Introduction
Pulmonary arterial hypertension (PAH) is a
refractory cardiovascular disease accompanied by varying degrees of
inflammation, which currently lacks a curative treatment method
(1). The major pathological
features of PAH are pulmonary vascular remodeling and progressive
obstruction, leading to increased pulmonary artery pressure, and
ultimately severe heart failure and death (2). Previous research has shown that the
10-year survival rate of patients is only 45-66% (3). Although there are various drugs that
can alleviate the clinical symptoms of patients with PAH to a
certain extent, the mortality rate remains high, and death from
failure of the right ventricle occurs in ~40% of patients within 5
years of diagnosis (4,5). However, the specific pathogenesis of
PAH has not been fully elucidated (6,7).
Therefore, there is an urgent need to explore the pathogenesis of
PAH and identify effective targets for its diagnosis and
treatment.
Pulmonary vascular remodeling is a key
pathophysiological mechanism in PAH progression and is often
accompanied by pathological changes such as injury and migration of
vascular endothelial cells, thickening of the pulmonary artery
media [mainly pulmonary artery smooth muscle cells (PASMCs)], and
pathological deposition of the extracellular matrix (8). Pulmonary arterioles (diameter,
<100 µm) are key remodeling vessels during PAH (9). Importantly, PASMC proliferation and
hypertrophy are considered significant pathological characteristics
of pulmonary vascular remodeling in PAH (10,11).
An increasing number of studies have revealed that senescence,
apoptosis and pyroptosis of PASMCs may participate in PAH
progression, and are accompanied by changes in various cytokines
and inflammatory factors (such as IL-1β and IL-6) that regulate the
proliferation and phenotypic transformation of PASMCs (12-14).
However, the mechanisms that regulate inflammatory factors to
promote PASMC proliferation in PAH remain unclear. Therefore,
identifying a novel pathophysiological mechanism that inhibits
PASMC proliferation is important for the prevention and treatment
of PAH.
Pyroptosis is a specialized form of programmed cell
death accompanied by a pro-inflammatory response (15). Nod-like receptor protein 3 (NLRP3)
is a typical pyroptotic protein. The NLRP3 inflammasome, a
cytosolic protein complex for early inflammatory responses, is
composed of apoptosis-associated speck-like protein containing a
caspase recruitment domain, NLRP3 and Caspase-1(16). After NLRP3 is activated,
pro-caspase-1 is cleaved into activated Caspase-1(17). After the activation of cysteine
protease by NLRP3, the activation and self-digestion of Caspase-1
leads to the cleavage of the substrate gasdermin D (GSDMD). GSDMD
initiates the formation of membrane pores. Several pores are formed
on the membrane, allowing inflammatory factors (such as IL-1β and
IL-18) to be cleaved into mature forms (18). Subsequently, pyroptotic cells
swell, and the cell membrane ruptures, further promoting
inflammatory factor release, activating a strong inflammatory
stress response, and eventually accelerating the development and
progression of PAH (19). Previous
studies have demonstrated that inhibiting pyroptosis in vascular
smooth muscle cells (VSMCs) is crucial for preventing the
occurrence and progression of vascular diseases. Xu et al
(20) confirmed that VSMC pyroptosis accelerates atherosclerotic
plaque formation and aggravates plaque instability (20). In addition, when a high-fat diet is
used in vivo in an animal study or the VSMCs are treated
with oxidized low-density lipoprotein in vitro, VSMC
pyroptosis promotes abnormal proliferation and migration of VSMCs,
leading to the progression of arteriosclerosis (21,22).
Studies have confirmed that VSMC pyroptosis is involved in
pathological processes such as acute lung injury, atherosclerosis
and vascular remodeling (23-25).
These studies suggest that VSMC pyroptosis plays a significant role
in cardiovascular disease. However, the pivotal role and mechanism
of VSMC pyroptosis in rats with monocrotaline (MCT)-induced PAH
remains unclear. MCT is a macrocyclic pyrrolizidine alkaloid that
causes a pulmonary vascular syndrome in rats characterized by
pulmonary hypertension (26). The
GSDM protein family oligomerizes to form large pores in the
membrane that drive swelling and membrane rupture (27), with various researchers defining
pyroptosis as a form of GSDMD-mediated programmed cell death
(28,29). In our preliminary experiments, it
was found that GSDMD protein expression was significantly
upregulated in vivo. Hence, it was hypothesized that PASMC
pyroptosis may be a novel pathophysiological mechanism for
promoting PAH, and its molecular mechanisms may be related to the
formation of GSDMD membrane pores and the secretion of interleukins
in MCT-induced PAH rats.
In the present study, PAH rats were treated with
MCT. The aim of this study was to demonstrate the role of
pyroptosis in pulmonary hypertension. With regard to the mechanism,
this study would show whether pyroptotic PASMCs promote PASMC
proliferation by secreting IL-1β and IL-18 in MCT-induced PAH. The
present study provides new insights into the pathophysiological
mechanisms of PAH, as well as a new direction for developing a
targeted and novel therapeutic option against PAH.
Materials and methods
Animals
Male Sprague-Dawley (SD) rats, aged 6-8 weeks,
weighing 185-205 g, were purchased from Hunan Silaike Jingda
Laboratory Animal Co., Ltd. and housed at the Laboratory Animal
Center of Hengyang Medical School (University of South China;
Hengyang, China). Rats were labelled and allowed to drink freely.
The ambient temperature was set to 18-22˚C and the humidity was
50-60%, with a 12-h light and 12-h darkness cycle. All experiments
were performed in strict accordance with the ARRIVE guidelines and
approved by The Animal Ethics Committee of the University of South
China (Hengyang, China; approval no. 446).
Animal experiments
A total of 30 male SD rats were acclimatized for one
week. After which, the rats were randomly divided into three groups
(n=10/group) including: i) Control group; ii) vehicle group; and
iii) MCT group. To establish the PAH rat models, SD rats in the MCT
group were injected intraperitoneally with MCT (cat. no. C2401;
Millipore Sigma) at a dose of 60 mg/kg. The rats in the vehicle
group were intraperitoneally injected with the same volume of the
vehicle (10% DMSO + 40% PEG400 + 50% saline), while the rats in the
control group were not treated.
A total of 40 male SD rats were acclimatized for one
week. The second batch of rats were randomly divided into four
groups (n=10/group) including: i) Control group; ii) vehicle group;
iii) MCT group; and iv) disulfiram-treated PAH group (MCT +
disulfiram group). The rats in the MCT + disulfiram group were
treated with disulfiram (cat. no. HY-B0240; MedChem Express)
injected intraperitoneally at a dose of 20 mg/kg, 24 h before MCT
treatment and 5, 10, 15 and 20 days after MCT treatment (the same
dose of MCT was used for these rats). Simultaneously, vehicle rats
were intraperitoneally injected with the same volume of the
vehicle. The MCT-induced and the control rats were treated in the
same manner as aforementioned.
The rats were weighed separately over seven days and
fed a normal diet. Rats were monitored closely for any severe
impairment in physiological or neurological function and were
euthanized when these signs became apparent. Each rat was
anesthetized via intraperitoneal injection with pentobarbital (40
mg/kg), and the right ventricular systolic pressure (RVSP) and mean
pulmonary arterial pressure (mPAP) were measured through the
jugular vein using a transvenous catheter, as previously described
(30). Subsequently, a total of
1.5 ml blood was collected from the right ventricle and placed in a
tube to determine the serum level of IL-1β and IL-18, and the rats
were sacrificed by exsanguination via the jugular vein and carotid
artery. Animal death was confirmed by the cessation of heart rate
and a lack of breathing. The right ventricle (RV), left ventricle
(LV) and interventricular septum (S) were isolated and weighed. The
tibial length of the right leg was also monitored. The RV/(LV + S)
and RV/tibial length ratios were assessed as they are considered
essential for evaluating right ventricular hypertrophy. Finally,
the pulmonary tissues were collected to detect differential protein
expression.
Hematoxylin-eosin (HE) staining
Lung tissues were used to assess pathological
alterations in pulmonary artery remodeling through HE staining.
Pulmonary arteries were evaluated by an experienced pathologist and
the isolated rat lung tissues were stained with HE to assess the
degree of pulmonary vascular remodeling. The samples were fixed in
10% formalin for 12 h at 4˚C, and then embedded in paraffin for
subsequent sectioning. The 5-µm specimens were first placed in
distilled water and then in an aqueous solution of hematoxylin for
staining for ~10 min at room temperature. After which, the slices
were placed into ammonia and acid water for several sec, and then
rinsed in running water for 1 h. The sections were placed in
distilled water for several sec, and after which, dehydrated in
alcohol at concentrations of 90 and 70% for 10 min each.
Subsequently, the sections were stained with eosin staining
solution for 2-3 min at room temperature. After staining, the
samples were dehydrated with 100% alcohol and placed in xylene. The
sections were sealed and placed in an incubator for drying. Images
were obtained and collected using a ZEISS LSM 880 Confocal
Microscope (Carl Zeiss AG). Image J (version 1.43; National
Institutes of Health) was used to analyze the images. The ratio of
the intima-media thickness to the outer diameter was calculated as
a percentage of the intima-media thickness (30-32).
The areaext (AE) represented the areas surrounded by external
elastic plates, while the areaint (AI) represented the areas
surrounded by internal elastic plates. The pulmonary artery wall
thickness (WT) was calculated as follows: (AE-AI)/AE x100.
Immunofluorescence
Paraffin sections of lung tissue from rats were
obtained from the embedded samples. The 5-µm paraffin sections were
heated at 60˚C oven for 1 h, dewaxed twice in xylene solutions for
15 min each and rehydrated in a descending alcohol series. Antigen
retrieval was performed with sodium citrate buffer (cat. no. C1031;
Beijing Solarbio Science & Technology Co., Ltd.) at 100˚C for
10 min. After which, the 5-µm sections were incubated with 0.5%
Triton X-100 and 5% BSA (cat. no. A8850-5; Beijing Solarbio Science
& Technology Co., Ltd.) for 30 min at room temperature. The
samples were incubated with the following primary antibodies at 4˚C
overnight: Ki-67 (cat. no. ab92742; 1:200; Abcam) and α-SMA (cat.
no. BM0002; 1:100; Boster Biological Technology). After which, the
samples were incubated the following secondary antibodies:
CoraLite488-conjugated Goat Anti-Mouse IgG (H + L; cat. no.
SA00013-1; 1:100; Proteintech Group, Inc.) and
CoraLite594-conjugated Goat Anti-Rabbit IgG (H + L; cat. no.
SA00013-4; 1:100; Proteintech Group, Inc.) for 1 h at 37˚C.
Finally, the nuclei were stained with DAPI for 10 min at 37˚C.
Images were visualized and captured using a Nikon fluorescence
microscope (Zeiss AG).
Detection of interleukin expression by
ELISA
The levels of IL-1β and IL-18 in rat serum were
determined using a Rat IL-18 ELISA Kit (cat. no. EK0592; Boster
Biological Technology) and a Rat IL-1β/IL1B ELISA Kit (cat. no.
EK0393; Boster Biological Technology). Experiments were performed
according to the manufacturer's instructions. The OD value was
determined, a standard curve was drawn and the sample concentration
was calculated.
Protein extraction and
quantification
Pulmonary arteries were isolated from SD rats. PBS
was added to the blank culture dish, and the isolated pulmonary
artery tissue was spread out in the culture dish. The adventitia of
the pulmonary artery tissue was scraped with forceps. The pulmonary
artery tissue was cut with ophthalmic scissors to expose the
intima. The tunica intima of the pulmonary artery tissue was
scraped with curved forceps, and the tunica media of the pulmonary
artery, termed pulmonary artery smooth muscle cells, were obtained.
Pulmonary artery smooth muscle cells were added to the lysate,
homogenized with a homogenizer, diluted in lysate and centrifuged
(at 4˚C for 15 min at 10,464 x g). The supernatant was used for the
subsequent analyses. Protein concentrations were determined using a
BCA kit (cat. no. AR1189; Boster Biological Technology) and sample
volumes were calculated. The SDS-PAGE loading buffer (cat. no.
CW0027S; CoWin Biosciences) was added and the sample was denatured
for 6-10 min at 100˚C. After cooling, the protein was stored at
-80˚C.
Western blotting
Samples were loaded at 20 µg per lane and
electrophoresed by 10% SDS-PAGE. After partitioning by gel
electrophoresis, the proteins were transferred onto polyvinylidene
difluoride membranes (MilliporeSigma). The membranes were blocked
in 5% non-fat milk for 2 h at room temperature and incubated for ~7
h at 4˚C with primary antibodies, including anti-GSDMD antibody
(cat. no. sc-81868; 1:1,000; Santa Cruz Biotechnology, Inc.),
anti-proliferating cell nuclear antigen (PCNA) antibody (cat. no.
13110S; 1:1,000; Cell Signaling Technology, Inc.), anti-NLRP3
antibody (cat. no. ab263899; 1:1,000; Abcam), anti-caspase-1
antibody (cat. no. NB100-56565; 1:2,000; Novus Biologicals, LLC),
anti-GAPDH antibody (cat. no. BM3876; 1:2,000; Boster Biological
Technology) and anti-β-actin antibody (cat. no. BM5422; 1:2,000;
Boster Biological Technology). Stable protein references were
selected based on the molecular weight of the target proteins.
GAPDH (molecular weight, 36 kDa) was selected as the reference for
NLRP3 (118 kDa), caspase-1 (53 kDa) and GSDMD (45 kDa), and β-actin
(molecular weight, 42 kDa) was selected as the reference for PCNA
(molecular weight, 36 kDa). Furthermore, the membranes were
incubated with the appropriate secondary antibodies: HRP Conjugated
AffiniPure Goat Anti-rabbit IgG (cat. no. BA1054; 1:5,000; Boster
Biological Technology) and HRP Conjugated AffiniPure Goat
Anti-mouse IgG (cat. no. BA1050; 1:5,000; Boster Biological
Technology) for ~1 h. Thereafter, the bands were visualized using
the Chemiluminescent Substrate kit (cat. no. PE0010; Beijing
Solarbio Science & Technology Co., Ltd.) combined with a Tanon
5200 (Tanon Science and Technology Co., Ltd.), and densitometry
analysis was performed using Image J (version 1.43; National
Institutes of Health).
Statistical analysis
All data were analyzed using SPSS Statistics 21 (IBM
Corp.) and graphics were generated using GraphPad Prism 8.0.2
(Dotmatics). Statistical values are expressed as the mean ± SEM and
all experiments were repeated three times. One-way ANOVA with
Tukey's post hoc test was used to compare multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
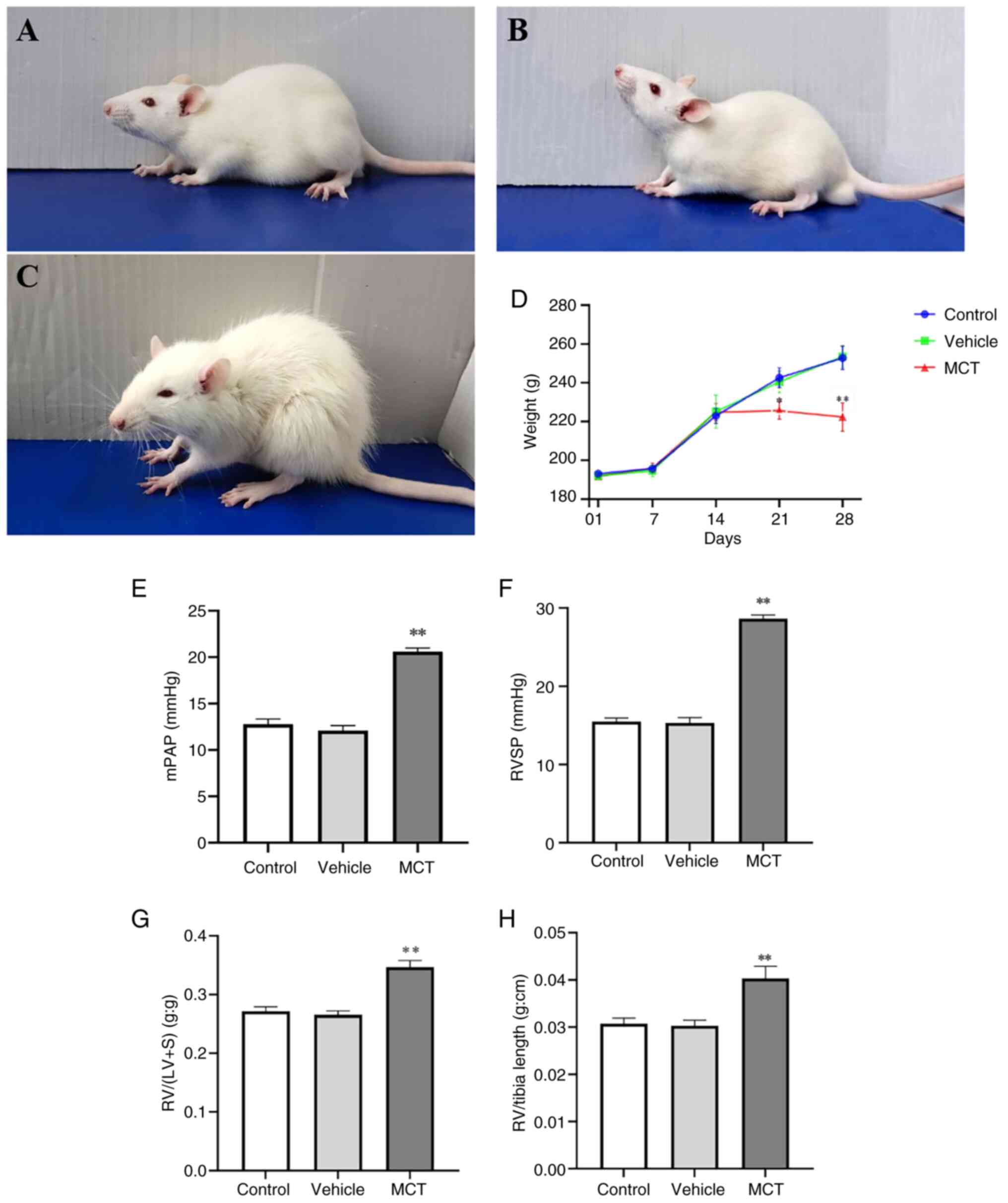
Increased vascular remodeling and
PASMC pyroptosis in MCT-induced PAH rats. Significant changes in
characteristics of rats and hemodynamic changes
In the present study, a classical rat model of
MCT-induced PAH was used to explore the specific mechanism of PASMC
pyroptosis in pulmonary vascular remodeling. Compared with the
control rats, MCT-treated rats showed notable mental distress, slow
response, loss of appetite, rough hair, shortness of breath and
emaciation (Fig. 1A-C). In
addition, the weight of the MCT-induced rats significantly
decreased during the final two weeks (Fig. 1D). To verify whether the modelling
was successful, the hemodynamic changes in different groups were
analyzed. The results demonstrated that RVSP, mPAP, RV/(LV + S) and
RV weight/right tibial length were significantly higher in the MCT
group than in the control group (P<0.01; Fig. 1E-H). Therefore, as aforementioned,
the MCT-induced PAH rat model was successfully established.
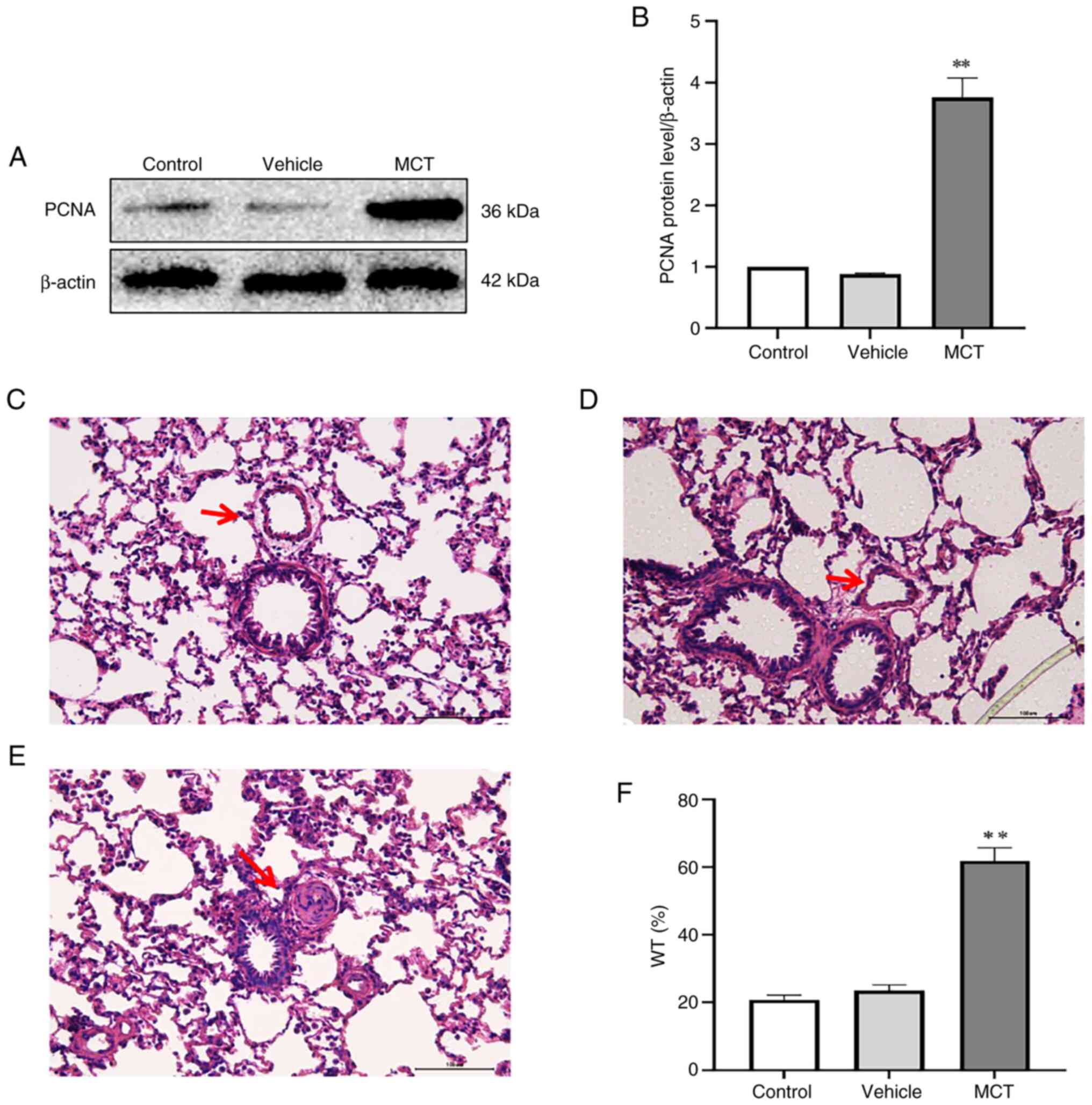
Significant proliferation in PASMCs.
Pulmonary artery smooth muscle cell proliferation obviously
reflects the pulmonary vascular remodeling. The proliferation of
PASMC was assessed by HE staining of lung tissues and PCNA protein
expression. The results indicated that PCNA expression in PASMCs
was significantly upregulated in the MCT group compared with the
control group (Fig. 2A and
B). It was found that the medial
membrane of the pulmonary artery was thickened in the MCT group
(Fig. 2C-E), and the percentage of
WT in the MCT group was significantly higher than that in the
control group (P<0.01; Fig.
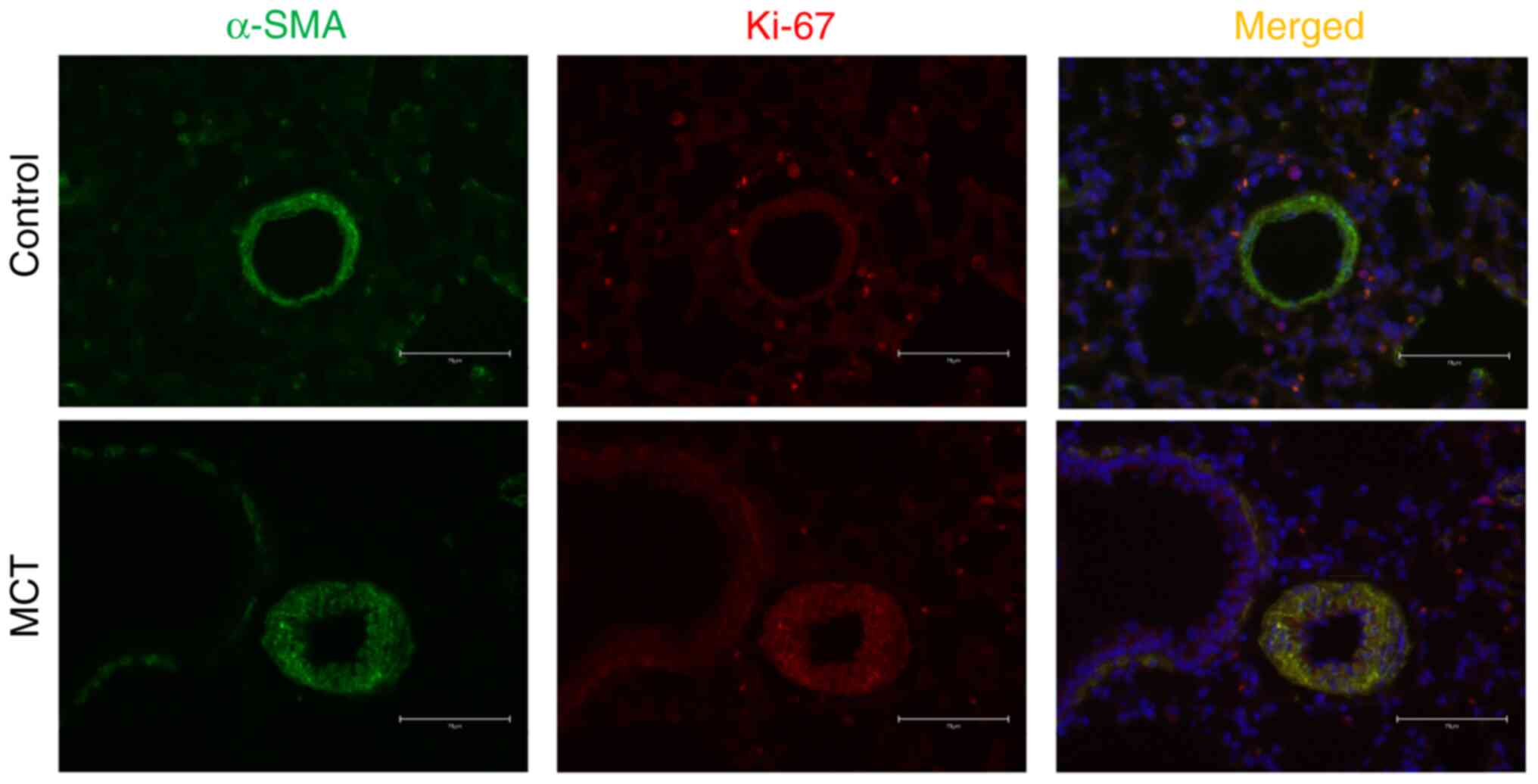
2F). Furthermore, immunofluorescence showed that the protein
expression of Ki-67 in PASMCs in MCT-PAH rats was increased
compared with that in the control rats (Fig. 3). These results indicated that
PASMC proliferation increased after treatment with MCT (60 mg/kg)
for 28 days. Based on these results, a rat model of MCT-induced PAH
was successfully established. In addition, the vehicles had almost
no impact on PASMC proliferation or pulmonary artery
remodeling.
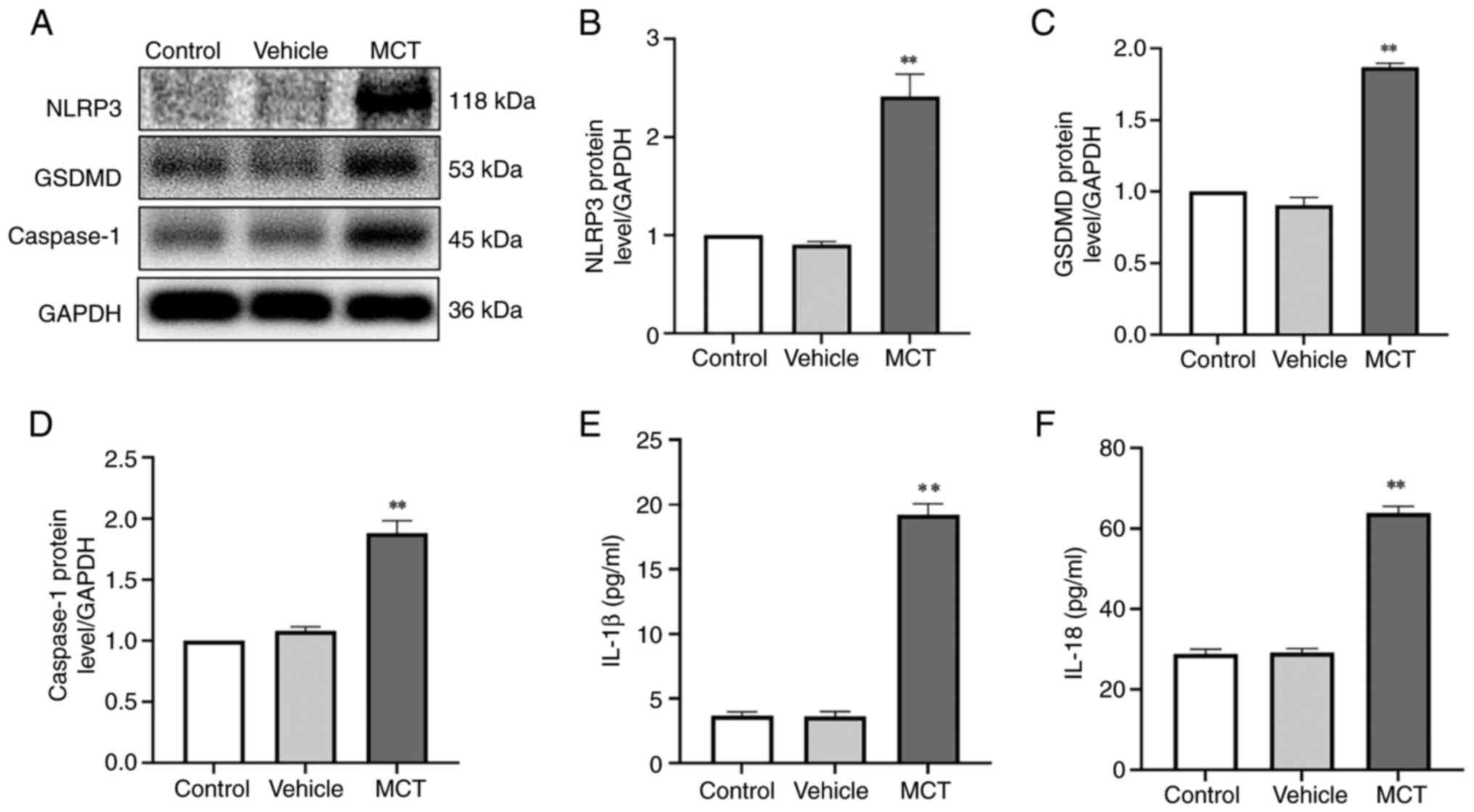
MCT induces PASMC pyroptosis. To determine
whether PASMC pyroptosis is involved in the regulation of pulmonary
vascular remodeling in MCT-induced PAH rats, the protein expression
levels of NLRP3, GSDMD and caspase-1 were measured. As shown in
Fig. 4, the protein expression
levels of NLRP3, GSDMD and caspase-1 in PASMCs in the MCT group
were significantly upregulated (P<0.01; Fig. 4A-D). These results indicated a
significant increase in MCT-induced pyroptosis of PASMCs in rats
with PAH.
PASMC pyroptosis promotes the proliferation of
PASMCs via the paracrine effects of IL-1β and IL-18. To explore the
regulatory mechanisms responsible for MCT-induced PASMC pyroptosis,
the concentrations of interleukins were determined using ELISA. The
levels of IL-1β and IL-18 in the MCT group were significantly
higher than those in the control group (Fig. 4E and F). The results indicated that pyroptotic
PASMCs could secrete IL-1β and IL-18. Therefore, PASMC pyroptosis
may play a crucial role in promoting pulmonary vascular remodeling
in MCT-induced PAH, and its molecular mechanism may be closely
related to IL-1β and IL-18.
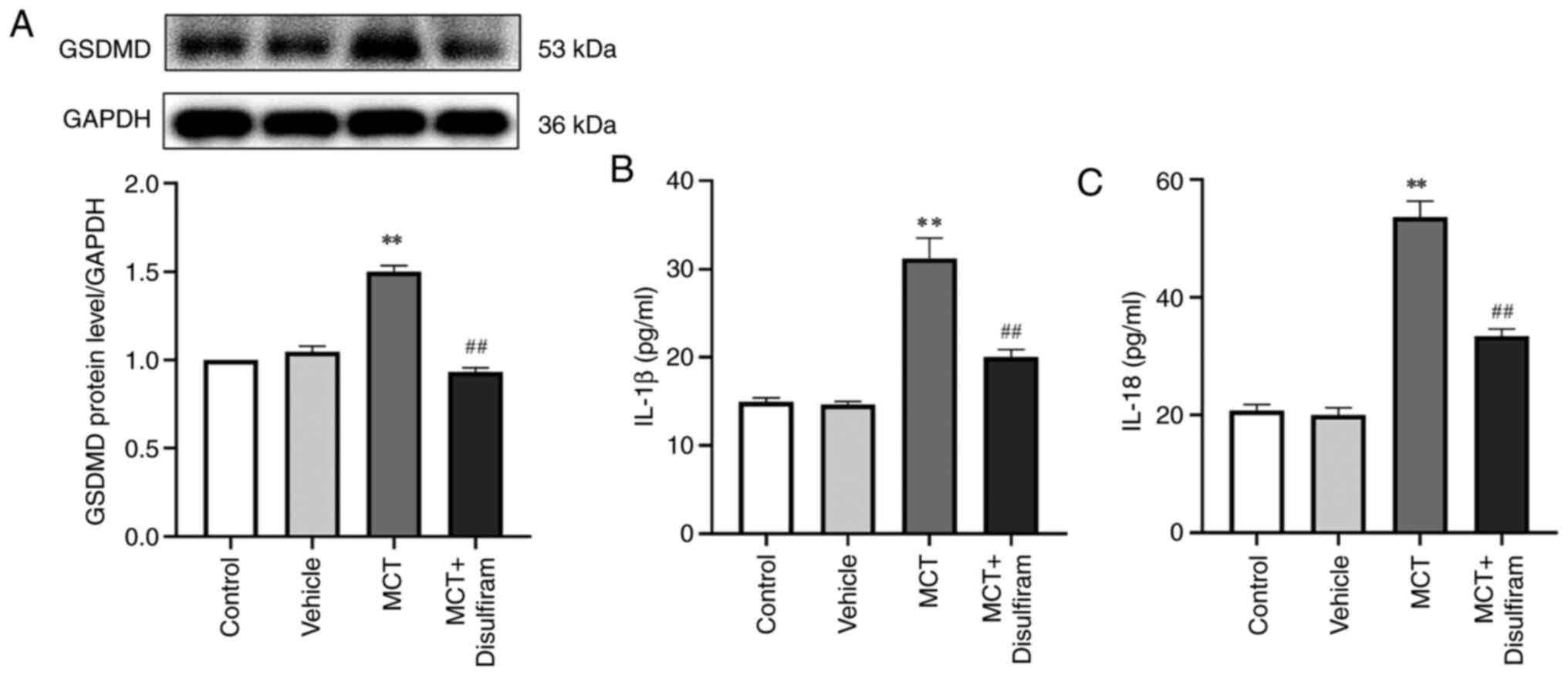
Disulfiram, a pyroptosis inhibitor,
reverses the upregulation of GSDMD and inhibits the secretion of
IL-1β and IL-18
To further clarify the important role of PASMC
pyroptosis in pulmonary vascular remodeling and its molecular
mechanisms, disulfiram, an inhibitor of GSDMD pore membrane and
pyroptosis, was used to treat PAH rats. The present study revealed
that GSDMD expression in disulfiram-treated PAH rats was
significantly downregulated compared with that in MCT-induced rats
(P<0.01; Fig. 5A). Increasing
evidence suggests that IL-1β and IL-18 are involved in cell
proliferation; however, it is unclear whether they influence PASMC
proliferation in PAH. To determine the underlying mechanism through
which PASMC pyroptosis indirectly promotes PASMC proliferation, the
levels of interleukins in the serum of MCT-induced PAH rats were
determined using ELISA. The results demonstrated that disulfiram
lowers the concentrations of IL-1β and IL-18 in the serum of
MCT-induced PAH rats (P<0.01; Fig.
5B and C). These results
suggest that disulfiram can reduce the concentrations of IL-1β and
IL-18 by inhibiting PASMC pyroptosis in MCT-induced PAH rats.
Inhibiting IL-1β and IL-18 paracrine
signaling attenuates PASMC proliferation and vascular
remodeling
To explore whether inhibiting IL-1β and IL-18
paracrine signaling can attenuate pulmonary artery remodeling, the
hemodynamic changes and the degree of cardiac and vascular
remodeling in each group of rats were further compared. It was
found that the RVSP, mPAP, RV/(LV + S) and RV weight/right tibia
length in the disulfiram-treated PAH rats were significantly lower
than those in the MCT-induced PAH rats (Fig. 6A-D). The aforementioned results
revealed that inhibiting IL-1β and IL-18 paracrine signaling
significantly attenuates right ventricular remodeling and inhibits
pulmonary arterial thickening in MCT-induced PAH rats. To further
prove that inhibiting IL-1β and IL-18 paracrine signaling
attenuated PASMC hyperplasia, an inhibitory group model was
established for comparative analysis. The results suggested that
PCNA expression in PASMCs in the MCT-induced rats was significantly
upregulated compared with that in the control rats. By contrast, it
was significantly downregulated in the disulfiram-treated PAH rats
compared with that in the MCT-induced rats (Fig. 6J). It was found that the pulmonary
arterioles were significantly thicker in MCT-treated rats, and the
percentage of WT was also significantly increased. Numerous
inflammatory cells, such as lymphocytes and neutrophils,
infiltrated the lung tissues of MCT-induced PAH rats. However, the
opposite results were observed in disulfiram-treated PAH rats
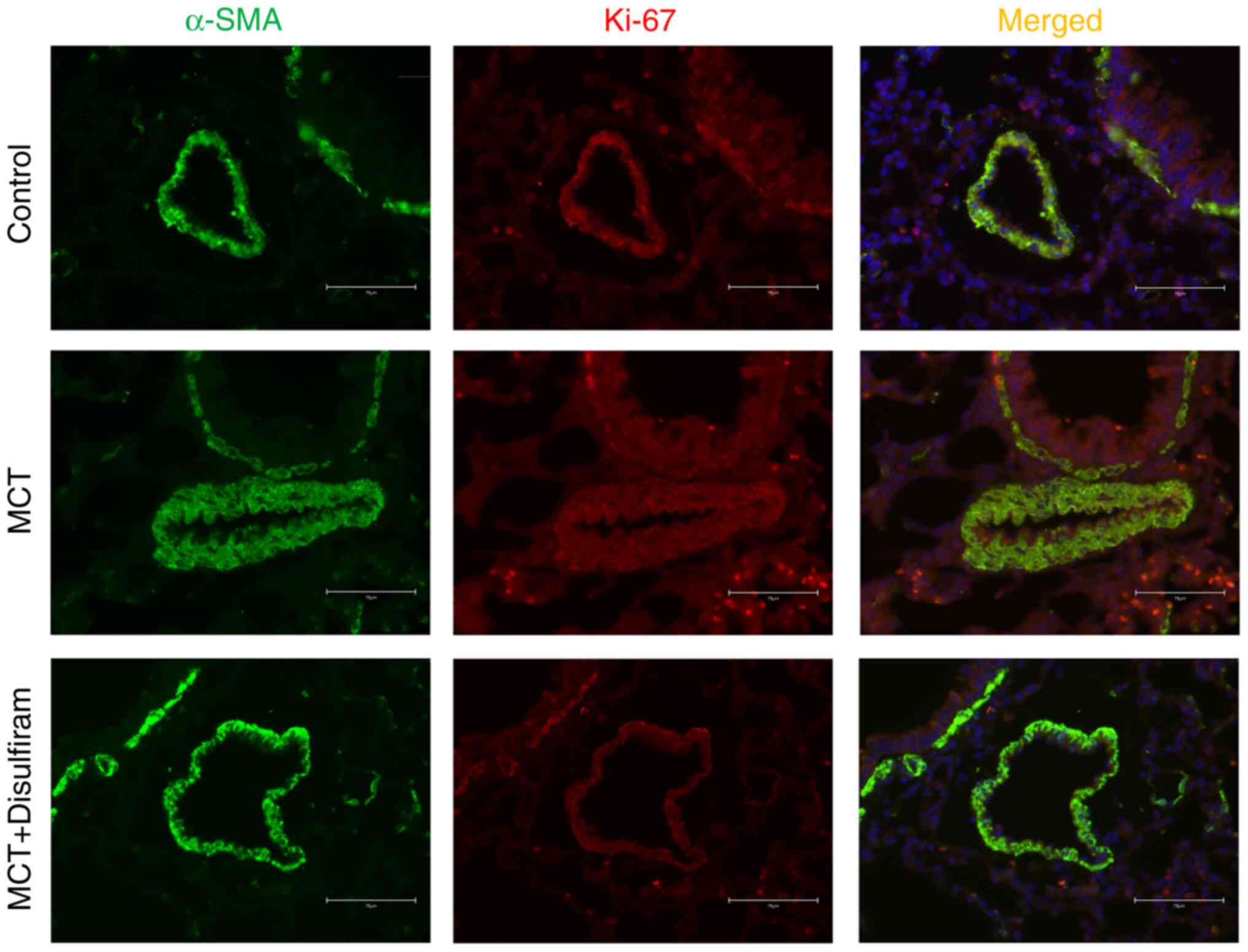
(Fig. 6E-I). In addition, it was
found that disulfiram downregulated the protein expression of Ki-67
in PASMCs compared with that in MCT-induced PAH rats by
immunofluorescence (Fig. 7). The
aforementioned results suggest that inhibiting IL-1β and IL-18
paracrine signaling can attenuate PASMC proliferation and reverse
vascular remodeling in MCT-induced PAH rats.
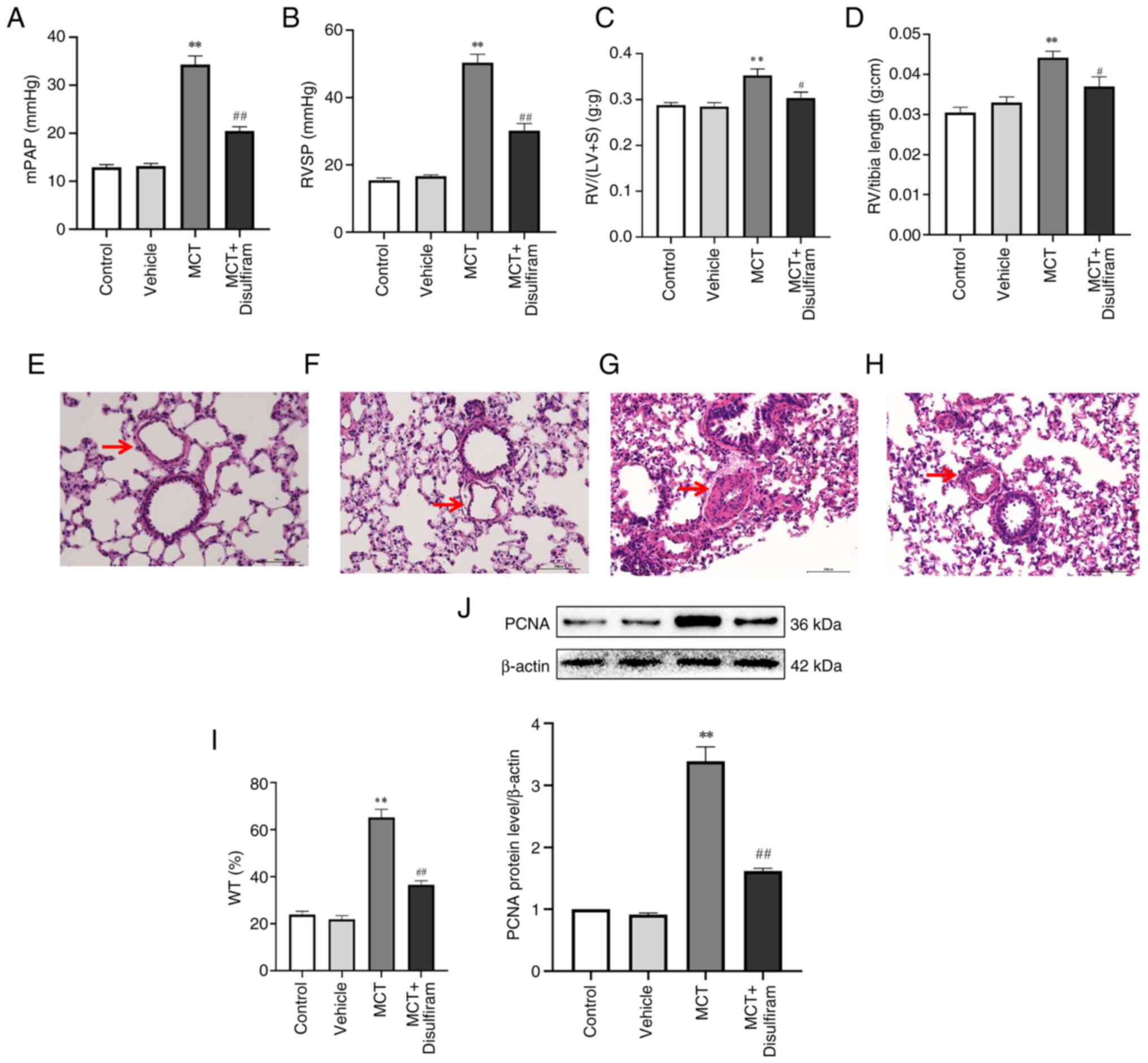 | Figure 6Inhibiting IL-1β and IL-18 paracrine
attenuates PASMC proliferation and vascular remodeling in
MCT-induced PAH rats. mPAP (A), RVSP (B), RV/(LV + S) (C) and
RV/tibia length (D) in the disulfiram-induced rats were
significantly decreased than those in the MCT-induced PAH rats.
Pulmonary vascular remodeling was observed using HE staining in (E)
control rats, (F) vehicle rats, (G) MCT rats and (H) MCT +
disulfiram rats (scale bar, 100 µm; magnification, 200x). The
arrows show the pulmonary arterioles. (I) Quantitative analysis of
WT. (J) PCNA protein expression was measured by western blotting.
Data are represented as the mean ± SEM (n=10)
**P<0.01 vs. control group; #P<0.05,
##P<0.01 vs. MCT group. MCT, monocrotaline; PAH,
pulmonary arterial hypertension; mPAP, mean pulmonary artery
pressure; RVSP, right ventricular systolic pressure; RV, right
ventricle; LV, left ventricle; S, interventricular septum; WT,
pulmonary artery wall thickness; PCNA, proliferating cell nuclear
antigen; PASMC, pulmonary artery smooth muscle cell. |
Discussion
PAH is a vascular disease characterized by pulmonary
artery remodeling, increased afterload and cardiac hypertrophy
(33). The proliferation of PASMCs
significantly contributes to the occurrence and progression of PAH
(11). The mechanism underlying
pulmonary artery remodeling induced by PASMC proliferation has not
yet been fully elucidated. Therefore, identifying new pathogenic
and effective targets for PASMC proliferation is important for
discovering preventative and early treatment therapies for PAH. The
present study revealed the significant effect of PAMSC pyroptosis
in PAH induced by MCT and clarifies that pyroptotic PASMCs can
secrete IL-1β and IL-18, promote the proliferation of PASMCs and
further facilitate pulmonary vascular remodeling. Furthermore, it
was demonstrated that disulfiram can attenuate the progression of
pulmonary hypertension, and the mechanisms may involve the
secretion of IL-1β and IL-18.
Pyroptosis manifests as a form of pro-inflammatory
cell death, accompanied by the formation of cell membrane pores and
the secretion of various pro-inflammatory factors, especially the
NLRP3 inflammasome and its downstream effector inflammatory factors
IL-1β and IL-18(15). Pyroptosis
is widely associated with the progression of atherosclerosis, liver
fibrosis, renal ischaemia/reperfusion injury and nervous system
diseases (34-38).
However, whether pyroptosis occurs in the PASMCs of MCT-induced PAH
rats has not yet been reported. In the present study, it was found
that pyroptosis significantly increased following MCT treatment in
rats. Notably, PASMC pyroptosis was accompanied by the secretion of
interleukins such as IL-1β and IL-18. GSDMD is a direct effector of
pyroptosis and is an important target for the release of IL-1β and
IL-18(39). As aforementioned,
PASMC pyroptosis may be crucial for MCT-induced pulmonary arterial
remodeling. However, the regulatory mechanisms underlying the PASMC
proliferation are unclear. Studies have indicated that the
paracrine effects of IL-18 and IL-1β can promote human aortic
smooth muscle cell migration and proliferation (40). Furthermore, a study on
atherosclerotic mice showed that IL-1β can promote the
proliferation, remodeling and structural maintenance of smooth
muscle cells and fibrous caps (41). Importantly, reducing the level of
IL-1β can inhibit smooth muscle cell proliferation and migration
(42). Additionally, clinical
studies have found that the levels of the interleukins IL-1β and
IL-18 in the serum of patients with PAH are significantly increased
(43-45).
Pyroptosis of PASMCs is significantly increased in PAH rats, and
interleukins play crucial roles in cardiovascular diseases. Thus,
it would be useful to explore whether pyroptotic PASMCs can
indirectly promote PASMC proliferation by secreting IL-18 and
IL-1β.
The pore-forming activity of GSDMD directly
determines the manner of death of cells, and GSDMD is the most
direct and necessary executor of pyroptosis (46,47).
The formation of membrane pores caused by GSDMD is a necessary step
for the occurrence of pyroptosis. Disulfiram is a newly developed
drug and Hu et al (48)
found that disulfiram specifically inhibits the formation of the
GSDMD pore membrane to prevent the secretion of inflammatory
factors, and inhibit pyroptosis. IL-18 and IL-1β play important
roles in cardiovascular disease (49,50).
Notably, receptors for IL-1β and IL-18 are expressed at high levels
on VSMCs (51,52). Moreover, high expression levels of
IL-1β and IL-18 promote the proliferation and migration of VSMCs
(53). To investigate whether
pyroptosis can directly or indirectly promote PASMC proliferation
and whether its mechanism is closely related to IL-1β and IL-18,
disulfiram (a pyroptosis inhibitor) was used to explore its
potential in reducing PASMC proliferation by inhibiting GSDMD pore
formation and interleukin secretion. In the current study, it was
confirmed that disulfiram can markedly downregulate the GSDMD
protein expression and the concentrations of serum IL-1β and IL-18,
reduce PASMC proliferation and reverse pulmonary vascular
remodeling in PAH rats. Therefore, it is hypothesized that PASMC
pyroptosis, a recently discovered mechanism of cell death, plays a
vital role in promoting PASMC proliferation and facilitating
pulmonary vascular remodeling. Notably, pyroptosis promotes PASMC
proliferation and aggravates pulmonary vascular remodeling,
possibly through paracrine IL-1β and IL-18.
PAH is a rare but fatal disease (1), and as the burden of PAH continues to
increase, the prevention, treatment and research of PAH must be
continuously strengthened. Increased pulmonary vascular resistance
in PAH is driven by vasoconstriction, inflammation and
proliferative remodeling of the intima and media of the pulmonary
arteries (8). The strengths of the
present study could be that experiments were carried out in an
in vivo model and indicated a critical role for PASMC
pyroptosis in MCT-induced PAH rats. In particular, an important
strength could be that the experiments showed the crucial role of
IL-1β and IL-18 in PASMC proliferation. The findings of the present
study indicate that targeted inhibition of PASMC pyroptosis could
effectively suppress PASMC proliferation and relieve pulmonary
vascular remodeling in PAH. It is also hypothesized that IL-1β and
IL-18 could be used as novel targets for the prevention and
treatment of PAH in the further research, and blocking the
secretion of IL-1β and IL-18 may reduce and delay the development
of PAH and improve the therapeutic effect of PAH. Therefore, this
research could provide a new preventive and therapeutic strategy
for PAH.
However, the present study had several limitations
that must be addressed further. Firstly, the study confirmed that
pyroptosis in PASMCs promotes their proliferation through the
secretion of inflammatory factors in vivo. However, its role
and mechanism of action have not been verified in vitro. At
present, the primary culture of PASMCs is being performed with the
intention of providing further evidence to reveal the effect of
IL-1β and IL-18 on PASMC proliferation. In addition, a stain that
specifically and targeted PASMC cells and their proliferation was
not used in the present study. Moreover, the lack of female rats
could be a limitation of the present study as estrogen may play a
unique pathogenic or protective role in pulmonary hypertension
(54,55). Male rats were used in the present
study for a variety of reasons, such as to reduce variability due
to hormonal differences between males and females, or to avoid
potential complications related to the estrous cycle in female
animals. IN addition, at present, researcher investigating the
pathogenesis of PAH have exclusively used male SD rats (56-58).
Finally, further studies with greater samples are required.
In conclusion, the present study confirmed the new
pathogenesis of MCT-induced PAH in rats. Pyroptosis of PASMCs plays
an important role in MCT-induced PAH in rats. PASMC pyroptosis is
significantly increased in PAH rats and can markedly promote PASMC
proliferation, possibly by secreting IL-1β and IL-18, which could
be useful for identifying novel therapeutic strategies against
PASMC pyroptosis to reverse PAH. Targeting the inhibition of GSDMD
pore membrane formation can markedly reduce the concentrations of
IL-1β and IL-18, inhibit PASMC proliferation and further reverse
pulmonary artery remodeling in MCT-induced PAH rats. The present
study demonstrated the crucial role and mechanism of PASMC
pyroptosis in pulmonary artery remodeling and provided an important
basis for developing new therapeutic strategies for PAH. Blocking
the pyroptosis of PVSMCs provides a novel therapeutic strategy of
PAH. With the advancing of related research and the improvement of
detection technology, treatment of anti-PASMC pyroptosis has
potential. In the future, exploring the inhibition of pyroptosis
and their intrinsic mechanisms, will make significance
contributions to the discovery of novel therapeutic targets and the
development of new drugs for PAH.
Acknowledgements
Not applicable.
Funding
Funding: This project was supported by The National Natural
Science Foundation of China (grant no. 81600040), The Natural
Science Foundation of the Province of Hunan (grant no.
2021JJ30601), Key Program of Education Department of Hunan Province
(grant no. 21A0274), Key Guidance Program of Health Commission of
Hunan Province (grant no. 20201905), The Clinical Medical Research
Center of Hunan (grant no. 2020SK4007) and Hunan Provincial Health
High-Level Talent Scientific Research Project (grant no.
R2023068).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
APW, XFM and YT conceived and designed the
experiments and obtained the funding. QYZ and WL wrote the article.
QYZ, APW and XFM revised the manuscript. QYZ and WL completed the
ELISA and western blotting experiments. QYZ, WL, APW, YT and XFM
analyzed all the data. SXG performed the HE staining. QYZ and WL
conducted animal experiments. YT, XFM and APW supervised the
project. APW and XFM confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
All animal experiments were performed in strict
accordance with the ARRIVE guidelines and were approved by The
Animal Ethics Committee of the University of South China (Hengyang,
China; approval no. 446).
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Breault NM, Wu D, Dasgupta A, Chen KH and
Archer SL: Acquired disorders of mitochondrial metabolism and
dynamics in pulmonary arterial hypertension. Front Cell Dev Biol.
11(1105565)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Galiè N, McLaughlin VV, Rubin LJ and
Simonneau G: An overview of the 6th world symposium on pulmonary
hypertension. Eur Respir J. 53(1802148)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Van Nuffel S, Quatredeniers M, Pirkl A,
Zakel J, Le Caer JP, Elie N, Vanbellingen QP, Dumas SJ, Nakhleh MK,
Ghigna MR, et al: Multimodal imaging mass spectrometry to identify
markers of pulmonary arterial hypertension in human lung tissue
using MALDI-ToF, ToF-SIMS, and hybrid SIMS. Anal Chem.
92:12079–12087. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Samokhin AO, Hsu S, Yu PB, Waxman AB, Alba
GA, Wertheim BM, Hopkins CD, Bowman F, Channick RN, Nikolic I, et
al: Circulating NEDD9 is increased in pulmonary arterial
hypertension: A multicenter, retrospective analysis. J Heart Lung
Transplant. 39:289–299. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Guignabert C, Savale L, Boucly A, Thuillet
R, Tu L, Ottaviani M, Rhodes CJ, De Groote P, Prévot G, Bergot E,
et al: Serum and pulmonary expression profiles of the activin
signaling system in pulmonary arterial hypertension. Circulation.
147:1809–1822. 2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tang H, Desai AA and Yuan JX: Genetic
insights into pulmonary arterial hypertension. application of
whole-exome sequencing to the study of pathogenic mechanisms. Am J
Respir Crit Care Med. 194:393–397. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Frid MG, McKeon BA, Thurman JM, Maron BA,
Li M, Zhang H, Kumar S, Sullivan T, Laskowsky J, Fini MA, et al:
Immunoglobulin-driven complement activation regulates
proinflammatory remodeling in pulmonary hypertension. Am J Respir
Crit Care Med. 201:224–239. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yun X, Philip NM, Jiang H, Smith Z,
Huetsch JC, Damarla M, Suresh K and Shimoda LA: Upregulation of
aquaporin 1 mediates increased migration and proliferation in
pulmonary vascular cells from the rat SU5416/hypoxia model of
pulmonary hypertension. Front Physiol. 12(763444)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Luo L, Hong X, Diao B, Chen S and Hei M:
Sulfur dioxide attenuates hypoxia- induced pulmonary arteriolar
remodeling via Dkk1/Wnt signaling pathway. Biomed Pharmacother.
106:692–698. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jia D, He Y, Zhu Q, Liu H, Zuo C, Chen G,
Yu Y and Lu A: RAGE-mediated extracellular matrix proteins
accumulation exacerbates HySu-induced pulmonary hypertension.
Cardiovasc Res. 113:586–597. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dean A, Gregorc T, Docherty CK, Harvey KY,
Nilsen M, Morrell NW and MacLean MR: Role of the aryl hydrocarbon
receptor in sugen 5416-induced experimental pulmonary hypertension.
Am J Respir Cell Mol Biol. 58:320–330. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Parpaleix A, Amsellem V, Houssaini A, Abid
S, Breau M, Marcos E, Sawaki D, Delcroix M, Quarck R, Maillard A,
et al: Role of interleukin-1 receptor 1/MyD88 signalling in the
development and progression of pulmonary hypertension. Eur Respir
J. 48:470–483. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zehendner CM, Valasarajan C, Werner A,
Boeckel JN, Bischoff FC, John D, Weirick T, Glaser SF, Rossbach O,
Jaé N, et al: Long noncoding RNA TYKRIL plays a role in pulmonary
hypertension via the p53-mediated regulation of PDGFRβ. Am J Respir
Crit Care Med. 202:1445–1457. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang AP, Yang F, Tian Y, Su JH, Gu Q, Chen
W, Gong SX, Ma XF, Qin XP and Jiang ZS: Pulmonary artery smooth
muscle cell senescence promotes the proliferation of PASMCs by
paracrine IL-6 in hypoxia-induced pulmonary hypertension. Front
Physiol. 12(656139)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhaolin Z, Guohua L, Shiyuan W and Zuo W:
Role of pyroptosis in cardiovascular disease. Cell Prolif.
52(e12563)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Duan H, Zhang X, Song R, Liu T, Zhang Y
and Yu A: Upregulation of miR-133a by adiponectin inhibits
pyroptosis pathway and rescues acute aortic dissection. Acta
Biochim Biophys Sin (Shanghai). 52:988–997. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gong T, Yang Y, Jin T, Jiang W and Zhou R:
Orchestration of NLRP3 inflammasome activation by ion fluxes.
Trends Immunol. 39:393–406. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Banerjee I, Behl B, Mendonca M,
Shrivastava G, Russo AJ, Menoret A, Ghosh A, Vella AT, Vanaja SK,
Sarkar SN, et al: Gasdermin D restrains type I interferon response
to cytosolic DNA by disrupting ionic homeostasis. Immunity.
49:413–426.e5. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli
VG, Wu H and Lieberman J: Inflammasome-activated gasdermin D causes
pyroptosis by forming membrane pores. Nature. 535:153–158.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xu YJ, Zheng L, Hu YW and Wang Q:
Pyroptosis and its relationship to atherosclerosis. Clin Chim Acta.
476:28–37. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pan J, Han L, Guo J, Wang X, Liu D, Tian
J, Zhang M and An F: AIM2 accelerates the atherosclerotic plaque
progressions in ApoE-/- mice. Biochem Biophys Res Commun.
498:487–494. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pan J, Lu L, Wang X, Liu D, Tian J, Liu H,
Zhang M, Xu F and An F: AIM2 regulates vascular smooth muscle cell
migration in atherosclerosis. Biochem Biophys Res Commun.
497:401–409. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cheng KT, Xiong S, Ye Z, Hong Z, Di A,
Tsang KM, Gao X, An S, Mittal M, Vogel SM, et al:
Caspase-11-mediated endothelial pyroptosis underlies
endotoxemia-induced lung injury. J Clin Invest. 127:4124–4135.
2017.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Zhaolin Z, Jiaojiao C, Peng W, Yami L,
Tingting Z, Jun T, Shiyuan W, Jinyan X, Dangheng W, Zhisheng J and
Zuo W: OxLDL induces vascular endothelial cell pyroptosis through
miR-125a-5p/TET2 pathway. J Cell Physiol. 234:7475–7491.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li P, Dong XR, Zhang B, Zhang XT, Liu JZ,
Ma DS and Ma L: Molecular mechanism and therapeutic targeting of
necrosis, apoptosis, pyroptosis, and autophagy in cardiovascular
disease. Chin Med J (Engl). 134:2647–2655. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wilson DW, Segall HJ, Pan LC, Lamé MW,
Estep JE and Morin D: Mechanisms and pathology of monocrotaline
pulmonary toxicity. Crit Rev Toxicol. 22:307–325. 1992.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kovacs SB and Miao EA: Gasdermins:
Effectors of pyroptosis. Trends Cell Biol. 27:673–684.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang L, Li K, Lin X, Yao Z, Wang S, Xiong
X, Ning Z, Wang J, Xu X, Jiang Y, et al: Metformin induces human
esophageal carcinoma cell pyroptosis by targeting the miR-497/PELP1
axis. Cancer Lett. 450:22–31. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
McKenzie BA, Mamik MK, Saito LB, Boghozian
R, Monaco MC, Major EO, Lu JQ, Branton WG and Power C: Caspase-1
inhibition prevents glial inflammasome activation and pyroptosis in
models of multiple sclerosis. Proc Natl Acad Sci USA.
115:E6065–E6074. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang AP, Li XH, Gong SX, Li WQ, Hu CP,
Zhang Z and Li YJ: miR-100 suppresses mTOR signaling in
hypoxia-induced pulmonary hypertension in rats. Eur J Pharmacol.
765:565–573. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Guo L, Li Y, Tian Y, Gong S, Chen X, Peng
T, Wang A and Jiang Z: eIF2α promotes vascular remodeling via
autophagy in monocrotaline-induced pulmonary arterial hypertension
rats. Drug Des Devel Ther. 13:2799–2809. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu B, Peng Y, Yi D, Machireddy N, Dong D,
Ramirez K, Dai J, Vanderpool R, Zhu MM, Dai Z and Zhao YY:
Endothelial PHD2 deficiency induces nitrative stress via
suppression of caveolin-1 in pulmonary hypertension. Eur Respir J.
60(2102643)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sharifi Kia D, Kim K and Simon MA: Current
understanding of the right ventricle structure and function in
pulmonary arterial hypertension. Front Physiol.
12(641310)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wei Y, Lan B, Zheng T, Yang L, Zhang X,
Cheng L, Tuerhongjiang G, Yuan Z and Wu Y: GSDME-mediated
pyroptosis promotes the progression and associated inflammation of
atherosclerosis. Nat Commun. 14(929)2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yang F, Qin Y, Wang Y, Li A, Lv J, Sun X,
Che H, Han T, Meng S, Bai Y and Wang L: LncRNA KCNQ1OT1 mediates
pyroptosis in diabetic cardiomyopathy. Cell Physiol Biochem.
50:1230–1244. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tonnus W, Maremonti F, Belavgeni A, Latk
M, Kusunoki Y, Brucker A, von Mässenhausen A, Meyer C, Locke S,
Gembardt F, et al: Gasdermin D-deficient mice are hypersensitive to
acute kidney injury. Cell Death Dis. 13(792)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gaul S, Leszczynska A, Alegre F, Kaufmann
B, Johnson CD, Adams LA, Wree A, Damm G, Seehofer D, Calvente CJ,
et al: Hepatocyte pyroptosis and release of inflammasome particles
induce stellate cell activation and liver fibrosis. J Hepatol.
74:156–167. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Moonen S, Koper MJ, Van Schoor E,
Schaeverbeke JM, Vandenberghe R, von Arnim CAF, Tousseyn T, De
Strooper B and Thal DR: Pyroptosis in Alzheimer's disease: Cell
type-specific activation in microglia, astrocytes and neurons. Acta
Neuropathol. 145:175–195. 2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Dai R, Ren Y, Lv X, Chang C, He S, Li Q,
Yang X, Ren L, Wei R and Su Q: MicroRNA-30e-3p reduces coronary
microembolism-induced cardiomyocyte pyroptosis and inflammation by
sequestering HDAC2 from the SMAD7 promoter. Am J Physiol
Cell Physiol. 324:C222–C235. 2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sukhanov S, Higashi Y, Yoshida T, Mummidi
S, Aroor AR, Jeffrey Russell J, Bender SB, DeMarco VG and
Chandrasekar B: The SGLT2 inhibitor empagliflozin attenuates
interleukin-17A-induced human aortic smooth muscle cell
proliferation and migration by targeting
TRAF3IP2/ROS/NLRP3/Caspase-1-dependent IL-1β and IL-18 secretion.
Cell Signal. 77(109825)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gomez D, Baylis RA, Durgin BG, Newman AAC,
Alencar GF, Mahan S, St Hilaire C, Müller W, Waisman A, Francis SE,
et al: Interleukin-1β has atheroprotective effects in advanced
atherosclerotic lesions of mice. Nat Med. 24:1418–1429.
2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Haldar S, Dru C, Choudhury D, Mishra R,
Fernandez A, Biondi S, Liu Z, Shimada K, Arditi M and Bhowmick NA:
Inflammation and pyroptosis mediate muscle expansion in an
interleukin-1β (IL-1β)-dependent manner. J Biol Chem.
290:6574–6583. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Saito T, Miyagawa K, Chen SY, Tamosiuniene
R, Wang L, Sharpe O, Samayoa E, Harada D, Moonen JAJ, Cao A, et al:
Upregulation of human endogenous retrovirus-K is linked to immunity
and inflammation in pulmonary arterial hypertension. Circulation.
136:1920–1935. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Mavrogiannis E, Hagdorn QAJ, Bazioti V,
Douwes JM, Van Der Feen DE, Oberdorf-Maass SU, Westerterp M and
Berger RMF: Pirfenidone ameliorates pulmonary arterial pressure and
neointimal remodeling in experimental pulmonary arterial
hypertension by suppressing NLRP3 inflammasome activation. Pulm
Circ. 12(e12101)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Choudhury P, Dasgupta S, Kar A, Sarkar S,
Chakraborty P, Bhattacharyya P, Roychowdhury S and Chaudhury K:
Bioinformatics analysis of hypoxia associated genes and
inflammatory cytokine profiling in COPD-PH. Respir Med.
227(107658)2024.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Chen X, He WT, Hu L, Li J, Fang Y, Wang X,
Xu X, Wang Z, Huang K and Han J: Pyroptosis is driven by
non-selective gasdermin-D pore and its morphology is different from
MLKL channel-mediated necroptosis. Cell Res. 26:1007–1020.
2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ding J, Wang K, Liu W, She Y, Sun Q, Shi
J, Sun H, Wang DC and Shao F: Pore-forming activity and structural
autoinhibition of the gasdermin family. Nature. 535:111–116.
2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hu JJ, Liu X, Xia S, Zhang Z, Zhang Y,
Zhao J, Ruan J, Luo X, Lou X, Bai Y, et al: FDA-approved disulfiram
inhibits pyroptosis by blocking gasdermin D pore formation. Nat
Immunol. 21:736–745. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Liu S, Deng X, Zhang P, Wang X, Fan Y,
Zhou S, Mu S, Mehta JL and Ding Z: Blood flow patterns regulate
PCSK9 secretion via MyD88-mediated pro-inflammatory cytokines.
Cardiovasc Res. 116:1721–1732. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Westphal E, Herzberg M, Neumann I, Beibei
L, Pilowski C, Li C, Werdan K and Loppnow H: Neutrophils process
interleukin-1beta and interleukin-18 precursors in a caspase-1-like
fashion-processing is inhibited by human vascular smooth muscle
cells. Eur Cytokine Netw. 17:19–28. 2006.PubMed/NCBI
|
|
51
|
Porritt RA, Zemmour D, Abe M, Lee Y,
Narayanan M, Carvalho TT, Gomez AC, Martinon D, Santiskulvong C,
Fishbein MC, et al: NLRP3 inflammasome mediates immune-stromal
interactions in vasculitis. Circ Res. 129:e183–e200.
2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sahar S, Dwarakanath RS, Reddy MA, Lanting
L, Todorov I and Natarajan R: Angiotensin II enhances
interleukin-18 mediated inflammatory gene expression in vascular
smooth muscle cells: A novel cross-talk in the pathogenesis of
atherosclerosis. Circ Res. 96:1064–1071. 2005.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Li P, Li YL, Li ZY, Wu YN, Zhang CC, A X,
Wang CX, Shi HT, Hui MZ, Xie B, et al: Cross talk between vascular
smooth muscle cells and monocytes through
interleukin-1β/interleukin-18 signaling promotes vein graft
thickening. Arterioscler Thromb Vasc Biol. 34:2001–2011.
2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Rodriguez-Arias JJ and García-Álvarez A:
Sex differences in pulmonary hypertension. Front Aging.
2(727558)2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Sun Y, Sangam S, Guo Q, Wang J, Tang H,
Black SM and Desai AA: Sex differences, estrogen metabolism and
signaling in the development of pulmonary arterial hypertension.
Front Cardiovasc Med. 8(719058)2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Huang Y, Lei C, Xie W, Yan L, Wang Y, Yuan
S, Wang J, Zhao Y, Wang Z, Yang X, et al: Oxidation of ryanodine
receptors promotes Ca2+ leakage and contributes to right
ventricular dysfunction in pulmonary hypertension. Hypertension.
77:59–71. 2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Yan X, Huang J, Zeng Y, Zhong X, Fu Y,
Xiao H, Wang X, Lian H, Luo H, Li D and Guo R: CGRP attenuates
pulmonary vascular remodeling by inhibiting the cGAS-STING-NFκB
pathway in pulmonary arterial hypertension. Biochem Pharmacol.
222(116093)2024.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Williams TL, Nyimanu D, Kuc RE, Foster R,
Glen RC, Maguire JJ and Davenport AP: The biased apelin receptor
agonist, MM07, reverses sugen/hypoxia-induced pulmonary arterial
hypertension as effectively as the endothelin antagonist
macitentan. Front Pharmacol. 15(1369489)2024.PubMed/NCBI View Article : Google Scholar
|