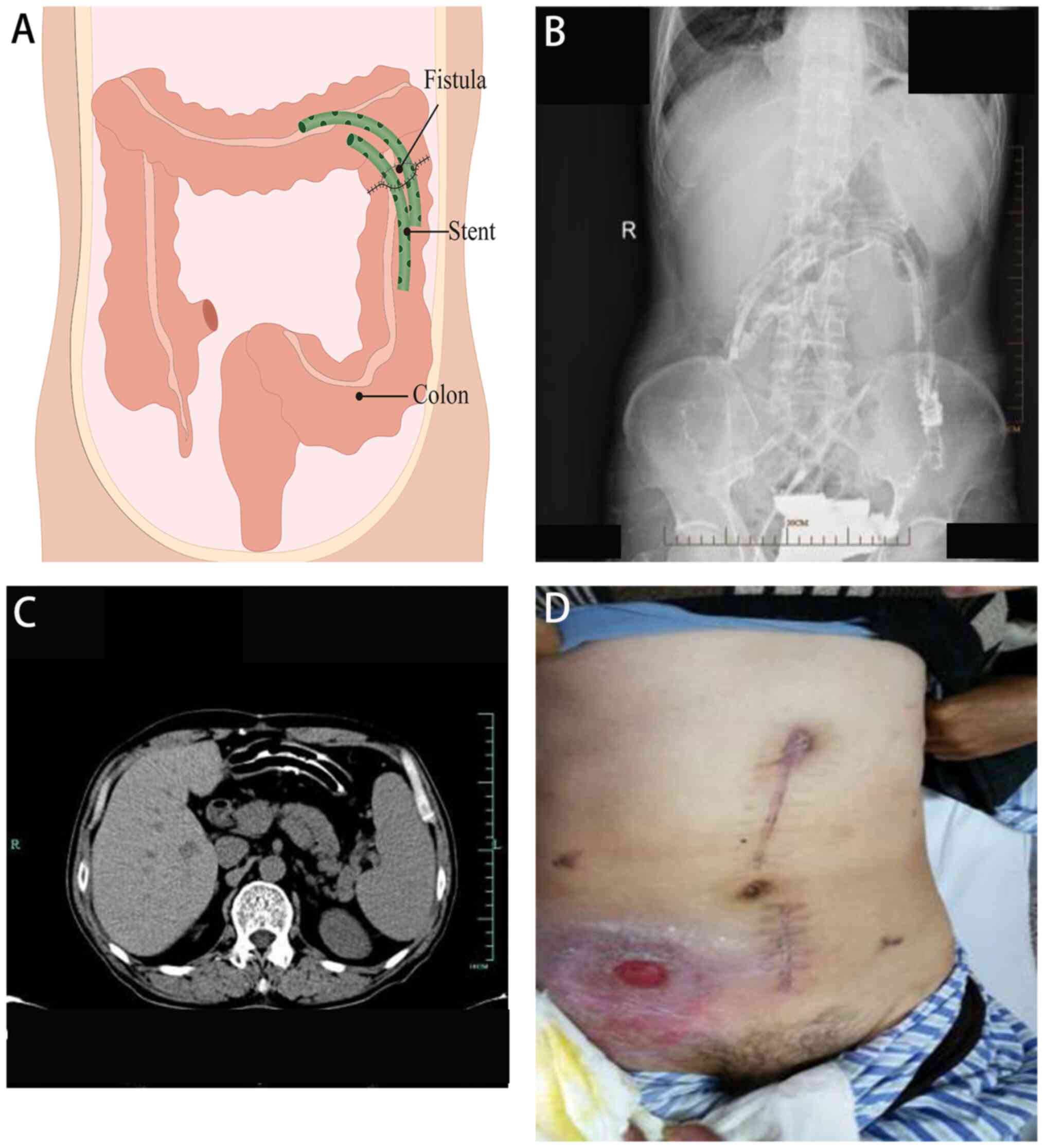
The third stage of treatment involved the insertion
of drainage stents into the colon (6-10 weeks after admission). Two
colon drainage tubes, each measuring 12 cm in length, were
utilized. Multiple side holes were cut along the tubes, which were
then bound together and fully inserted into the colonic cavity. To
prevent stent displacement due to intestinal peristalsis, they were
suspended and secured in place using 10# silk thread at the
midpoint. A total of 8 weeks after admission, a small amount of
leakage was observed at the incision site on the upper abdomen, and
the fixed thread was removed. A total of 10 weeks after admission,
skin leakage in the upper abdominal colon completely healed
(Fig. 3).
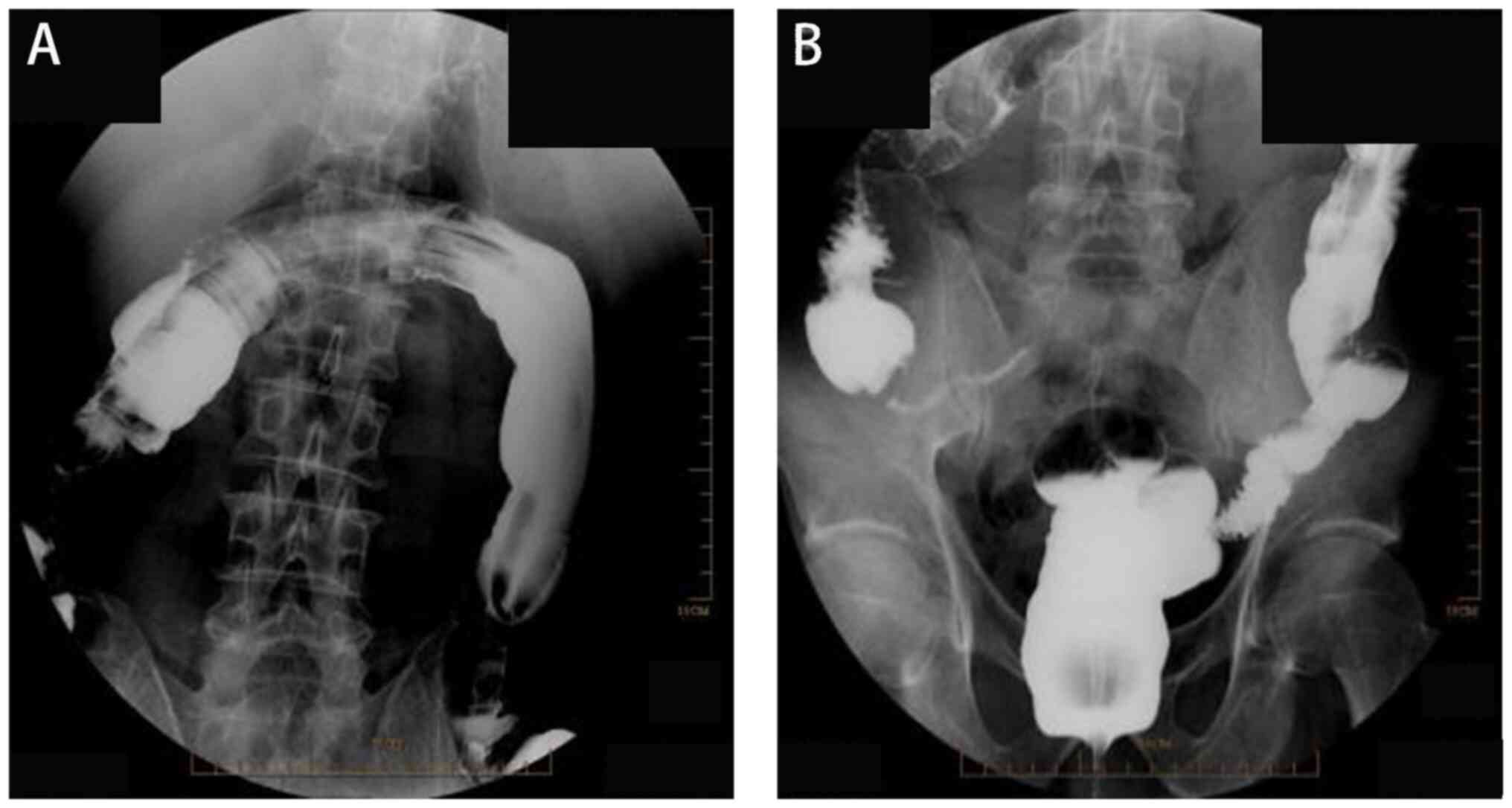
The fourth stage of treatment involved colonoscopy
and removal of the colonic drainage stent. A total of 1 month after
the healing of the colonic fistula, a retrograde colonoscopy of the
anus showed no leakage of the contrast agent at the site of the
colonic anastomosis fistula (Fig.
4). During colonoscopy, a drainage tube was identified ~40 cm
from the anus. Using a ligation device, the colonic drainage stent
tube inserted in the third stage was successfully removed.
Finally, the fifth stage of treatment involved small
intestinal fistula recovery. A total of 1 month after the removal
of the colon drainage stent, a small intestinal fistula reversion
operation was performed. Following the procedure, the patient
experienced a successful recovery. Physical examination and
abdominal computed tomography revealed favorable progress, and the
patient was discharged (Fig. 5).
The patient received satisfactory treatment outcomes through
diligent care provided by the medical team and nursing staff,
effectively averting further complications, such as fistula
formation and larger trauma (Fig.
6). The latest follow-up of the patient was in June 2023, and
the patient is currently undergoing an annual follow-up. The
follow-up entails blood tests to assess routine blood work, liver
function, kidney function and electrolyte levels, as well as a full
abdominal computed tomography or magnetic resonance imaging scan
and physical examination. The patient is currently in good
condition and is satisfied with the recovery.
Discussion
The patient underwent a full course of treatment for
6 months and ultimately recovered after five surgical procedures.
The latter four of these were minimally invasive procedures that
did not introduce more complications or cause pain to the patient.
Various techniques and surgical procedures for treating intestinal
fistulas have been reviewed (2,5,8).
Conservative treatment is usually the preferred treatment option
initially; however, patients with severe intestinal fistulas cannot
be cured through conservative treatment, and their condition can
worsen and thus put their lives at risk. Furthermore, surgical
enterostomies can increase trauma and pain.
Notably, ≥28% of patients with intestinal fistulas
have a complex fistula, which is identified as more than one
abnormal connection between the gastrointestinal tract and the
skin, or a fistula involving multiple bowel loops. Studies have
described a 44-100% success rate of fistula treatment methods,
including fibrin glue, vacuum-assisted closure devices,
over-the-scope clip endoscopic treatment and extracellular matrix
plug; however, the number of patients in the studies were too small
to draw a meaningful conclusion about the efficacy of these
techniques (9-12).
A total of 31 studies involving 1,381 patients have reported
fistula healing in 44.8% of the patients who received conservative
treatment (2). The fistula
recurrence rate after any type of treatment was revealed to be
15.1% in 41 studies (2,557 patients) and the mortality rate was
9.6% (2). After surgery for
fistulas, the fistula recurrence rate was 7.6%, the hernia
recurrence rate was 19.7% and the infection rate at the surgical
site was 36.5%. Mortality was primarily associated with the general
condition of the patients, including the presence of sepsis and
advanced cancer, malnutrition, American Society of Anesthesiologist
score, fistula recurrence and tobacco smoking (2,13-16).
In the present case, before being admitted to Hexi
University Affiliated Zhangye People's Hospital, the patient
underwent partial resection of the transverse and descending
colons, and ileostomy. Consequently, colonic leakage poses a risk
of abdominal abscess formation. Urgent surgical intervention and
prompt treatment of the infection are imperative in such scenarios.
The intestinal continuity can be restored after complete infection
control; therefore, acting hastily may not be advisable, as it
could exacerbate pain and increase the risk of complications,
particularly in cases of direct colostomy.
Treatment of intestinal fistulas requires a
multidisciplinary approach (17,18).
In the second stage of treatment in the present study, we
collaborated with the urology team to perform an endoscopy using
transurethral prostate resection instrumentation under direct
vision through the upper abdominal incision opening without
anesthesia. Placing a drainage tube in the intestinal tract and
rinsing it with physiological saline is beneficial for wound
healing. Transurethral prostate resection instrumentation, in which
a hard endoscope is used to guide resection, is commonly used in
urology. The compact design of the endoscope facilitates easy
handling during procedures. This instrument provides high-pressure
flushing of the operative field, thereby ensuring optimal
visibility. Moreover, the insertion of a guidewire catheter in
transurethral prostate resection instrumentation is comparatively
less invasive than laparoscopy, and offers greater convenience and
simplicity compared with colonoscopy (19,20).
Patients may experience postoperative complications, such as
recurrent fistula, infection or intestinal failure after
laparoscopic and open surgical procedures. Therefore, systemic
nutrition, which is the most important and first step in treatment,
is crucial in these patients. During instrument exploration, the
patient receives minimally invasive treatment without the need for
anesthesia; thus, there was no risk of surgical and anesthetic
complications for the patient. If minimally invasive treatment is
ineffective, open surgery can be performed, as it is the only hope
for patient survival or cure. It has previously been reported that
instrument-based exploration can effectively treat intestinal
diseases (21). Therefore, the
same prostate resection endoscopy instrumentation was reused for
the exploration, treatment and insertion of drainage stents. After
sufficient intestinal recovery, satisfactory treatment results were
achieved. Although the patient in this case had good treatment
outcomes and did not experience postoperative complications or
recurrence, this was an isolated case because there were no other
patients who underwent such treatment. Therefore, it is not
possible to determine the success rate of the treatment options,
which is a limitation of the present study.
In the present case, the treatment in each stage was
slow and orderly. First, the infection was controlled and
intestinal drainage was performed. Once intestinal continuity was
fully restored, the patient underwent small intestinal recovery
surgery. While the surgical procedures served a crucial role, it is
important to note that they were just one aspect of the thorough
treatment approach. The treatment of intestinal fistulas requires
comprehensive treatment, and the initial focus should be on dealing
with water-electrolyte-balance disorders, and actively preventing
and treating infection and sepsis. Adjusting the patient's
physiological and psychological state is optimal, and effective
parenteral nutrition support is essential. Patients with complex
intestinal fistulas can be cured through sufficient minimally
invasive drainage.
In conclusion, endoscopic guidance for intestinal
stent placement to promote intestinal recovery is a simple,
effective and minimally invasive treatment plan for complex
intestinal fistulas. At present, there are several treatment
options for intestinal fistulas, including conservative and
surgical treatment, but each has its advantages and disadvantages.
The treatment cycle is long, and the therapeutic effect is
uncertain, which is a major challenge faced by surgeons. This case
report offers valuable insights into employing a comprehensive
approach for treating complex intestinal fistulas using a colon
indwelling drainage stent under the guidance of transurethral
prostate resection instrumentation. By integrating multiple
techniques along with interdisciplinary collaboration, this method
demonstrates efficacy in managing such challenging cases.
Leveraging commonly utilized urological equipment proves to be
particularly advantageous, streamlining procedures and yielding
significant outcomes with minimal effort in patients undergoing
general surgery. The limitations of specialization of surgeons and
departments can be effectively addressed through interdisciplinary
collaboration, benefiting more patients.
Acknowledgements
Not applicable.
Funding
Funding: This study was funded by grants from the President Fund
Innovation Team Project of Hexi University (grant no.
CXTD2022012).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
CW, YQ and ST contributed to the drafting of the
manuscript and design of the study. JQ, FY and JY contributed to
the conceptualization and design of the study, as well as the
completion of the surgery. ST, FY and JY collected clinical
information and assisted with drafting the manuscript. JQ and JY
critically revised the intellectual content and confirm the
authenticity of all the raw data, and have given final approval of
the version to be published. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Ethics approval for this study was granted by the
Ethics Committee of Hexi University Affiliated Zhangye People's
Hospital (approval no. B2014-012).
Patient consent for publication
Written consent was obtained from the patient for
the publication of the data and images included in this case
report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bhama AR: Evaluation and management of
enterocutaneous fistula. Dis Colon Rectum. 62:906–910.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gefen R, Garoufalia Z, Zhou P, Watson K,
Emile SH and Wexner SD: Treatment of enterocutaneous fistula: A
systematic review and meta-analysis. Tech Coloproctol. 26:863–874.
2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Islam MS, Gafur MA, Mahmud AA, Mahiuddin
M, Khan SA, Reza E, Rahman MS, Mahmud M, Karim MR, Hoque MM, et al:
Clinicopathological study of enterocutaneous fistula in Mymensingh
Medical College Hospital. Mymensingh Med J. 27:513–519.
2018.PubMed/NCBI
|
|
4
|
Cattoni DI, Ravazzola C, Tüngler V,
Wainstein DE and Chara O: Effect of intestinal pressure on fistula
closure during vacuum assisted treatment: A computational approach.
Int J Surg. 9:662–668. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Assenza M, Rossi D, De Gruttola I and
Ballanti C: Enterocutaneous fistula treatment: Case report and
review of the literature. G Chir. 39:143–151. 2018.PubMed/NCBI
|
|
6
|
Ghimire P: Management of enterocutaneous
fistula: A review. JNMA J Nepal Med Assoc. 60:93–100.
2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xiong YC, Deng YH, Li Z, Huang Y and Qin
YL: Evaluation of comfort in patients with intestinal fistula and
analysis of influencing factors. Altern Ther Health Med.
29:170–179. 2023.PubMed/NCBI
|
|
8
|
Kluciński A, Wroński M, Cebulski W, Guzel
T, Witkowski B, Makiewicz M, Krajewski A and Słodkowski M: Surgical
repair of small bowel fistulas: Risk factors of complications or
fistula recurrence. Med Sci Monit. 25:5445–5452. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Smith TA, Hardman RL, Jenkins L, Marashi
K, O'Hara R and Cizman Z: Extracellular matrix enterocutaneous
fistula plugs show promise for low-flow colocutaneous and
enterocutaneous fistulae. J Vasc Interv Radiol. 32:128–134.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hwang TL and Chen MF: Randomized trial of
fibrin tissue glue for low output enterocutaneous fistula. Br J
Surg. 83(112)1996.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Young S, D'Souza D, Hunter D, Golzarian J
and Rosenberg M: The use of latex catheters to close
enterocutaneous fistulas: An institutional protocol and
retrospective review. AJR Am J Roentgenol. 208:1373–1377.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Amiot A, Setakhr V, Seksik P, Allez M,
Treton X, De Vos M, Laharie D, Colombel JF, Abitbol V, Reimund JM,
et al: Long-term outcome of enterocutaneous fistula in patients
with Crohn's disease treated with anti-TNF therapy: A cohort study
from the GETAID. Am J Gastroenterol. 109:1443–1449. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Owen RM, Love TP, Perez SD, Srinivasan JK,
Sharma J, Pollock JD, Haack CI, Sweeney JF and Galloway JR:
Definitive surgical treatment of enterocutaneous fistula: Outcomes
of a 23-year experience. JAMA Surg. 148:118–126. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Brenner M, Clayton JL, Tillou A, Hiatt JR
and Cryer HG: Risk factors for recurrence after repair of
enterocutaneous fistula. Arch Surg. 144:500–505. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Draus JM Jr, Huss SA, Harty NJ, Cheadle WG
and Larson GM: Enterocutaneous fistula: Are treatments improving?
Surgery. 140:570–576. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Redden MH, Ramsay P, Humphries T and
Fuhrman GM: The etiology of enterocutaneous fistula predicts
outcome. Ochsner J. 13:507–511. 2013.PubMed/NCBI
|
|
17
|
Papa A, Lopetuso LR, Minordi LM, Di
Veronica A, Neri M, Rapaccini G, Gasbarrini A and Papa V: A modern
multidisciplinary approach to the treatment of enterocutaneous
fistulas in Crohn's disease patients. Expert Rev Gastroenterol
Hepatol. 14:857–865. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Grainger JT, Maeda Y, Donnelly SC and
Vaizey CJ: Assessment and management of patients with intestinal
failure: A multidisciplinary approach. Clin Exp Gastroenterol.
11:233–241. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xu W, Qin Y, Yang F, Qian J, Dong Y, Tu S
and Yao J: Application of transurethral prostate resection
instrumentation for treating rectal anastomotic stenosis: Case
series. Medicine (Baltimore). 102(e33799)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang Z, Hu Z, Qin Y, Qian J, Tu S and Yao
J: Application of transurethral prostate resection instrumentation
for treating low rectal anastomotic leakage: A pilot study. Cancer
Manag Res. 14:1987–1994. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yan P, Qin Y, Zhang Z, Xu W, Qian J, Tu S
and Yao J: Application of prostate resection endoscopy for treating
acute obstruction associated with rectal cancer. J Cancer.
13:1679–1684. 2022.PubMed/NCBI View Article : Google Scholar
|