Introduction
Thrombosis is known to develop due to various risk
factors classified as blood flow stagnation, vascular endothelial
dysfunction and hypercoagulability. In patients with cancer,
hypercoagulability due to the cancer and other factors that may
present during cancer treatment, such as excessive bed rest,
surgery, central venous catheter placement, medication and
infection, are risk factors for thromboembolism development. Thus,
since patients with cancer may have several of these risk factors,
they are considered to be at high risk for thrombosis development.
With the increase in the number of patients with cancer and the
development of chemotherapy, cancer- and chemotherapy-related
thromboses are being increasingly observed (1). This increase is in proportion to an
increase in the number of patients with cancer globally (2-4),
which is attributable to a broadening array of treatment modalities
(5) and improvements in the
accuracy of diagnosis, such as CT imaging examinations (6). To the best of our knowledge, no
studies have discussed superior mesenteric vein (SMV) thrombosis
that was discovered incidentally during postoperative chemotherapy.
In the present case, a patient with asymptomatic SMV thrombosis
noted on contrast-enhanced CT after postoperative chemotherapy for
colorectal cancer was treated with direct-acting oral
anticoagulants (DOACs). In addition, the literature was reviewed,
with a focus on the risk of SMV thrombosis during the perioperative
period and cancer chemotherapy. Treatment strategies, depending on
the presence or absence of symptoms, and methods for the early
detection of asymptomatic venous thromboembolism were also
discussed.
Case report
In May 2020, a 71-year-old woman was diagnosed with
occult blood in their stool during a health check. A mass was also
detected in the rectum during a colonoscopy, following which the
patient was referred to Gifu University Hospital (Gifu Japan) that
same month. The patient had a history of arrhythmia and had been
taking pilsicainide orally at a dosage of 150 mg three times a day
since the previous year. After multiple examinations using computed
tomography (CT) scans, endoscopy and fluoroscopy, among others, the
patient was diagnosed with colorectal cancer and underwent a
laparoscopic high anterior resection that same month (May 2020). In
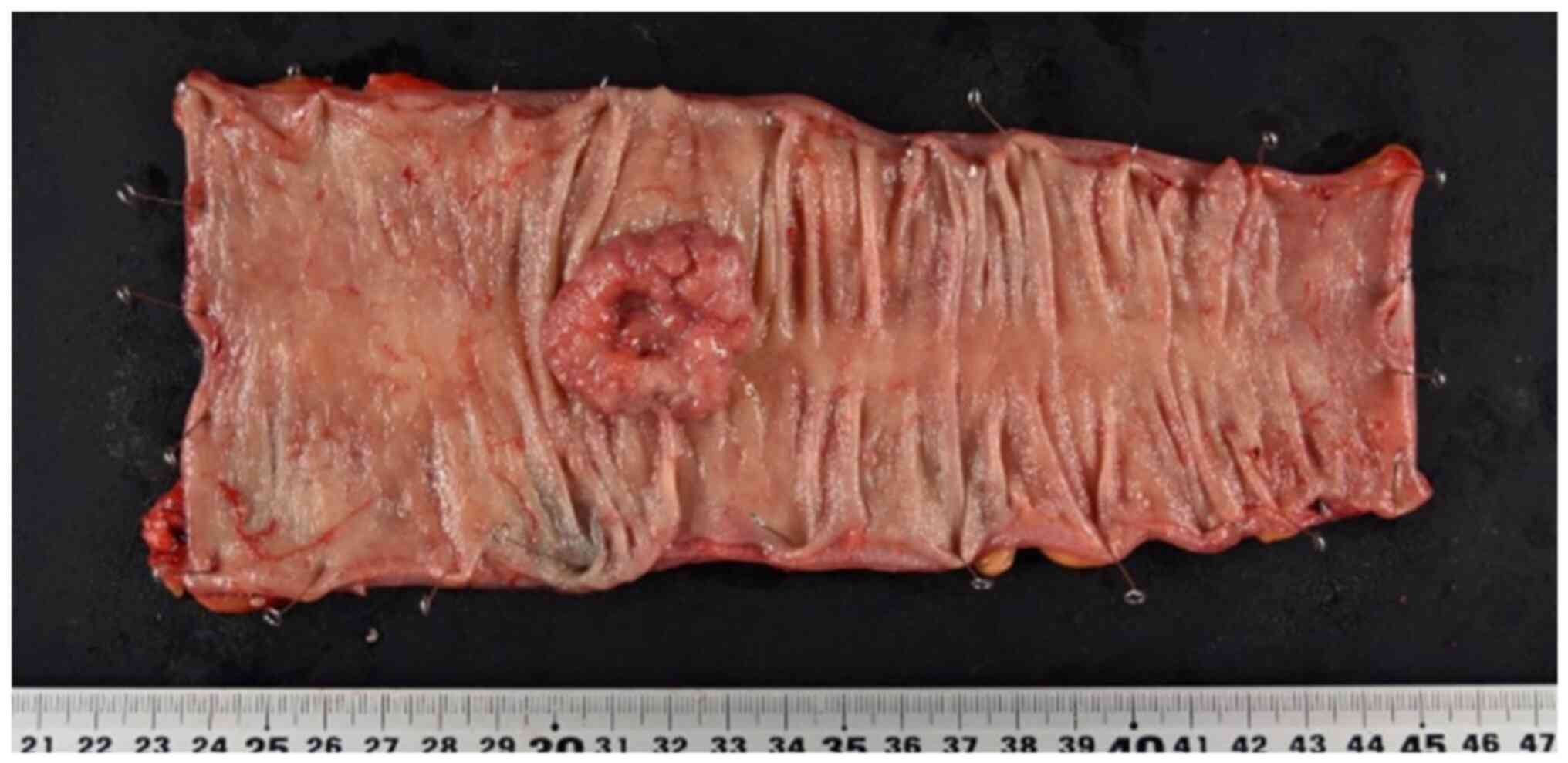
the resected specimen, the tumor was centrally depressed, and the
border of the peritumor was clearly defined. (Fig. 1). The postoperative course was
uneventful, and the patient was discharged on postoperative day 12.
The pathological findings were T3, N0, M0, and Stage IIA according
to the Union for International Cancer Control 8th edition
classification (7). The patient
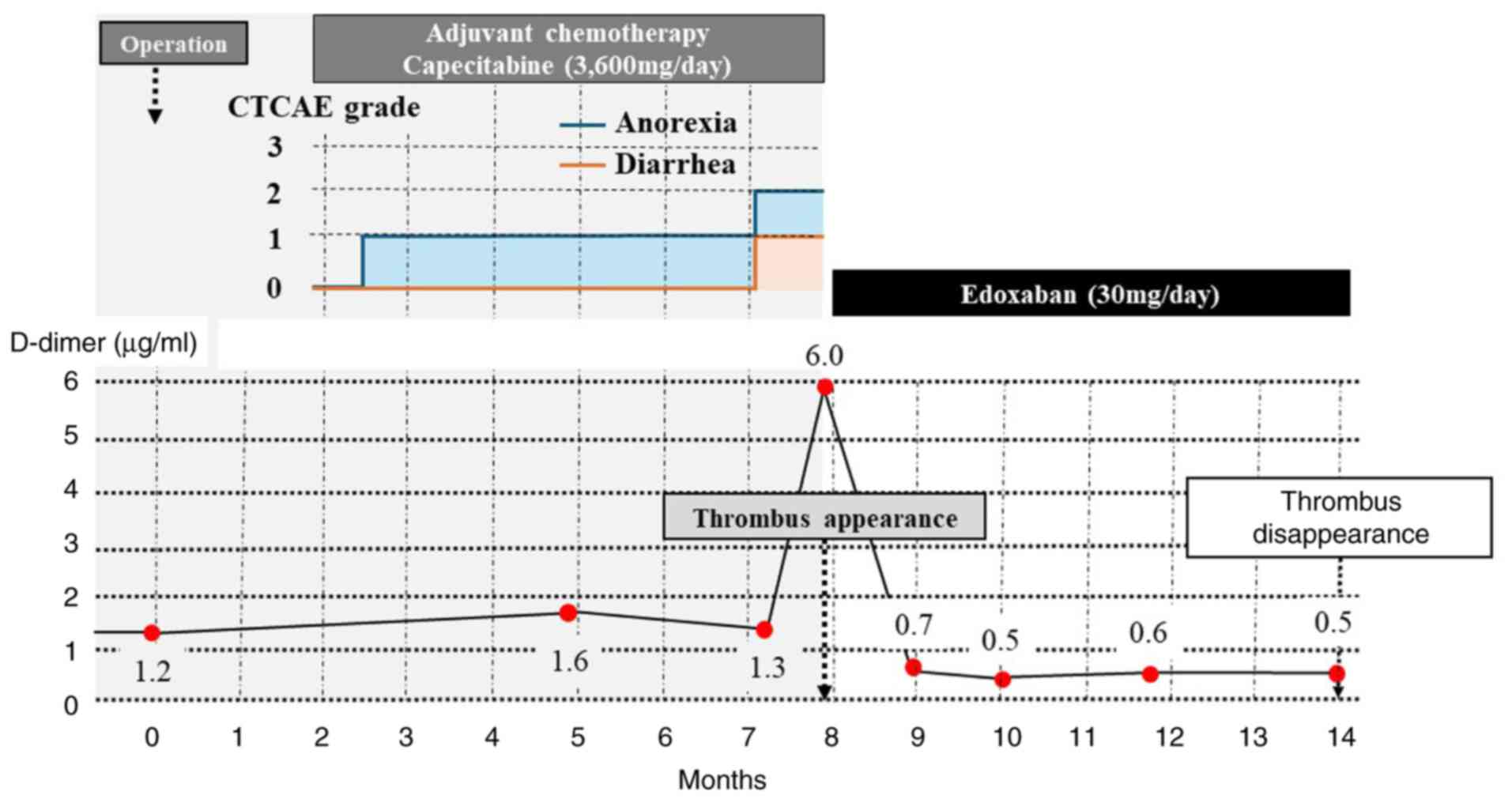
subsequently underwent postoperative adjuvant chemotherapy with
capecitabine. Capecitabine (3,600 mg/day) was administered for 2
weeks, followed by a 1-week break. This was counted as 1 course,
and a total of 8 courses were administered, but suffered from Grade
1 diarrhea and Grade 2 anorexia during the regimen of capecitabine
(grades according to the National Cancer Institute Common
Terminology Criteria for Adverse Events, version 5.0(8).
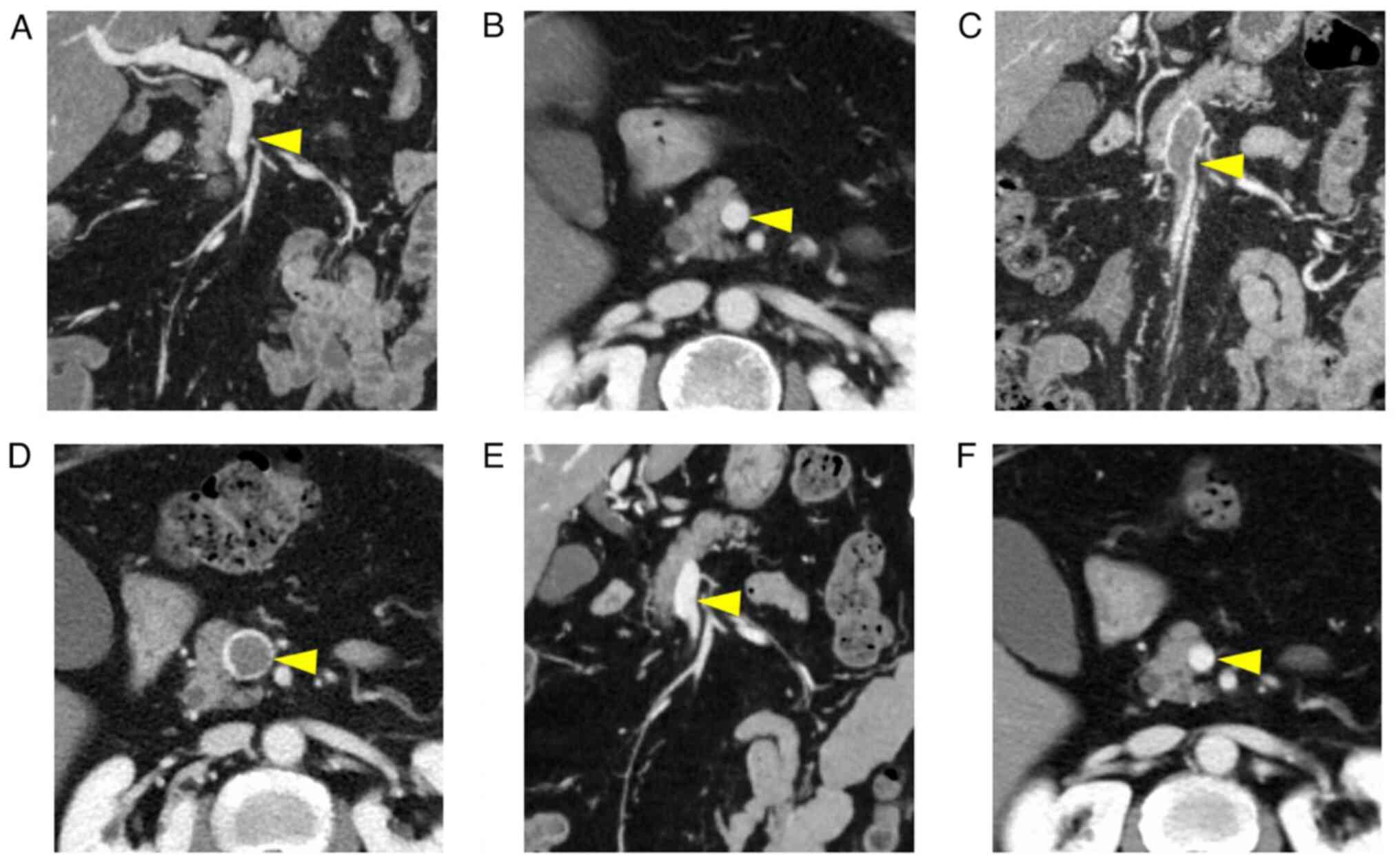
After completing chemotherapy, contrast-enhanced CT
was performed in December 2020 to detect recurrence. Although no
recurrence was observed, a thrombus completely occupying the SMV
lumen was observed (Fig. 2A-F).
Preoperative contrast-enhanced CT showed no thrombi. The patient
had no abdominal symptoms after the chemotherapy. Blood tests
revealed no liver dysfunction or coagulation abnormalities. D-dimer
levels were 6.0 µg/ml, anti-cardiolipin antibody was negative,
protein C level was 57% and protein S level was 140%. No
abnormalities were evident in anti-cardiolipin antibody or protein
C or S activity. Furthermore, no venous thrombus could be detected
by lower limb venous ultrasonography, and no pulmonary artery
thrombosis was observed on contrast-enhanced CT. Based on these
findings, the patient was diagnosed with SMV thrombosis. Since no
abdominal symptoms or obvious intestinal ischemia could be detected
on CT, conservative treatment with anticoagulants was decided.
Treatment with oral edoxaban (30 mg/day) was initiated, following
which D-dimer levels normalized after 1 month. Subsequently, 6
months later, a follow-up examination revealed complete resolution
of the thrombus and no recurrence of colorectal cancer in June
2021. Therefore, edoxaban treatment was discontinued (Fig. 3). No hemorrhagic events were
observed during this treatment period. Subsequent follow-up is
presently being conducted in an outpatient clinic to monitor
recurrence and thrombus formation, and to date, no thrombus or
cancer recurrence has been noted.
Discussion
To the best of our knowledge, the present study is
the first to report a cure for asymptomatic SMV thrombosis in an
outpatient case of a patient recovering from colorectal cancer
after receiving adjuvant chemotherapy using edoxaban.
SMV thrombosis was first reported by Warren and
Eberhard (9) in 1935 as a disorder
that causes congestive infarction due to impaired blood flow in the
mesenteric veins. It is a relatively rare disorder that accounts
for 5-15% of all occlusive lesions in mesenteric vessels (10,11).
The causative factors can be classified as primary, which occurs
idiopathically, and secondary, which is caused by a thrombogenic
predisposition or an underlying disease. Secondary factors
contributing to thrombosis can include general thrombophilia and
inflammatory conditions of the abdomen, such as an abnormal
coagulation-fibrinolytic system, liver disease, surgery,
malignancy, and chemotherapy (12). In both cases, the underlying cause
is damage to the vascular endothelium, impaired blood flow, or
thrombus formation due to hypercoagulation caused by inflammation
or mechanical stimulation (12).
In the present case, no abnormalities were found in coagulability,
such as protein C or S deficiency, and no history of liver disease
was noted. However, this patient underwent postoperative
chemotherapy for a malignant tumor (colorectal cancer) and was
considered to be at high risk of SMV thrombosis.
Heit et al (13) previously reported on the
relationship among patients with cancer, anticancer drugs and
thrombosis, stating that the risk of thromboembolism in individuals
with cancer is 4 times higher compared with that in those without
cancer, which increases further to 6.5 times with chemotherapy.
Cancer treatment has been reported to be an independent risk factor
for recurrent venous thromboembolism (VTE) (14). In the present case, capecitabine
was administered as postoperative chemotherapy for 6 months.
Therefore, the possibility that capecitabine is a risk factor for
thrombus formation cannot be ruled out. Additionally, the frequency
of diarrhea associated with oral capecitabine administration is
~60%, indicating that intravascular dehydration may be a risk
factor for thrombosis (15). The
present patient had grade 1 diarrhea and grade 2 anorexia during
the administration of capecitabine, both of which may have been the
causes of SMV thrombosis due to intravascular dehydration.
The onset of SMV thrombosis varies from acute to
chronic, as the rate of thrombus formation and extent of occlusion
differ with the degree of collateral blood vessel development
(16). The acute form is
characterized by abdominal pain, hemorrhage, and vomiting, which
occurs rapidly due to organ necrosis caused by congestive reflux
obstruction resulting from the venous obstruction. By contrast, the
chronic form may be asymptomatic without reflux obstruction owing
to the development of collateral blood vessels caused by the slower
vascular occlusion (16).
Warshauer et al (17) previously performed a retrospective
study of 43 patients with SMV thrombosis and found that 6 (14%) had
no apparent symptoms. Anticoagulation therapy was administered to
the 6 asymptomatic patients, all of whom showed favorable progress;
however, another systematic review of 604 patients with SMV
thrombosis by Acosta and Salim (18) revealed a small bowel resection rate
of 43.9% for this disease. Therefore, considering the risk of small
bowel necrosis and resection, anticoagulant therapy should be
initiated even if the patient is asymptomatic.
Contrast-enhanced CT is the most effective
diagnostic method for mesenteric venous thrombosis (successful in
90% of cases) (16).
Contrast-enhanced CT images provide an effective means of
confirming the site of obstruction, extent of ischemia, and the
presence of perforation to make a definitive diagnosis and
determine treatment options (19).
Notably, treatment guidelines for colorectal cancer recommend a CT
scan every 6 months postoperatively. Therefore, CT is not
frequently performed (5).
There is a score known as the ‘Khorana score,’ which
is used to evaluate the risk of VTE in patients with malignant
tumors (20). During chemotherapy,
no abnormalities in blood cells were observed, and the
retrospective Khorana score in the present study was 0 points.
Furthermore, D-dimer has been reported to be a useful indicator of
thrombus development. The American Society of Clinical Oncology
guidelines recommend measuring D-dimer levels at the beginning of a
new chemotherapy regimen (21). A
previous large cohort study assessing a list of risk factors for
symptomatic VTE revealed that abnormal D-dimer values were a
significant risk factor (14).
Given that SMV thrombosis occurred either in the
perioperative period or during adjuvant chemotherapy in the present
case, D-dimer measurement could have detected clots earlier.
Treatment guidelines for colorectal cancer recommend measuring
tumor markers, such as CA19-9 and CEA, every 3 months. Therefore,
D-dimer levels should be measured simultaneously. Tanaka et
al (22) reported that in
addition to D-dimer, soluble fibrin monomer complex (SFMC) may also
be a beneficial biomarker for thrombosis in patients with
esophageal cancer, where measuring both D-dimer and SFMC levels is
believed to enhance the reliability of thrombus detection.
In terms of anticoagulant therapy, vitamin K
antagonists or low-molecular-weight heparin sodium are frequently
used (23,24). Cytochrome P450 (CYP) is a key
enzyme in the metabolic pathway of a variety of chemotherapeutic
drugs (25), including
capecitabine. Capecitabine inhibits the DNA synthesis of CYP2C9,
resulting in the reduction of vitamin K antagonist-metabolizing
enzyme levels to enhance the effects of vitamin K antagonists
(26). Therefore, edoxaban, which
exerts its antithrombotic effects by selectively but reversibly
inhibiting activated blood coagulation factor Xa (23), is metabolized by CYP3A4 at <10%
of the dose, meaning it may be safer for patients using
capecitabine (27). Additionally,
edoxaban requires no volume adjustment according to clotting factor
values, reaches maximum blood concentration quickly, and its
effects diminish relatively quickly after drug withdrawal (28). For these reasons, although no
reports exist on the treatment of asymptomatic SMV thrombosis with
DOAC to the best of our knowledge, edoxaban was selected in the
present case. Although a previous HOKUSAI Cancer VTE study
demonstrated non-inferiority of the efficacy of edoxaban to
dalteparin, caution was needed for cases of major bleeding
(23). In addition, there is an
antagonist against DOACs. In the event of uncontrolled bleeding,
andexanet α, a DOAC antagonist, has been designed to reverse the
anticoagulant effects of DOAC (29). Even when performing conservative
treatments for SMV thrombosis, as in the present case, there is
always a risk of bleeding due to mechanical stimulation from the
insertion of a gastric or ileus tube and bloody stool due to poor
intestinal blood flow. Therefore, the use of edoxaban, an
antagonist available on standby, would be highly beneficial.
In conclusion, the present case documents a patient
with asymptomatic SMV thrombosis that developed during anticancer
treatment for rectal cancer and was treated safely with edoxaban.
Cancer and chemotherapy are risk factors for thrombosis; therefore,
regular D-dimer measurements may be necessary during cancer
treatment. In addition, edoxaban may be considered an effective
therapeutic tool for SMV thrombosis, even in seemingly severe
cases.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TH and YT contributed to the design of the case
report. YS, KY, SK, and NM analyzed the data. MF, IY, RA and JYT
performed data collection. SK and NM confirm the authenticity of
the raw data. RA and JYT designed the study and prepared the
manuscript. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of the manuscript, including any identifying images
or data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
JCS Joint Working Group: Guidelines for
the diagnosis, treatment and prevention of pulmonary
thromboembolism and deep vein thrombosis (JCS 2009). Circ J, 2017.
(In Japanese).
|
|
2
|
Endo M, Tanaka Y, Sato Y, Sato Y, Ohno S
and Yoshida K: Asymptomatic pulmonary thromboembolism diagnosed
based on prolonged fever after gastric cancer surgery: A case
report with literature review. Int J Surg Case Rep.
92(106836)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Suetsugu T, Tanaka Y, Sato Y, Takaha R,
Fukada M, Yasufuku I, Okumura N, Matsuhashi N, Takahashi T and
Yoshida K: Gastric cancer receiving robotic surgery with
postoperative pulmonary thromboembolism: A case report with
literature review. Ann Vasc Surg. 8(1133)2021.
|
|
4
|
Sharma R and Rakshit B: Spatial and
temporal patterns of colorectal cancer in Asia, 1990-2019. Int J
Clin Oncol. 28:255–267. 2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hashiguchi Y, Muro K, Saito Y, Ito Y,
Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M,
et al: Japanese society for cancer of the colon and rectum (JSCCR)
guidelines 2019 for the treatment of colorectal cancer. Int J Clin
Oncol. 25:1–42. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Alastair J, Jason W, Murthy R, Lloyd R,
Tanabe Y and Prabhakar R: Imaging of acute pulmonary embolism: An
update. Cardiovasc Diagn Ther. 8:225–243. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Brierley JD, Gospodarowicz MK and
Wittekind C (eds): TNM Classification of Malignant Tumours. 8th
edition. Wiley-Blackwell, Hoboken, NJ, 2016.
|
|
8
|
U.S. Department of Health and Human
Services: Common Terminology Criteria for Adverse Events (CTCAE)
version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf.
Accessed July 31, 2024.
|
|
9
|
Warren S and Eberhard TP: Mesenteric
venous thrombosis. Surg Gynecol Obstet. 61:102–121. 1935.
|
|
10
|
Hashizume Y, Nomura T, Suzuki S and Kondo
M: Superior mesenteric vein thrombosis treated with edoxaban. J Gen
Fam Med. 18:169–170. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Kumar S, Sarr MG and Kamath PS: Mesenteric
venous thrombosis. N Engl J Med. 345:1683–1688. 2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Matsumiya M, Koizumi M, Kasahara N, Endo
K, Sasanuma H, Sakuma Y, Horie H, Hosoya Y, Kitayama J and Sata N:
Intestinal resection for delayed bowel stenosis after subacute
superior mesenteric venous thrombosis: A case report. Jpn J
Gastroenterological Surg. 54:538–547. 2021.
|
|
13
|
Heit JA, Silverstein MD, Mohr DN,
Petterson TM, O'Fallon WM and Melton LJ III: Risk factors for deep
vein thrombosis and pulmonary embolism: A population-based
case-control study. Arch Intern Med. 160:809–815. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ohashi Y, Ikeda M, Kunitoh H, Sasaki M,
Okusaka T, Mukai H, Fujiwara K, Nakamura M, Oba MS, Kimura T, et
al: One-year incidence of venous thromboembolism, bleeding, and
death in patients with solid tumors newly initiating cancer
treatment: Results from the Cancer-VTE Registry. Thromb Res.
213:203–213. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schmoll HJ, Cartwright T, Tabernero J,
Nowacki AF, Figer A, Maroun J, Price T, Lim R, Cutsem EV, Oark YS,
et al: Phase III trial of capecitabine plus oxaliplatin as adjuvant
therapy for stage III colon cancer: A planned safety analysis in
1,864 patients. J Clin Oncol. 25:102–109. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lim KH, Jang J, Yoon HY and Park J: Acute
superior mesenteric vein thrombosis associated with abdominal
trauma: A rare case report and literature review. Med (Baltim).
96(e8863)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Warshauer DM, Lee JK, Mauro MA and White
GC II: Superior mesenteric vein thrombosis with radiologically
occult cause: A retrospective study of 43 cases. AJR Am J
Roentgenol. 177:837–841. 2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Acosta S and Salim S: Management of acute
mesenteric venous thrombosis: A systematic review of contemporary
studies. Scand J Surg. 110:123–129. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Morasch MD, Ebaugh JL, Chiou AC, Matsumura
JS, Pearce WH and Yao JS: Mesenteric venous thrombosis: A changing
clinical entity. J Vasc Surg. 34:680–684. 2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Khorana AA, Kuderer NM, McCrae K,
Milentijevic D, Germain G, Laliberté F, MacKnight SD, Lefebvre P,
Lyman GH and Streiff MB: Cancer associated thrombosis and mortality
in patients with cancer stratified by Khorana score risk levels.
Cancer Med. 9:8062–8073. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Key NS, Khorana AA, Kuderer NM, Bohlke K,
Lee AY, Arcelus JI, Wong SL, Balaban EP, Flowers CR, Francis CW, et
al: Venous thromboembolism prophylaxis and treatment in patients
with cancer: ASCO clinical practice guideline update. J Clin Oncol.
38:496–520. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tanaka Y, Yamada A, Hirata S, Tanaka H,
Sakuratani T, Matsuhashi N, Yamaguchi K, Shimokawa T and Yoshida K:
Efficacy and safety of enoxaparin for prophylaxis of postoperative
venous thromboembolism after esophagectomy: A single-center
prospective randomized controlled phase II study. Anticancer Res.
39:2615–2625. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Raskob GE, van Es N, Verhamme P, Carrier
M, Nisio MD, Garcia D, Grosso MA, Kakkar AK, Kovacs MJ, Mercuri MF,
et al: Edoxaban for the treatment of cancer-associated venous
thromboembolism. N Engl J Med. 378:615–624. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mandalà M, Falanga A and Roila F: ESMO
guidelines working group. Management of venous thromboembolism
(VTE) in cancer patients: ESMO clinical practice guidelines. Ann
Oncol. 22:vi85–vi92. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhou Y, Ingelman SM and Lauschke VM:
Worldwide distribution of cytochrome P450 alleles: A meta-analysis
of population-scale sequencing projects. Clin Pharmacol Ther.
102:688–700. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Bellesoeur A, Thomas-Schoemann A, Allard
M, Smadja D, Vidal M, Alexandre J, Goldwasser F and Blanchet B:
Pharmacokinetic variability of anticoagulants in patients with
cancer-associated thrombosis: Clinical consequences. Crit Rev Oncol
Hematol. 129:102–112. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mar PL, Gopinathannair R, Gengler BE,
Chung MK, Perez A, Dukes J, Ezekowitz MD, Lakkireddy D, Lip GYH,
Miletello M, et al: Drug interactions affecting oral anticoagulant
use. Circ-Arrhythmia Electrophysiol. 15(e007956)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Leentjens J, Middeldrop S and Jung C: A
short review of ciraparantag in perspective of the currently
available anticoagulant reversal agents. J Drug Discov Today.
27(103332)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Escolar G, Diaz-Ricart M and
Arellano-Rodrigo E: Andexanet alfa: A recombinant mimetic of human
factor Xa for the reversal of anticoagulant therapies. Drugs Today
(Barc). 53:271–282. 2017.PubMed/NCBI View Article : Google Scholar
|