1. Introduction
Carpal tunnel syndrome (CTS) is a common peripheral
nervous system disorder and is the most frequent entrapment
neuropathy. CTS is more common in women than men, and it is most
often diagnosed at 45-60 years of age (1,2). A
compression of the median nerve in the wrist causes entrapment
neuropathy. The cause of CTS is not completely understood, despite
the previous identification of risk factors. Several factors
contribute to the development of CTS, including old age, smoking,
obesity, repetitive hand movements and vibration tools. Rheumatic
diseases (RDs), hypothyroidism and diabetes are also common
factors. CTS has a high prevalence and a significant impact on the
quality of life of patients. The condition is associated with
numbness, weakness and pain in the hand and arm, and is a marked
cause of disability (3).
RDs and musculoskeletal diseases affect millions of
people worldwide due to degenerative, inflammatory and immune
conditions. Chronic pain, joint damage and increasing disability
may occur in patients with these diseases (4). There are several RDs associated with
CTS, including osteoarthritis (OA), rheumatoid arthritis (RA),
psoriatic arthritis (PsA) and some connective tissue conditions,
such as systemic lupus erythematosus (SLE), systemic sclerosis
(SSc) and Sjögren's syndrome (SS). In general, RDs and related
musculoskeletal disorders are prevalent throughout the world, but
when their prevalence was compared, in different countries, for
each disorder, variations in their prevalence were identified (e.g.
the prevalence of OA may vary from 2.3-20.4%) (5-7).
The pathogenesis of RDs such as SLE, SSc, SS or RA
appears to be associated with the presence of autoantibodies
(8,9). The causes of neurological involvement
in RD are not fully understood (10,11).
In the present narrative review, neurological complications related
to the peripheral nerve system (PNS) in RD are discussed. As the
prevalence of CTS is on the rise, understanding the common
mechanism and making an early diagnosis are necessary to limit
unnecessary pain and cost. When patients with RD manifest symptoms
such as wrist pain, tingling sensations or numbness in their
fingers, CTS should be suspected. This suspicion should not be
interpreted in terms of RD. Strong interdisciplinary collaborations
would benefit the patient, since an early diagnosis can result in
full recovery of the median nerve lesion. However, late diagnosis
in severe CTS cases has limited recovery prospects. Collaborations
can only be achieved by identifying common problems. The current
review aims to raise awareness about these issues, which are often
overlooked.
2. Epidemiological data
CTS
It is estimated that 3.8% of the general population
worldwide suffer from CTS, with a higher prevalence among women
(male to female ratio, 1.4) (12).
A study published by Atroshi et al (12) found that 1 in 5 symptomatic
patients had CTS on clinical examination and electrophysiology
testing. According to a recent study, CTS diagnoses and surgical
decompressions are on the rise in the general population (13). While a CTS diagnosis is
approximately two times more common in women than in men and
increases with age, surgical intervention rates are similar between
men and women (13).
RDs
A total of >200 RDs affect the joints, bones,
muscles and connective tissue, with major categories including
inflammatory conditions, such as RA, and non-inflammatory
conditions, such as OA (14).
Almutairi et al (15)
revealed a global prevalence of RA of 0.46% between 1980 and 2018.
This was approximately two times higher than the estimated RA
prevalence of 0.24% reported by the Global Burden of Disease study
(16,17). The prevalence and incidence of SS
vary depending on diagnostic criteria and study designs used, and
it can be challenging to estimate trends in SS prevalence and
incidence at different geographic and temporal levels (18). The prevalence of hand OA is high,
ranging between 2.0 and 6.2% in adults, but range between 4.7 and
20.4% in elderly individuals (19).
CTS related to RD
Hand OA is a heterogeneous group of disorders and
not a single disease; it can be classified into three distinct
types: i) Erosive; ii) nodal; and iii) first carpometacarpal joint
OA (20). OA is a common
degenerative condition that often co-exists with CTS (21). Shin et al (22) concluded that the prevalence of
basal thumb OA was not higher in a CTS group compared with that in
a non-CTS group, despite several previous studies reporting a
causal link between CTS and basal thumb OA (23,24).
The results of an extensive systematic review and meta-analysis
revealed the risk of CTS to be approximately two times as high
among subjects with RA or OA, compared with individuals who don't
suffer from these RDs (11).
In RA, autoantibodies, persistent synovitis and
widespread inflammation are common (25). Entrapment, vasculitis and drug
toxicity can also cause neuropathy in RA. CTS is the most common
neurological finding in RA (26).
According to a study by Agarwal et al (27), 10.1% of 108 patients with RA had
CTS. In a study by Gray and Gottlieb (28), those patients with RA and flexor
tendinopathies develop CTS more frequently than those with RA
without flexor tendinopathies (47% vs. 13%). In a recent study by
Sakthiswary and Singh (29), there
was no conclusive and convincing evidence of a link between
laboratory or clinical parameters in RA and the involvement of the
median nerve. The study pooled data from eight studies with a
random selection of patients with RA, and it was revealed that
86/1,561 (5.5%) patients with RA had CTS. By contrast, subclinical
CTS exhibited a pooled prevalence of 14.0%. The study concluded
that the prevalence of CTS in RA is no different from the
prevalence in the general population, and no correlation was
observed between the median nerve characteristics and the clinical
parameters of the disease. A recent cross-sectional study showed
that 95.9% of the wrists of patients with RA had CTS (30). There was a high incidence of CTS in
this study compared with that in other studies using neuromuscular
ultrasound (NMUS) to assess CTS in patients with RA; The NMUS can
provide a greater level of accuracy in the diagnosis of CTS in
patients with clinical symptoms and negative NCS results (31). Smerilli et al (32) revealed that CTS was diagnosed in
26.3% patients with RA, and Karadag et al (33) also reported CTS in 18% of patients
with RA (32,33). By contrast, Lee et al
(34) found that the incidence
rate of CTS in patients with RA was similar to the incidence rate
of CTS in the general population, and CTS occurrence was not
correlated with RA duration or activity.
PsA is an inflammatory erosive arthritis that
affects the peripheral joints as RA does (26). PsA occurs in ≤30% of patients with
psoriasis and can have severe debilitating effects on the
peripheral joints, tendon insertions and spine (35). Tezcan et al (36) showed that the CTS frequency was
higher in PsA compared with that in healthy control groups. In
30.76% of patients with PsA, an electrodiagnostic examination (EDX)
detected CTS. In addition, NMUS or magnetic resonance imaging (MRI)
was used to support the diagnosis in patients who could not undergo
EDX (36). As reported in a recent
study by Subaşı et al (26), electrophysiological and
ultrasonographic findings indicated that CTS occurred more
frequently in patients with RA and in patients with PsA compared
with that in controls; CTS was detected in 13.2 and 15.4% of
patients with RA and PsA with hand involvement, respectively, and
in 3.5% of controls (26).
SS belongs to the group of autoimmune RDs. Patients
with SS often experience peripheral neuropathies. Symptoms of
autoimmune disease may precede the onset of neuropathy (37). An estimated 0.2-1% of the
population is affected by SS, and it appears to be as common as RA
(38,39). Based on a retrospective,
comparative study by Hsu et al (10) in 2021, patients with RA and SS have
a high risk of CTS. The rate of CTS in SS patients is high (54%),
and Jaskólska et al hypothesized this is due to inflammation
and synovitis overgrowth (37).
As an autoimmune disorder, scleroderma causes
inflammation, blood vessel injury and organ fibrosis. The condition
can be divided into two groups: SSc and localized scleroderma.
Patients with SSc most frequently suffered from myopathy (51.8%),
trigeminal neuropathy (16.52%), peripheral sensorimotor
polyneuropathy (PNP; 14.25%) and CTS (6.56%) (40).
Behcet's disease (BD) is a multisystemic disorder
with unknown pathogenesis (41).
Recurrent oral and genital ulcers, skin lesions, recurrent uveitis,
articular, vascular and neurological symptoms are the clinical
symptoms of BD. In patients with BD, neurological involvement
occurs in 2.2-49% of cases and is a major cause of morbidity
(42). BD, a chronic, widespread
inflammatory condition, can be associated with peripheral nerve
involvement, therefore CTS can occasionally occur in those with BD
(41). An analysis of 1,750
patients with BD in a study by Lee et al (42) found a 0.8% prevalence of CTS. The
clinical severity of CTS or nerve conduction study (NCS) findings
of CTS was not notably correlated with the disease activity of BD
(42). In total, 45-60% of
patients with musculoskeletal involvement experience arthritis or
arthralgia; it can be observed in the knees, elbows, hands and
ankles, and can mimic RA due to non-erosive, oligoarticular or
monoarticular involvement (43).
A chronic, autoimmune disease known as SLE has
heterogeneous clinical manifestations that may affect every system
and organ in the body (44). In
certain cases, SLE damages the central nervous system and PNS for
numerous years before other symptoms appear. CTS could be the first
sign of SLE (45). In the study by
Sivri et al (46), 23.6% of
patients with SLE had peripheral nerve conduction slowing and
almost half of them were asymptomatic. The study also suggested
that electrophysiological tests should be conducted during early
SLE in asymptomatic patients to detect nervous system involvement.
The proportion of patients with CTS in this study was 44.7%. In
another study, patients with SLE without clinical or
electrophysiological neuropathy showed marked differences with
respect to several NCS parameters of the upper and lower limbs
compared with matched healthy controls of the same age and sex.
According to these findings, SLE may have an early effect on PNS.
However, it may also suggest the beginning of an insidious and
gradual process leading to PNS, the mechanisms of which are unknown
(47). Omdal et al
(48) conducted a retrospective
study on 30 patients with SLE and assessed the neurological
complications caused by the disease. In 23% of the patients, there
was CTS and muscular weakness. A study by Toledano et al
(49) found a 17.7% overall
prevalence of PNS involvement (93 out of 524 patients presented
with manifestation of PNS). PNS involvement was the only
manifestation of SLE in ~50% of the study participants. In the
study, CTS was reported by only 4.2% of patients with SLE, a value
that is within range found for the general population (3.8-4.9%).
According to this study, SLE is not, generally, the direct cause of
CTS (49).
Table I summarizes
the most relevant epidemiological studies on CTS and its
association with RDs that have been published in the last 30
years.
 | Table IMost relevant epidemiological studies
on CTS and RD in the last 30 years. |
Table I
Most relevant epidemiological studies
on CTS and RD in the last 30 years.
| First author,
year | Total patients,
n | Patients with RD,
n | CTS, % | (Refs.) |
|---|
| Karadag et
al, 2012 | 100 | 100 RA | 17.0 | (33) |
| Agarwal et
al, 2008 | 108 | 108 RA | 10.1 | (27) |
| Smerilli et
al, 2021 | 82 | 57 RA | 26.3 | (32) |
| Lee et al,
2015 | 1,060 | 1,060 RA | 3.5 | (34) |
| Sakthiswary and
Singh, 2017 | 1,561 (8
studies) | 1,561 RA | 5.5 | (29) |
| Subaşı et
al, 2021 | 179 | 70 RA/39 PsA | 13.2/15.4 | (26) |
| Mahmoud et
al, 2022 | 74 | 74 RA | 95.9 | (30) |
| Tezcan et
al, 2023 | 67 | 39 PsA | 30.8 | (36) |
| Jaskólska et
al, 2020 | 50 | 50 SS | 54.0 | (37) |
| Amaral et
al, 2013 | 9,506 (182
studies) | 1,628 SSc | 6.56 | (40) |
| Lee et al,
2015 | 1,750 | 1,750 BD | 0.8 | (42) |
| Sivri et al,
1995 | 58 | 38 SLE | 44.7 | (46) |
| Toledano et
al, 2017 | 524 | 524 SLE | 4.2 | (49) |
| Florack et
al, 1992 | 246 | 256 OA | 43.0 | (23) |
3. Diagnosis of CTS in patients with
RDs
Clinical
The symptoms of acroparesthesia include tingling,
numbness, reduced sensation and prickling in the extremities
(fingers and toes). Despite the frequency of the condition, data
regarding a diagnostic approach and its management are limited.
There are several diseases that can be revealed by acroparesthesia
(50). Some patients may
experience acroparesthesia along with rheumatic complaints such as
arthritis, or it may result from mononeuropathies such as CTS
(50).
CTS involves a constellation of signs and symptoms
caused by different pathogenetic mechanisms, all resulting in
median nerve compression. A clinical history and physical
examination are the first steps in diagnosing CTS (51). It is possible to categorize CTS
symptoms and signs into three stages. At the beginning of the
disease, patients with typical CTS often experience paresthesia,
numbness and tingling in 1-3 fingers and the radial half of the
ring finger, especially at night. The second stage involves the
appearance of symptoms during the day. They may be experienced by
patients performing repeated hand or wrist movements or remaining
in the same position for a long period of time. Reduced grip
strength is one of the symptoms experienced by patients. Atrophy or
hypotrophy of the thenar eminence constitutes the third stage. At
this stage, sensory symptoms may not be felt at all (52). Among the numerous tests that can be
used to diagnose CTS clinically, Tinel's sign and Phalen's
maneuvers are two of the most widely used (53). The Boston carpal tunnel
questionnaire is a widely used, self-administered questionnaire
that assesses symptom severity and functional status in patients
with CTS (53).
RA is characterized by symmetric polyarthritis, but
the disease often involves extra-articular structures, and it does
not only affect the joints. PNS may be asymptomatic in its early
stages, or it may present with a variety of symptoms such as pain,
paresthesia and weakness of the muscles. There may be similarities
and overlaps between these symptoms and those associated with
arthritis (27).
Patients with OA also experience considerable pain,
with reduced grip strength and joint mobility, as well as impaired
functional abilities, particularly when grip strength is required
to twist the hands (19,54).
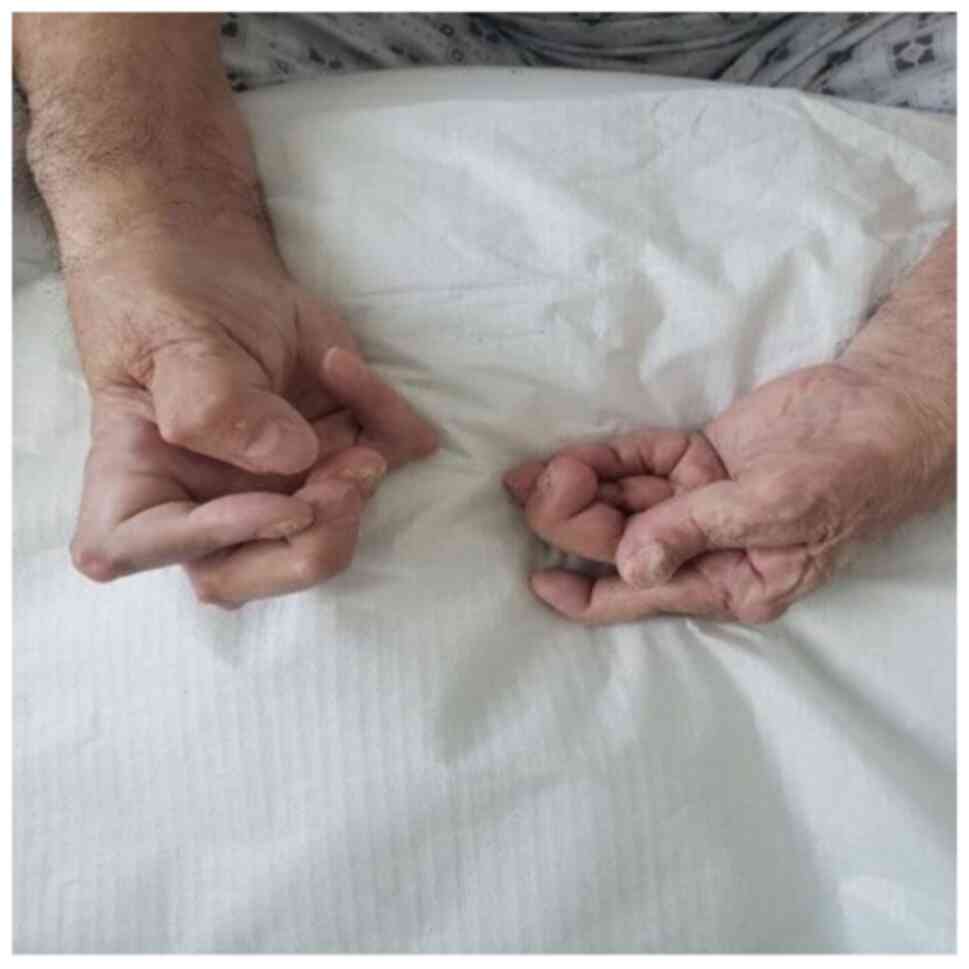
Fig. 1 shows a
patient suffering from specific RA changes and deformities of the
joints, with an exclusive focus on the proximal interphalangeal
joint, localized rheumatoid nodules on the skin and bilateral
thenar atrophy (secondary to severe bilateral CTS).
Pain and paresthesia at night as well as weakness,
loss of dexterity and thenar atrophy, are common complaints in the
patients with RA, and they are presented late for the EDX study, as
CTS in patients with RA is not always obvious (55).
Paraclinical. EDX
Considering the limited diagnostic value of clinical
tests, complementary EDX is recommended when CTS is suspected in a
patient with RD. These tests include NCS and if necessary,
electromyography. Due to high specificity (at least 94%) and
sensitivity (56-85%) percentages, NCS is the gold standard for
diagnosing CTS (52). EDX is used
to diagnose the severity of CTS in RA and PsA based on NCS results,
emphasizing the importance of this test for treatment (26,30).
EDX can be used to determine the location and
severity of nerve compression, monitor its course after therapy and
exclude other causes of median pain, including cervical
radiculopathies, brachial plexopathies, PNPs and mononeuropathies
(1).
It is recommended that EDX should be performed when
quantifying the severity of CTS in individuals aged >70 years
(56). In patients with clinical
symptoms and NCS results, the NMUS provides a more accurate
diagnosis of CTS (56). In a study
of patients with clinical CTS but normal NCS, Aseem et al
(57) reported abnormal NMUS
findings, which were compatible with CTS, such as median nerve
enlargement at the wrist (mean area 16.3 mm2) and an
increased wrist-forearm ratio.
Role of NMUS in studying CTS in
patients with RD
The use of NMUS has grown over the past 20 years,
and several diagnostic laboratories are now routinely performing
the technique. For the diagnosis of CTS, it has been shown to be
sensitive and specific (57).
Through NMUS examinations, the morphological changes
to the nerve can be evaluated, as well as their severity.
Furthermore, the examination can identify some anatomical
conditions or variants that may be contraindicated by minimally
invasive treatments, and it is also useful in evaluating patients
whose surgical outcomes were unfavorable (1). The test can also identify secondary
causes of CTS, such as serous or hyperplastic tenosynovitis, carpal
tunnel lesions, including ganglion cysts, giant cell tumors of the
tendon sheath and vascular malformations, and gout and pseudogout
crystallized deposits (1). A
limited number of studies (26,30,32,36)
have applied NMUS to evaluate the local causes, with a greater
focus on wrist arthritis and tenosynovitis as the main causes of
entrapment neuropathy of the median nerve in RA (30). Also, clinicians can use NMUS to
diagnose CTS in patients with clinical symptoms but negative nerve
conduction test results (31).
The use of NMUS has proven to be beneficial in
identifying the etiopathogenesis of CTS, especially in patients
with OA or RA, for which causes other than synovial inflammation
must be considered (19,30). According to Smerilli et al
(32), patients with RA and CTS
have distinct sonographic patterns compared with patients with
idiopathic CTS. CTS in patients with RA exhibits a characteristic
inflammatory pattern, defined by finger flexor tenosynovitis and/or
radio-carpal joint synovitis. On the other hand, idiopathic CTS is
characterized by a marked swelling of the median nerve (32). According to Yagci et al
(58), the median nerves in
patients with SSc lose their elasticity, while the cross-sectional
area (CSA) is in the normal range. This suggests that the increased
peripheral nerve involvement in SSc is due to increased nerve
stiffness.
MRI in studying CTS in patients with RD.
Recently, MRI of the peripheral nerves has been used as a
complementary diagnostic modality in patients with CTS to assess
nerve and carpal tunnel anatomical parameters (59). With MRI, the flexor retinaculum and
carpal bones could be reliably mapped, thus defining the borders of
the carpal tunnel. Carpal tunnel flexor tendons are distinguished
from median nerves in all cases by their ovoid shape and moderate
signal intensity (60).
MRI is used less in clinical practice than
electrophysiological evaluation in CTS diagnosis. This is because
it is very costly, time-consuming and not readily available
(52). When clinical and EDX
findings are inconsistent, MRI can be helpful in identifying
patients who could benefit from surgery. Also, it may be an
important tool for assessing the persistence and recurrence of CTS
(52).
In RD, MRI plays a marked role. As part of OA, PsA
or RA, synovitis plays a key role, particularly in the inflammatory
phenotype (61,62). MRI findings for patients with PsA
usually include periostitis and synovitis of the proximal
interphalangeal joints, while MRI findings for patients with RA
typically include synovitis with erosions of the wrist, and the
midcarpal, carpometacarpal and metacarpophalangeal joints (61). In patients with RA, MRI can also
detect abnormalities such as bifid median nerves, persistent median
arteries and accessory muscle bundles that contribute to CTS
(34).
4. Pathogenesis of CTS and RD
The development of CTS can be caused by a variety of
factors, including thickening of the tendon or flexor retinaculum,
synovitis, the accumulation of fluid and alterations of the
subsynovial connective tissue (63). CTS results from mechanical trauma
or increased pressure (carpal tunnel shrinkage or increased median
nerve size), which subsequently leads to ischemic injury to the
median nerve (64).
All the aforementioned RDs have a complex autoimmune
substrate that has not been fully elucidated. The rheumatoid wrist
experiences synovial expansion, joint erosions and ligamentous
laxity. This results in a reduction in the size of the carpal
tunnel and an increase carpal tunnel pressure. Consequently, median
nerve and vessel compression in the perineurium result in impaired
axonal transport and median nerve ischemia. Another plausible cause
of CTS in RDs is drug toxicity, vasculitis and amyloidosis
(29).
A combination of synovial inflammation and local
causes such as persistent median artery and accessory muscle bundle
may contribute to the etiology of CTS in patients with RA (30). With time, inflammation of the
joints results in their destruction, with loss of cartilage and
bone erosions, which changes the conformation of the carpal tunnel
(65). The carpal tunnel is formed
from the carpal bones and the transverse carpal ligament. Within
the tunnel, there are nine flexor tendons, as well as the median
nerve (52).
Filippucci et al (66) found that ~50% of the 90 recruited
patients with RA had at least one inflamed tendon, and an
equivalent percentage of those had at least one damaged tendon. A
variety of pathological conditions can lead to neurotendinous
abnormalities, including edema of the tendon, invasion of the
synovial tissue, tear of the tendon and scar formation Filippucci
et al (66) found a high
rate of both inflammation and tears in the flexor tendons of
fingers II, III and IV, as well as in the extensor carpi ulnaris
tendon in 90 patients with RA.
RA for a long period can result in histopathological
changes to the wrist tendon, including synovial proliferation and
tendon damage (66). In RA,
synovial hyperplasia and the formation of invasive synovial tissue,
called the pannus, occur. CTS compression can be caused by an
infiltrating pannus narrowing the space in the tunnel (55,67).
Demyelination, on the other hand, is a notable reaction to nerve
injury in the median nerve. As a result of compression,
demyelination spreads to the intermodal segment, where the axons
remain intact. In continuous compression, there is an interruption
of the blood flow to the endoneurial capillary system, resulting in
changes in the blood-nerve barrier and endoneurial edema. The
result is ischemia, venous congestion and local metabolic changes
(3).
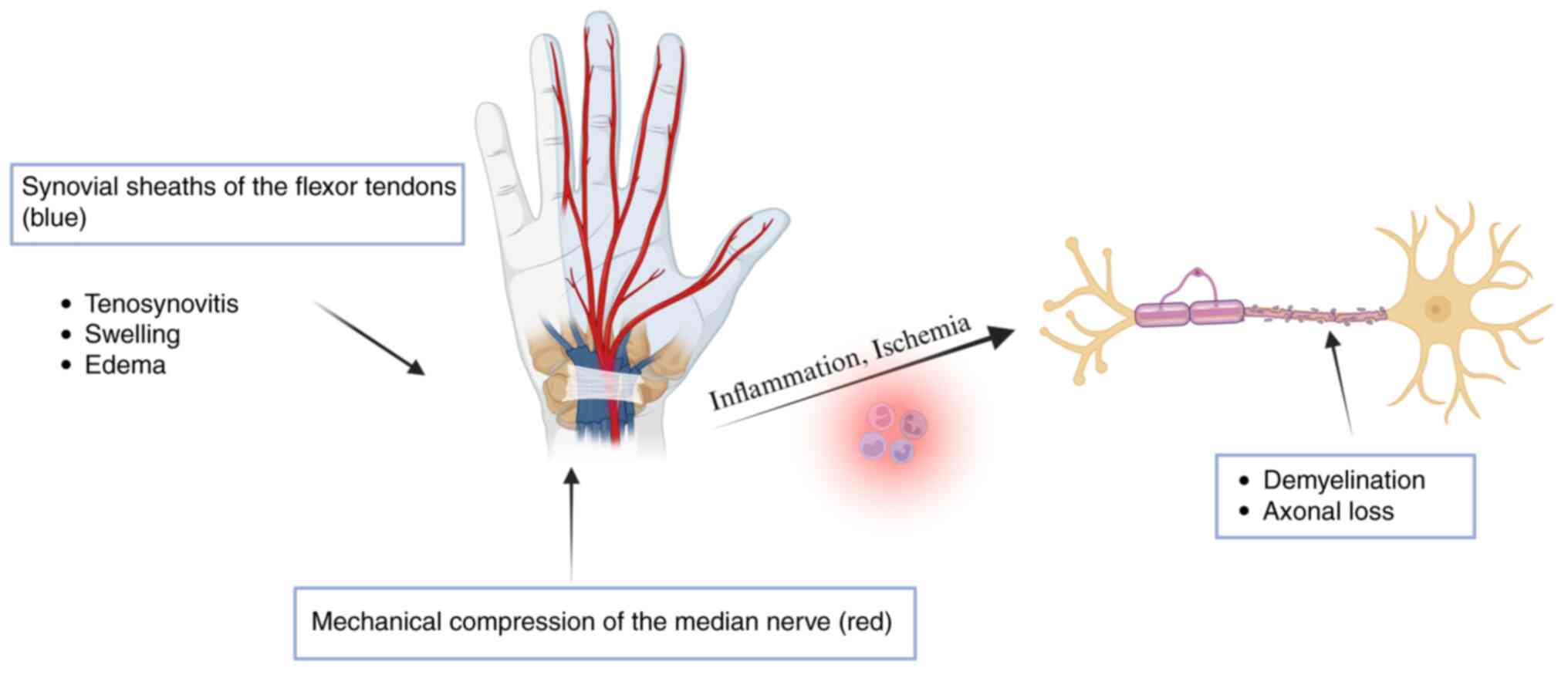
In Fig. 2, the
pathogenesis of CTS related to RA is briefly illustrated. Symptoms
of CTS appear to resolve quickly after surgery for carpal tunnel
release, indicating ischemic injury (64).
Studies conducted on RA and PsA that focused on
vascular changes showed that synovial membrane vascularity and
modification of the vascular morphology in patients with PsA were
markedly increased compared with those in patients with RA
(68,69). The symptoms of CTS are more common
in patients with psoriatic arthritis due to increased inflammation
and angiogenesis in the synovium, which causes narrowing of the
carpal tunnel and compression of the median nerve in the wrist area
(68,69).
Several fibrotic disorders are associated with
connective tissue growth factor (CTGF) expression, according to
Pierce et al (70). It has
also been demonstrated that tenosynovial samples from patients with
CTS and certain associated comorbidities, specifically SLE and RA,
show marked increases in CTGF levels compared with those found in
patients with idiopathic CTS (35).
As a chronic inflammatory autoimmune disease, SS is
characterized by infiltrating lymphocytic cells in the exocrine
glands, such as the salivary and lacrimal glands. Primary SS is the
result of an independent condition, while secondary SS occurs in
other autoimmune diseases, such as RA, SSc and SLE (37).
Patients with SSc may experience involvement of the
nerve connective tissue, suggesting multisystem involvement
(58). Patients with SSc
experience an increase in the stiffness of the median nerve. There
are still several questions regarding the involvement of the median
nerve in SSc and how it differs between patients with CTS and
healthy individuals (58).
CTS can also develop in patients with BD secondary
to inflammation in the connective tissues, tendons and vessels, as
well as nerve involvement in BD itself (41,42).
5. Discussion
According to numerous published studies (23,26,27,29,30,32-34,36,37,40,42,46,49),
CTS is more common in patients suffering from RD compared to those
without these conditions In addition, other risk factors or
diseases may contribute to CTS, as some researchers have discovered
that the incidence rate of CTS in patients with RA is similar to
that in the general population (29).
Several of the studies included in the present
review have limitations due to their retrospective nature (34). As a result, some of the data may
have been underestimated. Certain studies also included a small
patient group, which was partially justified by the low incidence
of some of the diseases in the general population, such as BD or
SSc (41,42).
The incidence of RA is closely associated with that
of CTS (67). Patients with PsA
have a higher risk of CTS compared with healthy individuals, as
evidenced by increased median nerve CSA on NMUS and MRI (36). CTS can occur due to BD inflammation
and can also be the presenting symptom of nerve involvement in BD.
Therefore, there should be a high level of suspicion for CTS in
patients with BD and vice versa, although the exact association is
uncertain (42).
EDX evaluation is essential in the diagnostic
process for first determining whether there is a lesion of the
median nerve, and then ruling out other pathologies causing similar
symptoms, and assessing the severity of the lesion and treatment
accordingly. The diagnosis of CTS in RD mostly depends on
electrophysiological assessment. NMUS has become a crucial
complementary test to electrophysiological examinations in CTS
detection. When used together, EDX and NMUS are more informative
than when used separately (56).
MRI and NMUS are the two primary imaging modalities used to assess
synovitis in OA, PsA and RA (61,71).
As concluded by Agarwal et al (27), neuropathy in RA is common and
mostly subclinical. To evaluate patients with RA accurately, a
detailed electrophysiological examination should be part of the
evaluation process.
CTS and other neurological manifestations such as
PNP may be the first symptom of RD, and patients are initially
referred for neurological evaluation. Considering the marked
association between CTS and RD, some studies recommend that
rheumatologists refer patients with RD for neurological assessment,
and neurologists who diagnose CTS should refer patients for
rheumatological examination.
6. Conclusions
There are more studies that examine the associations
between CTS and PsA, RA and OA than those that examine the
associations between CTS and SLE, BD, SS and SSc. In part, this can
be explained by the lower incidence of the latter diseases, but
also by the prevalence of CTS associated with them. The pathogenic
mechanisms of RA, PsA and OA are similar, which may explain why CTS
is associated more often with these diseases. According to previous
research, SS, BD and SLE are not directly associated with CTS.
However, CTS should be investigated in these patients due to their
systemic nature and impact on other connective tissue
disorders.
A patient with RD and symptoms suggestive of CTS
will undergo similar paraclinical investigations to a patient
without such conditions. The diagnosis of CTS is based on clinical
symptoms, physical examination and electrophysiological findings.
EDX is helpful in identifying CTS frequency and the correlation
with disease activity in patients with RD. NMUS is useful for
identifying local causes, such as synovial inflammation and
anomalous variations contributing to CTS; however, EDX remains
essential for comprehensive evaluation and management. Due to the
ability of ultrasound to detect inflammatory patterns and nerve
swelling, it is essential for assessing CTS in RD, particularly in
OA, RA and PsA. The use of MRI in the diagnosis and assessment of
CTS in patients with RD is generally considered to be a valuable
tool, as it provides detailed insights into the inflammatory and
anatomical factors contributing to this condition.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
LB, GAV, CDA and DIC were responsible for study
conceptualization. LB and DIC were involved in validation of the
study. LB and GAV prepared the original draft. LB, GAV, IRP and CDA
reviewed and edited the manuscript. DMT supervised the study. All
authors have read and agreed to the final version of the
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the use of the image in Fig. 1.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gervasio A, Stelitano C, Bollani P,
Giardini A, Vanzetti E and Ferrari M: Carpal tunnel sonography. J
Ultrasound. 23:337–347. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Osiak K, Elnazir P, Walocha JA and
Pasternak A: Carpal tunnel syndrome: State-of-the-art review. Folia
Morphol (Warsz). 81:851–862. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sevy JO, Sina RE and Varacallo M: Carpal
tunnel syndrome. In: StatPearls [Internet]. StatPearls Publishing,
Treasure Island, FL, 2024.
|
|
4
|
Al Maini M, Adelowo F, Al Saleh J, Al
Weshahi Y, Burmester GR, Cutolo M, Flood J, March L,
McDonald-Blumer H, Pile K, et al: The global challenges and
opportunities in the practice of rheumatology: white paper by the
world forum on rheumatic and musculoskeletal diseases. Clin
Rheumatol. 34:819–829. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Woolf AD and Gabriel S: Overcoming
challenges in order to improve the management of rheumatic and
musculoskeletal diseases across the globe. Clin Rheumatol.
34:815–817. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chopra A: The WHO ILAR COPCORD Latin
America: Consistent with the world and setting a new perspective. J
Clin Rheumatol. 18:167–169. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Peláez-Ballestas I, Pons-Estel BA and
Burgos-Vargas R: Epidemiology of rheumatic diseases in indigenous
populations in Latin-Americans. Clin Rheumatol. 35 (Suppl 1):S1–S3.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Didier K, Bolko L, Giusti D, Toquet S,
Robbins A, Antonicelli F and Servettaz A: Autoantibodies Associated
with connective tissue diseases: What meaning for clinicians? Front
Immunol. 9(541)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Poshattiwar RS, Acharya S, Shukla S and
Kumar S: Neurological manifestations of connective tissue
disorders. Cureus. 15(e47108)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hsu PC, Chiu JW, Yang YC and Jeng MJ:
Carpal tunnel syndrome in autoimmune rheumatic diseases and
inflammatory bowel diseases: Retrospective population cohort study.
Am J Phys Med Rehabil. 100:760–765. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shiri R: Arthritis as a risk factor for
carpal tunnel syndrome: A meta-analysis. Scand J Rheumatol.
45:339–346. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Atroshi I, Gummesson C, Johnsson R,
Ornstein E, Ranstam J and Rosén I: Prevalence of carpal tunnel
syndrome in a general population. JAMA. 282:153–158.
1999.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tadjerbashi K, Åkesson A and Atroshi I:
Incidence of referred carpal tunnel syndrome and carpal tunnel
release surgery in the general population: Increase over time and
regional variations. J Orthop Surg (Hong Kong).
27(2309499019825572)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wright V: Introduction to rheumatic
disease. J R Soc Health. 110:153–155. 1990.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Almutairi K, Nossent J, Preen D, Keen H
and Inderjeeth C: The global prevalence of rheumatoid arthritis: A
meta-analysis based on a systematic review. Rheumatol Int.
41:863–877. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Safiri S, Kolahi AA, Hoy D, Smith E,
Bettampadi D, Mansournia MA, Almasi-Hashiani A, Ashrafi-Asgarabad
A, Moradi-Lakeh M, Qorbani M, et al: Global, regional and national
burden of rheumatoid arthritis 1990-2017: A systematic analysis of
the global burden of disease study 2017. Ann Rheum Dis.
78:1463–1471. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cross M, Smith E, Hoy D, Carmona L, Wolfe
F, Vos T, Williams B, Gabriel S, Lassere M, Johns N, et al: The
global burden of rheumatoid arthritis: Estimates from the global
burden of disease 2010 study. Ann Rheum Dis. 73:1316–1322.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Beydon M, McCoy S, Nguyen Y, Sumida T,
Mariette X and Seror R: Epidemiology of Sjögren syndrome. Nat Rev
Rheumatol. 20:158–169. 2024.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kloppenburg M and Kwok WY: . Hand
osteoarthritis-a heterogeneous disorder. Nat Rev Rheumatol.
8:22–31. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Favero M, Belluzzi E, Ortolan A, Lorenzin
M, Oliviero F, Doria A, Scanzello CR and Ramonda R: Erosive hand
osteoarthritis: Latest findings and outlook. Nat Rev Rheumatol.
18:171–183. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
George RE, Seitz AJ, Moura SP, Mclaughlin
MT, Crawford SB, Attaluri PK, Edalatpour A and Michelotti BF:
Osteoarthritis increases the frequency and duration of
postoperative hand clinic visits after carpal tunnel release. Plast
Reconstr Surg Glob Open. 12(e5631)2024.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shin CH, Paik NJ, Lim JY, Kim TK, Kim KW,
Lee JJ, Park JH, Baek GH and Gong HS: Carpal tunnel syndrome and
radiographically evident basal joint arthritis of the thumb in
elderly Koreans. J Bone Joint Surg Am. 94:e1201–e1206.
2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Florack TM, Miller RJ, Pellegrini VD,
Burton RI and Dunn MG: The prevalence of carpal tunnel syndrome in
patients with basal joint arthritis of the thumb. J Hand Surg Am.
17:624–630. 1992.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kim YH, Han EY, Kim J, Seo KB, Jeon YT and
Im SH: Associations between hand function and electrophysiological
measurements in hand osteoarthritis patients of different ages with
or without carpal tunnel syndrome. Sci Rep.
10(19278)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Joaquim AF and Appenzeller S:
Neuropsychiatric manifestations in rheumatoid arthritis. Autoimmun
Rev. 14:1116–1122. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Subaşı KP, Güler T, Yurdakul FG, Ataman Ş
and Bodur H: Carpal tunnel syndrome in patients with rheumatoid
arthritis and psoriatic arthritis: An electrophysiological and
ultrasonographic study. Rheumatol Int. 41:361–368. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Agarwal V, Singh R, Wiclaf Chauhan S,
Tahlan A, Ahuja CK, Goel D and Pal L: A clinical,
electrophysiological, and pathological study of neuropathy in
rheumatoid arthritis. Clin Rheumatol. 27:841–844. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gray RG and Gottlieb NL: Hand flexor
tenosynovitis in rheumatoid arthritis. Prevalence, distribution,
and associated rheumatic features. Arthritis Rheum. 20:1003–1008.
1977.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sakthiswary R and Singh R: Has the median
nerve involvement in rheumatoid arthritis been overemphasized? Rev
Bras Reumatol Engl Ed. 57:122–128. 2017.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
30
|
Mahmoud W, Mansour M, El-Naby H and Awad
AA: Carpal tunnel syndrome in rheumatoid arthritis patients: The
role of combined ultrasonographic and electrophysiological
assessment. Egypt Rheumatol Reh. 49(62)2022.
|
|
31
|
Aktürk S, Büyükavcı R and Ersoy Y: Median
nerve ultrasound in carpal tunnel syndrome with normal
electrodiagnostic tests. Acta Neurol Belg. 120:43–47.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Smerilli G, Di Matteo A, Cipolletta E,
Carloni S, Incorvaia A, Di Carlo M, Grassi W and Filippuci E:
Ultrasound assessment of carpal tunnel in rheumatoid arthritis and
idiopathic carpal tunnel syndrome. Clin Rheumatol. 40:1085–1092.
2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Karadag O, Kalyoncu U, Akdogan A, Karadag
YS, Bilgen SA, Ozbakır S, Filippuci E, Kiraz S, Ertneli I, Grassi W
and Calgüneri M: Sonographic assessment of carpal tunnel syndrome
in rheumatoid arthritis: Prevalence and correlation with disease
activity. Rheumatol Int. 32:2313–2319. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lee KH, Lee CH, Lee BG, Park JS and Choi
WS: The incidence of carpal tunnel syndrome in patients with
rheumatoid arthritis. Int J Rheum Dis. 18:52–57. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ritchlin CT, Colbert RA and Gladman DD:
Psoriatic arthritis. N Engl J Med. 376:957–970. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tezcan EA, Levendoglu F, Durmaz MS, Kara
H, Batur EB, Gezer IA and Korez MK: Carpal tunnel syndrome in
patients with psoriatic arthritis: Ultrasonography and magnetic
resonance imaging findings. J Rheum Dis. 30:36–44. 2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jaskólska M, Chylińska M, Masiak A,
Siemiński M, Ziętkiewicz M, Czuszyńska Z, Somoleńska Z and
Zdrojewski Z: Neuro-Sjögren: Uncommon or underestimated problem?
Brain Behav. 10(e01665)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Westhoff G and Zink A: Epidemiology of
primary Sjörgren's syndrome. Z Rheumatol. 69:41–49. 2010.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
39
|
Binard A, Devauchelle-Pensec V, Fautrel B,
Jousse S, Youinou P and Saraux A: Epidemiology of Sjögren's
syndrome: Where are we now? Clin Exp Rheumatol. 25:1–4.
2007.PubMed/NCBI
|
|
40
|
Amaral TN, Peres FA, Lapa AT, Marques-Neto
JF and Appenzeller S: Neurologic involvement in scleroderma: A
systematic review. Semin Arthritis Rheum. 43:335–347.
2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Birol A, Ulkatan S, Koçak M and Erkek E:
Peripheral neuropathy in Behçet's disease. J Dermatol. 31:455–459.
2004.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lee J, Cho S, Kim DY, Zheng Z, Park H and
Bang D: Carpal tunnel syndrome in Behçet's disease. Yonsei Med J.
56:1015–1020. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Bulur I and Onder M: Behçet disease: New
aspects. Clin Dermatol. 35:421–434. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sciascia S, Roccatello D, Radin M, Parodis
I, Yazdany J, Pons-Estel G and Mosca M: Differentiating between
UCTD and early-stage SLE: From definitions to clinical approach.
Nat Rev Rheumatol. 18:19–21. 2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sidiq M, Kirsner AB and Sheon RP: Carpal
tunnel syndrome. First manifestation of systemic lupus
erythematosus. JAMA. 222:1416–1417. 1972.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Sivri A, Hasçelik Z, Celiker R and Başgöze
O: Early detection of neurological involvement in systemic lupus
erythematosus patients. Electromyogr Clin Neurophysiol. 35:195–199.
1995.PubMed/NCBI
|
|
47
|
Fong SY, Raja J, Wong KT and Goh KJ:
Systemic lupus erythematosus may have an early effect on peripheral
nerve function in patients without clinical or electrophysiological
neuropathy: Comparison with age- and gender-matched controls.
Rheumatol Int. 41:355–360. 2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Omdal R, Mellgren SI and Husby G: Clinical
neuropsychiatric and neuromuscular manifestations in systemic lupus
erythematosus. Scand J Rheumatol. 17:113–117. 1988.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Toledano P, Orueta R, Rodríguez-Pintó I,
Valls-Solé J, Cervera R and Espinosa G: Peripheral nervous system
involvement in systemic lupus erythematosus: Prevalence, clinical
and immunological characteristics, treatment and outcome of a large
cohort from a single centre. Autoimmun Rev. 16:750–755.
2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Slouma M, Ben Dhia S, Cheour E and
Gharsallah I: Acroparesthesias: An overview. Curr Rheumatol Rev.
20:115–126. 2024.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Padua L, Coraci D, Erra C, Pazzaglia C,
Paolasso I, Loreti C, Caliandro P and Hobson-Webb LD: Carpal tunnel
syndrome: Clinical features, diagnosis, and management. Lancet
Neurol. 15:1273–1284. 2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Ghasemi-Rad M, Nosair E, Vegh A, Mohammadi
A, Akkad A, Lesha E, Mohammadi MH, Sayed D, Davarian A,
Maleki-Miyandoab T and Hasan T: A handy review of carpal tunnel
syndrome: From anatomy to diagnosis and treatment. World J Radiol.
6:284–300. 2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Levine DW, Simmons BP, Koris MJ, Daltroy
LH, Hohl GG, Fossel AH and Katz JN: A self-administered
questionnaire for the assessment of severity of symptoms and
functional status in carpal tunnel syndrome. J Bone Joint Surg Am.
75:1585–1592. 1993.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Kjeken I, Dagfinrud H,
Slatkowsky-Christensen B, Mowinckel P, Uhlig T, Kvien TK and Finset
A: Activity limitations and participation restrictions in women
with hand osteoarthritis: Patients' descriptions and associations
between dimensions of functioning. Ann Rheum Dis. 64:1633–1638.
2005.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Feldon P and Terrono AL: Carpal tunnel
syndrome in rheumatoid arthritis. Tech Orthop. 21:42–47. 2006.
|
|
56
|
Pelosi L, Arányi Z, Beekman R, Bland J,
Coraci D, Hobson-Webb LD, Padua L, Pondar S, Simon N, Alfen N, et
al: Expert consensus on the combined investigation of carpal tunnel
syndrome with electrodiagnostic tests and neuromuscular ultrasound.
Clin Neurophysiol. 135:107–116. 2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Aseem F, Williams JW, Walker FO and
Cartwright MS: Neuromuscular ultrasound in patients with carpal
tunnel syndrome and normal nerve conduction studies. Muscle Nerve.
55:913–915. 2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Yagci I, Kenis-Coskun O, Ozsoy T, Ozen G
and Direskeneli H: Increased stiffness of median nerve in systemic
sclerosis. BMC Musculoskelet Disord. 18(434)2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Daliri M, Ebrahimnejad M, Najafi S,
Aminzadeh B, Emadzadeh M, Moradi E and Moradi A: Magnetic resonance
imaging and sonographic features before and after surgery in carpal
tunnel syndrome: Association with clinical findings. Clin Orthop
Sur. 14:603–612. 2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Kumari A, Singh S, Garg A, Prakash A and
Sural S: Tingling hand: Magnetic resonance imaging of median nerve
pathologies within the carpal tunnel. Pol J Radiol. 84:e484–e490.
2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Shiraishi M, Fukuda T, Igarashi T,
Tokashiki T, Kayama R and Ojiri H: Differentiating rheumatoid and
psoriatic arthritis of the hand: Multimodality imaging
characteristics. Radiographics. 40:1339–1354. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Hayashi D, Roemer FW, Jarraya M and
Guermazi A: Update on recent developments in imaging of
inflammation in osteoarthritis: A narrative review. Skeletal
Radiol. 52:2057–2067. 2023.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Uchiyama S, Itsubo T, Nakamura K, Kato H,
Yasutomi T and Momose T: Current concepts of carpal tunnel
syndrome: Pathophysiology, treatment, and evaluation. J Orthop Sci.
15:1–13. 2010.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Genova A, Dix O, Saefan A, Thakur M and
Hassan A: Carpal tunnel syndrome: A review of literature. Cureus.
12:316–320. 2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
López-Ferrer A, Laiz A and Puig L:
Psoriatic arthritis. Med Clin (Barc). 159:40–46. 2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Filippucci E, Gabba A, Di Geso L,
Girolimetti R, Salaffi F and Grassi W: Hand tendon involvement in
rheumatoid arthritis: An ultrasound study. Semin Arthritis Rheum.
41:752–760. 2012.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Guan W, Lao J, Gu Y, Zhao X, Rui J and Gao
K: Case-control study on individual risk factors of carpal tunnel
syndrome. Exp Ther Med. 15:2761–2766. 2018.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Fromm S, Cunningham CC, Dunne MR, Veale
DJ, Fearon U and Wade SM: Enhanced angiogenic function in response
to fibroblasts from psoriatic arthritis synovium compared to
rheumatoid arthritis. Arthritis Res Ther. 21(297)2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Veale D, Yanni G, Rogers S, Barnes L,
Bresnihan B and Fitzgerald O: Reduced synovial membrane macrophage
numbers, ELAM-1 expression, and lining layer hyperplasia in
psoriatic arthritis as compared with rheumatoid arthritis.
Arthritis Rheum. 36:893–900. 1993.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Pierce CW, Tucci MA, Lindley S, Freeland A
and Benghuzzi HA: Connective tissue growth factor (CTGF) expression
in the tenosynovium of patients with carpal tunnel syndrome-biomed
2009. Biomed Sci Instrum. 45:30–35. 2009.PubMed/NCBI
|
|
71
|
Guermazi A, Roemer FW, Haugen IK, Crema MD
and Hayashi D: MRI-based semiquantitative scoring of joint
pathology in osteoarthritis. Nat Rev Rheumatol. 9:236–251.
2013.PubMed/NCBI View Article : Google Scholar
|