Introduction
Psoriasis is a chronic inflammatory skin disease
with the characteristics of hyperkeratosis, thickening of the
stratum spinosum and dermal inflammation (1). Psoriasis usually contributes to the
chronic inflammatory response in the joints, nails and other organs
(2). The inflammatory response
increases the incidence of systemic inflammatory diseases and may
lead to severe arthritic dysfunction (3). In addition, adhesive inflammation
occurs in the collateral ligaments near the joints, resulting in
frequent spread of the inflammation to the nail bed, leading to
nail involvement (4). Long-term
chronic inflammation may increase the risk of depression of
patients and is associated with numerous diseases, including
hypertension, diabetes and metabolic syndrome that pro-inflammatory
cytokines and adipocytokines from psoriasis contribute to, which
markedly lowers the quality of life of most patients with psoriasis
(3,5). Keratinocytes stimulated by initial
triggers release their own nucleotides and antimicrobial peptides,
participating in the initiation of psoriasis. After cytokine
stimulation, activated keratinocytes influence the psoriasis
process in terms of inflammatory infiltration, epidermal
proliferation, innate immunity and tissue reorganization. In
addition, keratinocytes also act as amplifiers of psoriatic
inflammation during the maintenance phase (6). Due to their high proliferative
capacity, once activated by pro-inflammatory cytokines,
keratinocytes can produce abundant chemokines and other
inflammatory mediators, inducing innate immunity and amplifying
inflammation. Moreover, keratinocytes, together with fibroblasts
and endothelial cells, accelerate psoriasis plaque formation
through the activation and proliferation of endothelial cells and
the deposition of extracellular matrix (7,8).
Thus, keratinocytes serve as the end-target cells of the local
immune response in psoriasis and the abnormal proliferation and
differentiation of keratinocytes promote the psoriatic plaques
(9).
Hyperproliferation and defective keratinocyte
differentiation in psoriasis may impair epidermal barrier function,
resulting in the destruction of the protective barrier of the skin
(10). The main therapeutic drugs
for psoriasis include biological agents, herbal agents and small
molecule targeted drugs, which can also cause adverse reactions
(11). Therefore, identifying
clinical therapeutic drugs with fewer adverse reactions for the
treatment of psoriasis is of great significance.
Peptidyl-prolyl cis/trans isomerase (PPIase),
NIMA-interacting 1 (PIN1) belongs to the PPIase family (12). PIN1, which has wide existence in
living organisms, specifically recognizes and binds to
phosphorylated serine/threonine motifs in proteins, catalyzes
cis-trans isomerization of the amide bond therein, and subsequently
regulates the bioactivity, stability, phosphorylation level and
subcellular localization of the proteins (13,14).
PIN1 activates a series of proteins that promote cell
proliferation/oncogenesis and also inhibits a series of factors
that block cell proliferation/oncogenesis (15). PIN1 has been suggested to be a
critical regulator in the differentiation, maintenance and
proliferation of numerous types of stem cells, including totipotent
stem cells, neural stem cells, dental pulp stem cells and mammary
stem cells (16-18).
Since the mammary gland also originates from a single epithelial
layer of ectoderm during embryonic formation, it is similar to the
skin in terms of the molecular mechanisms of advancement and
pathogenesis (19). A previous
study revealed that the number of CD24+CD29+
mammary stem cells was reduced, the non-adherent mammospheres
formed by cultured stem cells in vitro were smaller, and the
ability to reconstruct the mammary gland was reduced in PIN1
knockout mice (20). In addition,
the PIN1 protein is widely expressed in skin tissues of patients
with atopic dermatitis, and PIN1 serves a key role in regulating
IL-33 expression in HaCaT cells (21). However, PIN1 expression in
psoriasis and its role in the advancement of psoriasis remain
unclear. Therefore, the present study was designed to verify the
role of PIN1 in five cytokines (M5)-induced HaCaT cells and to
clarify the mechanism underlying its effects.
Materials and methods
Cell lines
The HaCaT human immortalized keratinocyte cell line
(cat. no. iCell-h066; with STR profiling) provided by Cellverse
Bioscience Technology Co., Ltd. was cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (FBS, Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin at 37˚C with 5%
CO2. To induce psoriatic inflammation-like conditions,
HaCaT cells were exposed to M5 (IL-17A, TNF-α, IL-1α, IL-22 and
Oncostatin-M; final concentration, 10 ng/ml; PeproTech, Inc.),
followed by the addition of the mitochondrial autophagy inhibitor
mitochondrial division inhibitor-1 (Mdivi-1; 10 µM; MedChemExpress)
(22,23) for 1 h at 37˚C, while the control
group received no treatment.
Cell transfection
The specific short hairpin RNA (shRNA/sh) targeting
PIN1 and the corresponding negative control were constructed by
Shanghai GenePharma Co., Ltd. HaCaT cells were transfected with
shRNAs (100 nM) using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) for 48 h at 37˚C according to the
manufacturer's protocol. The sequences were as follows: sh-NC
forward, 5'-AACAAGATGAAGAGCACCAA-3' and reverse,
5'-TTGGTGCTCTTCATCTTGTT-3'; sh-PIN1-1 forward,
5'-GCTACATCCAGAAGATCAA-3' and reverse, 5'-TTGATCTTCTGGATGTAGC-3';
and sh-PIN1-2 forward, 5'-GCCGAATTGTTTCTAGTTA-3' and reverse,
5'-TAACTAGAAACAATTCGGC-3'. After 48 h transfection, cells were
collected for subsequent experiments.
Cell Counting Kit-8 (CCK-8) assay
HaCaT cells were seeded into a 96-well plate at
1x103 cells per well with 100 µl complete medium (Gibco;
Thermo Fisher Scientific, Inc.) and cultured at 37˚C, and 10 µl
CCK-8 solution (Beyotime Institute of Biotechnology) was added to
each well, After 24 h, plates were incubated at 37˚C for 2 h, and
then the absorbance at 450 nm was measured with Microplate Reader
(Bio-Rad Laboratories, Inc.). All experiments were performed in
triplicate.
5-ethynyl-2'-deoxyuridine (EdU)
assay
Following inoculation into 6-well plates
(4x105 cells/well), HaCaT cells were cultured at 37˚C
overnight. Subsequently, HaCaT cells underwent fixation in 4%
polyformaldehyde at room temperature for 1 h and exposure to 0.5%
Triton X-100 for 15 min at room temperature. Cells were stained by
Cell-Light™ EdU Apollo®488 in vitro imaging kit (Thermo
Fisher Scientific, Inc.) for 20 min at room temperature, and
subsequently counterstained with DAPI (5 µg/ml) for 10 min in
darkness at room temperature. The positive cells were counted under
a fluorescence microscope (Nikon Corporation).
ELISA
The levels of IL-1β, IL-6, IL-8 and IL-23A in
supernatants from HaCaT cells were examined using ELISA kits (cat.
no. H002-1-2, H007-1-1, H008-1-1 and H020, respectively; Nanjing
Jiancheng Bioengineering Institute) according to the recommended
protocols. The optical density value was determined using a BioTek
microplate reader (BioTek; Agilent Technologies, Inc.) at 450
nm.
Immunofluorescence colocalization
analysis
Cells that were cultured on slides in 6-well plates
(3x105 cells/ml) were cultured with MitoTracker Red (500
nM; Invitrogen; Thermo Fisher Scientific, Inc.) at 37˚C for 8 min,
followed by fixation in 4% paraformaldehyde for 1 h at room
temperature and permeation with 0.1% Triton-X-100 for 15 min at
room temperature. Subsequently, cells were successively exposed to
LC3B antibody (1:1,000; cat. no. ab232940; Abcam) for 1 h at room
temperature and a fluorescent Alexa Fluor® 488-conjugated goat
anti-rabbit secondary antibody (1:400; cat. no. ab150077; Abcam). A
fluorescence microscope (Nikon Corporation) was used to capture
images.
JC-1 staining
For examination of the mitochondrial membrane
potential (MMP), JC-1 staining was implemented. Briefly, the
collected HaCaT cells were cultured with JC-1 (cat. no. 420200-5MG;
MilliporeSigma) for 15 min at 37˚C, and then evaluated using a
fluorescence microscope. Red fluorescence represented a
potential-dependent aggregation in the mitochondria, reflecting the
mitochondrial membrane potential. Green emission of the dye
represented the monomeric form of JC-1, appearing in the cytosol
after mitochondrial membrane depolarization. Excitation and
emission wavelengths of 514 and 529 nm, respectively, were applied
for the detection of the monomeric form of JC-1, while excitation
and emission wavelengths of 585 and 590 nm, respectively, were used
to detect the aggregation of JC-1.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from sample HaCaT cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Reverse transcription of first-strand cDNA was performed using
PrimeScript RT Master Mix (Takara Bio, Inc.), followed by qPCR
using the SYBR Premix Ex Taq™ II kit (Takara Bio, Inc.) according
to the manufacturer's protocol. The PCR program was 95˚C for 3 min
and 35 cycles of denaturation at 95˚C for 30 sec, annealing at 60˚C
for 30 sec and extension at 72˚C for 1 min, with a final extension
step at 72˚C for 7 min. The primer sequences used for qPCR were as
follows: PIN1 forward, 5'-CCGCAGCTCAGGCCG-3' and reverse,
5'-GCAAACGAGGCGTCTTCAAA-3'; and GAPDH forward,
5'-GGGAAACTGTGGCGTGAT-3' and reverse, 5'-GAGTGGGTGTCGCTGTTGA-3'.
The relative mRNA level was normalized to that of GAPDH using the
2-ΔΔCq method (24).
Western blot analysis
Total protein was isolated from HaCaT cells using
RIPA buffer (Auragene Bioscience Co. BCA Protein Assay kit (Beijing
Dingguo Changsheng Biotechnology Co., Ltd.) was performed to detect
the protein concentration according to the manufacturer's
instructions. A total of 30 µg protein per well were resolved by
10% SDS-PAGE (Bio-Rad Laboratories, Inc.) and transferred to PVDF
membranes (MilliporeSigma). Subsequently, the membranes were
blocked with 5% skim milk for 1 h at 25˚C, and successively
incubated with primary antibodies against PIN1 (1:1,000; cat. no.
ab192036; Abcam), keratin (KRT)1 (1:1,000; cat. no. ab185628;
Abcam), KRT6B (1:1,000; cat. no. ab154313; Abcam), cyclooxygenase-2
(Cox2; 1:1,000; cat. no. ab179800; Abcam), inducible nitric oxide
synthase (iNOS; 1:1,000; cat. no. ab178945; Abcam), LC3B (1:1,000;
cat. no. ab63817; Abcam), Beclin-1 (1:1,000; cat. no. ab207612;
Abcam), PTEN induced kinase 1 (PINK1; 1:1,000; cat. no. ab216144;
Abcam), Parkin (1:1,000; cat. no. ab77924; Abcam), p62 (1:1,000;
cat. no. ab207305; Abcam), COX IV (1:1,000; cat. no. ab16056;
Abcam) or GAPDH (1:1,000; cat. no. ab8245; Abcam) overnight at 4˚C.
Then, the membranes were incubated with anti-mouse or anti-rabbit
secondary antibodies (cat. nos. sc-2004 or sc-2005; 1:5,000; Santa
Cruz Biotechnology, Inc.). An ECL detection system (Amersham;
Cytiva) was adopted for the visualization of protein bands in
accordance with the recommended specifications, while the band
density was semi-quantified using ImageJ software (version 1.49;
National Institutes of Health).
Statistical analysis
All experimental data were analyzed using SPSS 23.0
software (IBM Corp.) and are presented as the mean ± SD from at
least three independent experiments. For the analysis of
differences between two groups, unpaired, two-tailed Student's
t-test was employed, while one-way ANOVA with the Bonferroni post
hoc test was adopted for comparisons among multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
PIN1 is highly expressed in M5-induced
HaCaT cells
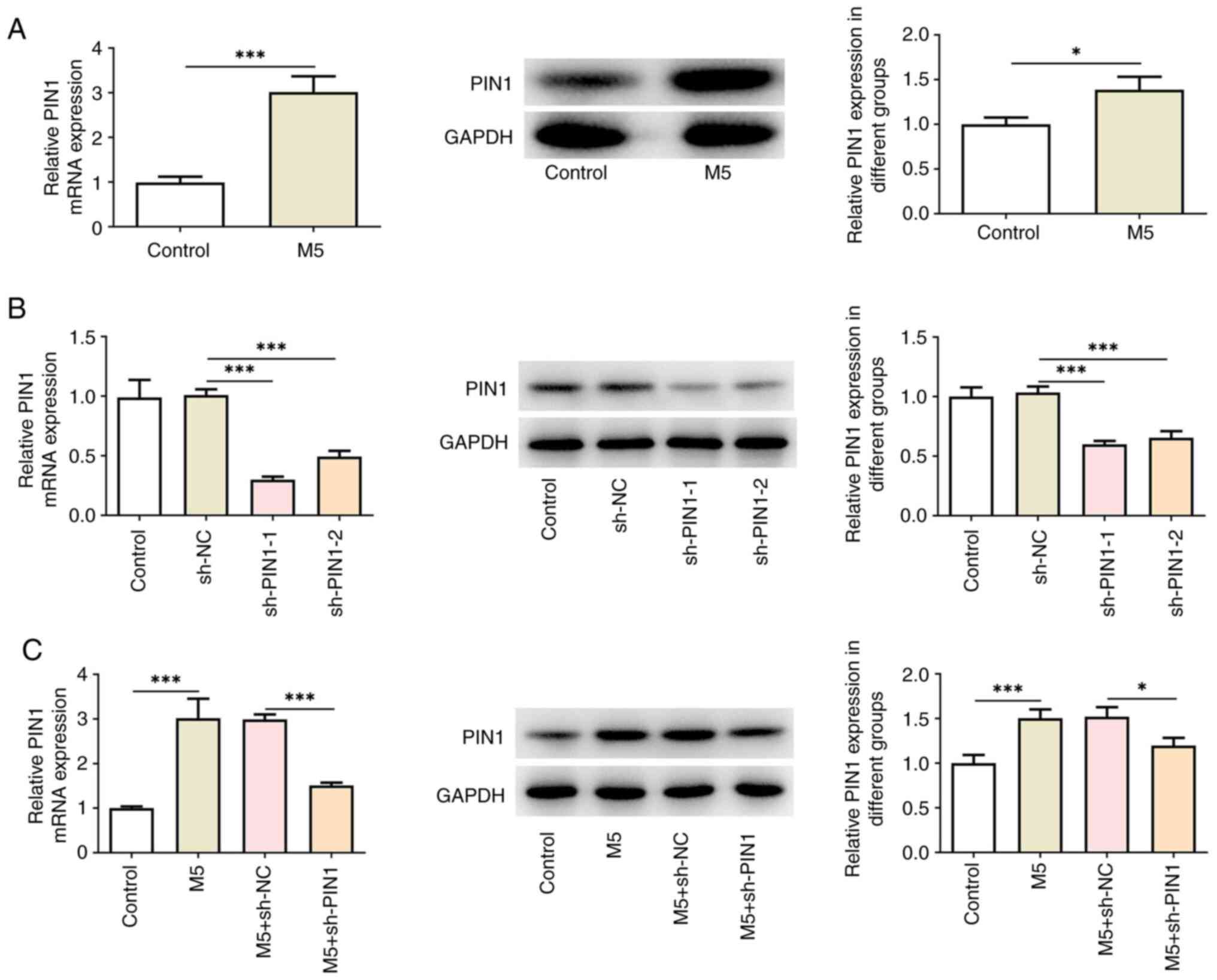
To investigate the role of PIN1 in psoriasis, the
expression levels of PIN1 in M5-induced HaCaT cells were first
detected. RT-qPCR and western blotting showed that PIN1 expression
was significantly upregulated in M5-induced HaCaT cells compared
with untreated HaCaT cells (Fig.
1A). Subsequently, PIN1 was silenced and the transfection
efficiency is demonstrated in Fig.
1B. Of note, sh-PIN1-1 had an improved knockdown effect, and
thus, was selected for subsequent assays (referred to as sh-PIN1).
In addition, sh-PIN1 also reduced PIN1 expression in HaCaT cells
treated with M5 (Fig. 1C).
PIN1 silencing inhibits M5-induced
hyperproliferation and inflammation in HaCaT cells
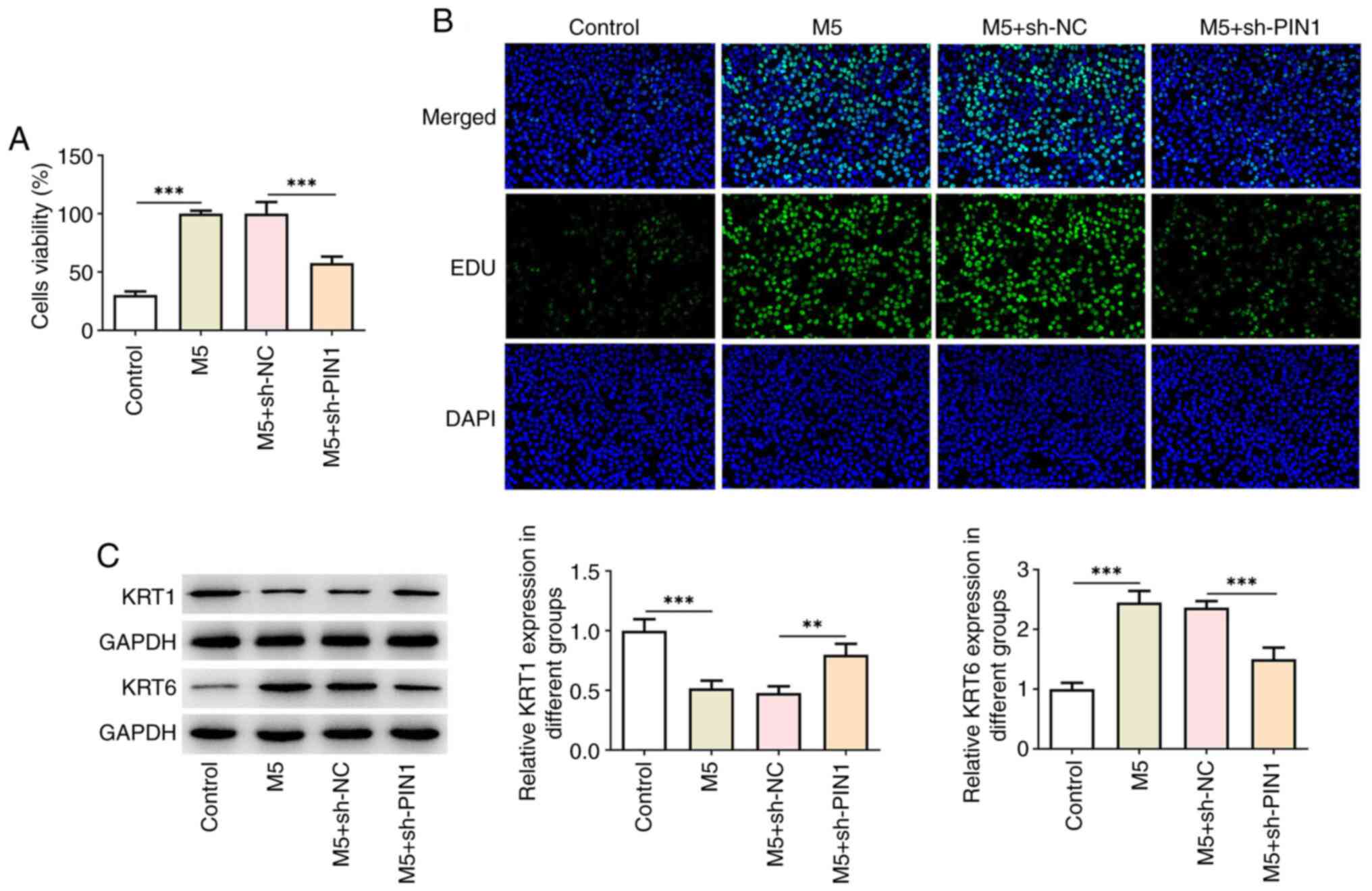
To investigate the biological roles of PIN1 in
M5-induced HaCaT cells, cell proliferation was initially examined
using a CCK-8 assay. As shown in Fig.
2A, M5 increased the cell viability, while PIN1 silencing
repressed the proliferation of M5-induced HaCaT cells. Furthermore,
EdU staining revealed that M5 increased the number of
positive-green cells, which was then reduced following transfection
with sh-PIN1 (Fig. 2B). In
addition, M5 reduced KRT1 levels and increased KRT6 levels, which
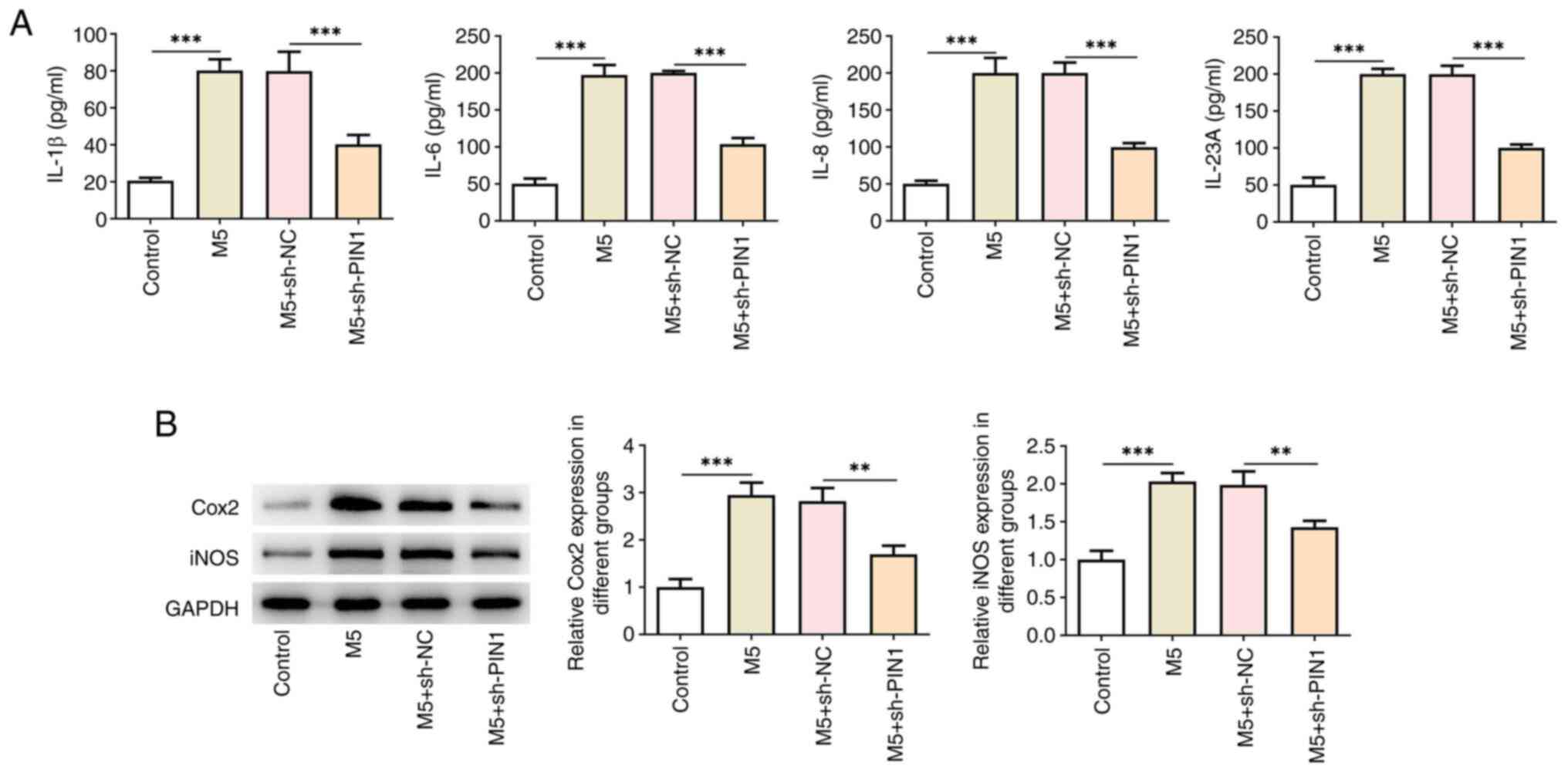
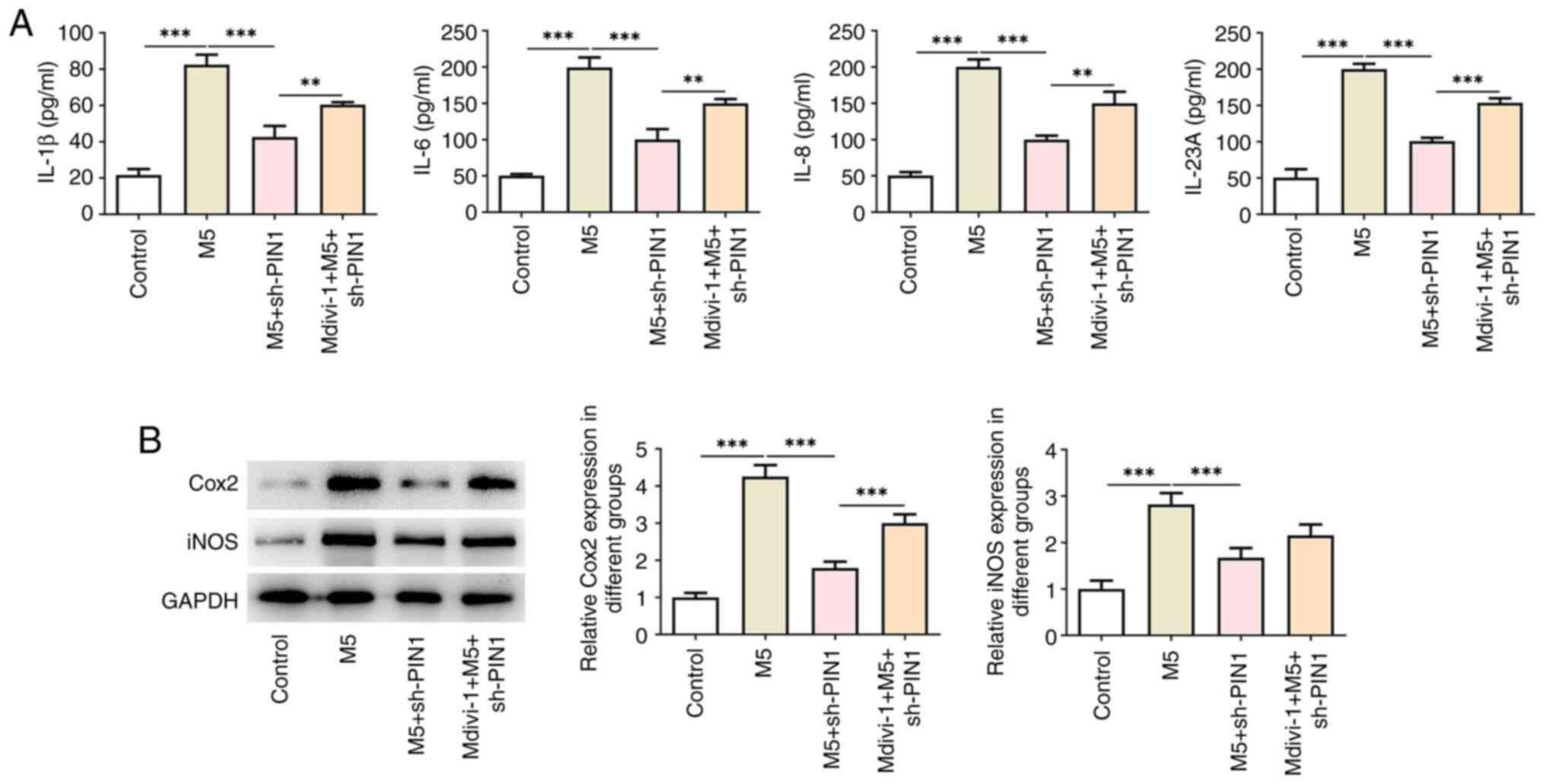
was reversed after silencing of PIN1 (Fig. 2C). ELISA results illustrated that
the increased levels of IL-1β, IL-6, IL-8 and IL-23A in HaCaT cells
due to M5 stimulation were decreased by sh-PIN1 (Fig. 3A). Western blotting indicated the
increase in the levels of Cox2 and iNOS following M5 treatment,
while PIN1 silencing had the opposite effect (Fig. 3B).
Knockdown of PIN1 activates M5-induced
mitochondrial autophagy in HaCaT cells
As revealed in Fig.
4A, LC3 and MitoTraker levels in double-stained cells were
examined using an immunofluorescence assay. The data indicated that
M5 stimulation markedly reduced the levels of LC3 and MitoTraker,
whereas knockdown of PIN1 reversed the effects of M5 on the
suppressive levels of LC3 and MitoTraker in HaCaT cells.
Additionally, M5 stimulation decreased the protein levels of
LC3II/LC3I, Beclin-1, PINK1 and Parkin (mitochondria), whereas it
promoted the production of p62 and Parkin (cytoplasm). However, the
trend was reversed by PIN1 silencing (Fig. 4B). Furthermore, JC-1 staining
demonstrated that M5 induction increased JC-1 aggregates, whereas
it reduced JC-1 monomers, while PIN1 silencing had the opposite
effect (Fig. 4C).
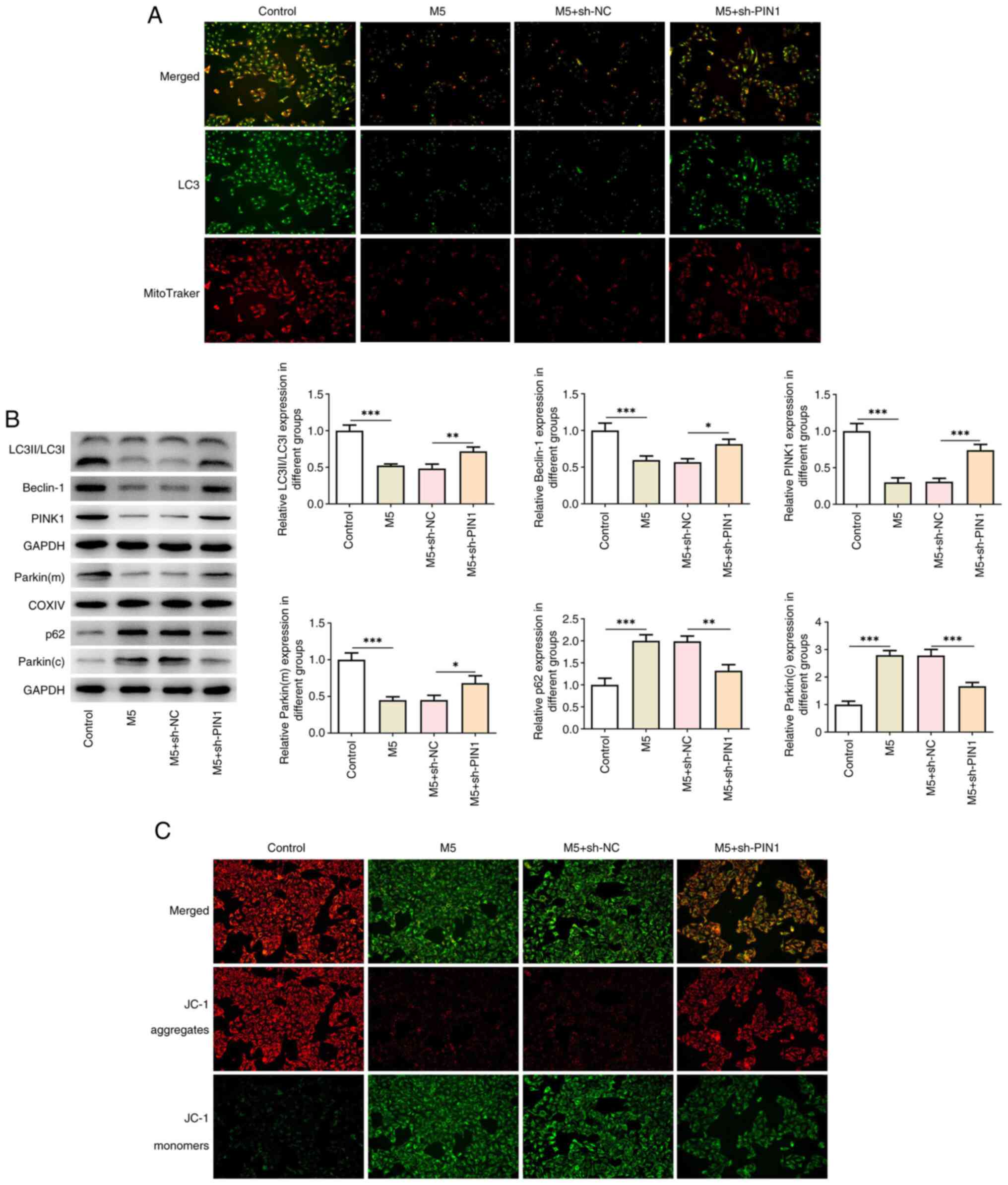 | Figure 4Knockdown of PIN1 activates M5-induced
mitochondrial autophagy in HaCaT cells. (A) The levels of LC3 and
MitoTraker in double-stained cells were measured by
immunofluorescence assay (magnification, x200). (B) The protein
levels of LC3II/LC3I, Beclin-1, PINK1, Parkin (mitochondrion), p62
and Parkin (cytoplasm) in M5-induced HaCaT cells transfected with
sh-PIN1 were detected by western blot analysis. (C) JC-1 staining
was used to assess the mitochondrial membrane potential
(magnification, x200). Results are the mean ± SD.
*P<0.05, **P<0.01 and
***P<0.001. PIN1, peptidyl-prolyl cis/trans
isomerase, NIMA-interacting 1; Cox, cyclooxygenase; shRNA, short
hairpin RNA; NC, negative control; SD, standard deviation. |
PIN1 silencing ameliorates M5-induced
hyperproliferation and inflammation of HaCaT cells by activating
mitochondrial autophagy
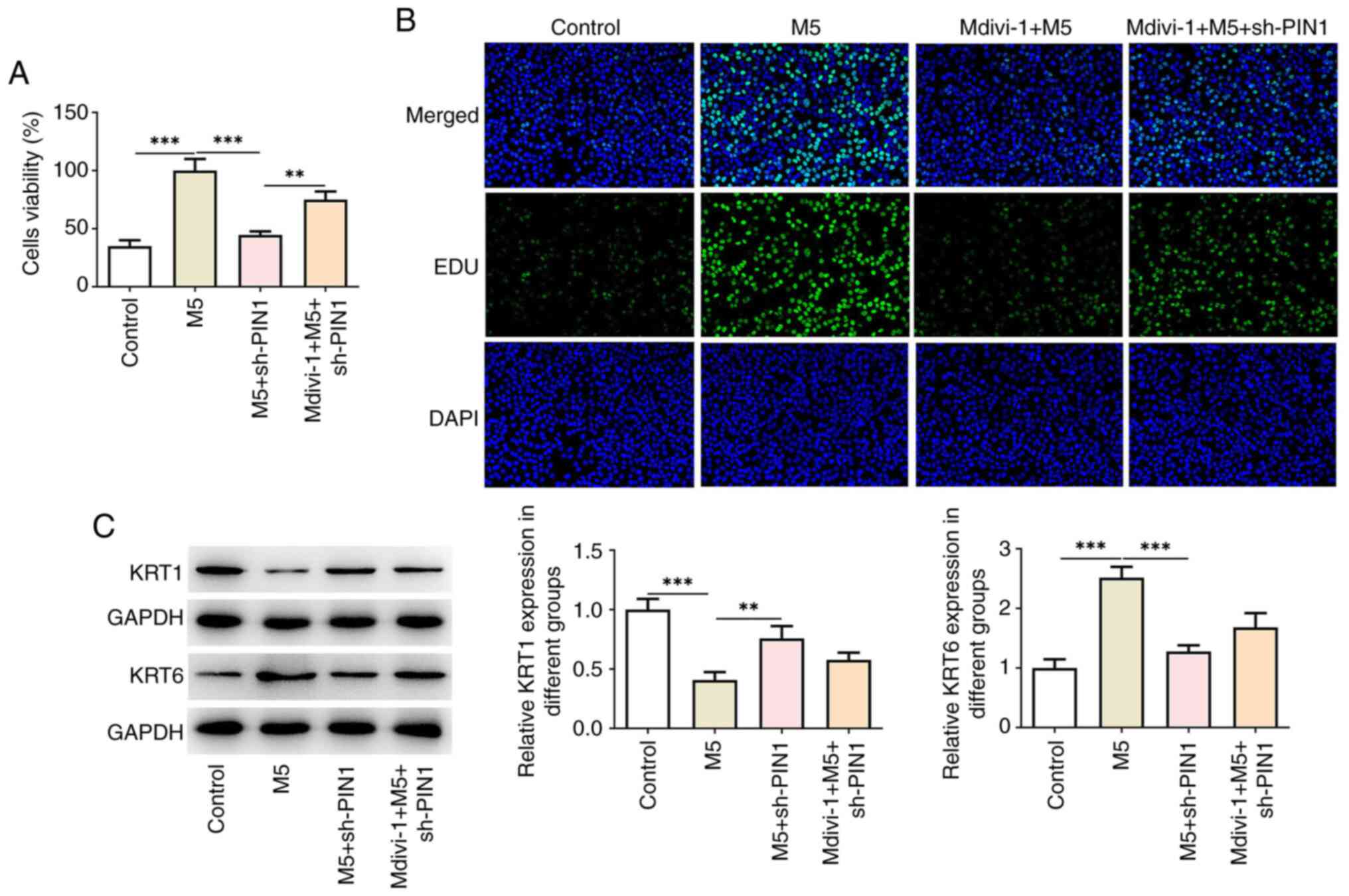
To explore the role of mitochondrial autophagy in
PIN1 silencing, the mitochondrial autophagy inhibitor Mdivi-1 was
used to treat cells. As illustrated in Fig. 5A, Mdivi-1 significantly increased
the reduced cell proliferation following PIN1 silencing.
Consistently, EdU staining revealed that the number of positive
cells was increased after Mdivi-1 treatment (Fig. 5B). In addition, western blotting
indicated that the KRT1 levels were decreased, while the KRT6
levels were increased following treatment with Mdivi-1 (Fig. 5C). Furthermore, Mdivi-1 treatment
increased the levels of IL-1β, IL-6, IL-8 and IL-23A in
PIN1-silenced HaCaT cells (Fig.
6A), which was consistent with the results of western blotting,
which indicated that the levels of Cox2 and iNOS were increased in
M5-induced HaCaT cells transfected with sh-PIN1 (Fig. 6B).
Discussion
Psoriasis results from a combination of genetic and
environmental factors, with environmental triggers including
stress, infections, alcohol, tobacco, drugs and obesity (25). Keratinocytes can function as innate
immune cells, secreting inflammatory factors, especially
chemokines, and also expressing various pattern recognition
receptors, such as toll-like receptors, which are capable of
initiating an intrinsic immune response, presenting antigens to T
cells, and further exacerbating inflammatory responses in the skin
(26,27).
PIN1 is a unique enzyme that isomerizes the target
protein proline residues (28). It
has been reported that PIN1 downregulated serine/threonine kinase 3
(STK3) by promoting its ubiquitination and leads to the
dysregulation of Hippo signaling, thereby causing carcinogenic
signaling and melanoma. Hippo signaling pathway consists of a core
kinase cascade of STK3, LATS1/2, YAP and TAZ. When STK3 was
downregulated, the Hippo signaling was repressed (29). Jeong et al (30) reported that PIN1 stimulation
facilitated the expression of pro-inflammatory proteins by
triggering NF-κB, cyclic AMP response element-binding protein
(CREB) and CCAAT/enhancer binding protein (C/EBP), suggesting that
PIN1 is a prospective therapeutic target for the treatment of
rheumatoid arthritis. It has also been reported that PIN1 inhibitor
could improve experimental autoimmune encephalomyelitis, and reduce
inflammation and demyelination of the central nervous system,
indicating the pivotal role of PIN1 in chronic inflammation
(31). In the present study, PIN1
expression was revealed to be increased in M5-induced HaCaT cells.
Silencing of PIN1 had inhibitory effects on HaCaT cell
proliferation and the inflammatory response. A previous study
revealed that the natural PIN1 inhibitor Juglone inhibited wound
healing by promoting skin cell migration via the Rac1/cell division
cycle 42/PAK pathway, and it may be a potential candidate for wound
healing and skin regeneration (32). In addition, the inhibition of PIN1
has been reported to suppress the activation of NF-κB, CREB and
C/EBP induced by UVA irradiation, which is associated with the
malignant transformation of epidermal cells (33).
Activation of vitamin D receptor has been described
to attenuate venous endothelial cell dysfunction by decreasing
PIN1-mediated mitochondrial translocation of p66Shc, and thus,
reducing mitochondrial reactive oxygen species (ROS) (34). Feng et al (35) demonstrated that the inhibition of
PIN1 expression markedly decreased mitochondrial translocation of
p66Shc and subsequent ROS generation and apoptosis, thus mitigating
intestinal injury and secondary lung injury by using superior
mesenteric artery occlusion-induced rat I/R model and
hypoxia/reoxygenation (H/R)-induced Caco-2 cells. Stress factors
such as ROS lead to a gradual accumulation of mitochondrial DNA
mutations, as well as a reduction in intracellular MMP and
depolarization damage, and ultimately lead to cell death (36). Inhibition of PIN1 increases the
level of autophagy in senescent cells and cochlear hair cells
(37). A different study reported
that the transfection of SW-48 cells with PIN1 small interfering
RNA injured cancer cell proliferation and migration, while it
facilitated apoptosis and autophagy (38). Taken together, the aforementioned
studies suggested that PIN1 may regulate mitochondrial autophagy.
Additionally, sirtuin 3 triggers mitochondrial autophagy in HaCaT
cells by triggering the FOXO3a/Parkin pathway, thus ameliorating
TNF-α-induced psoriasis (39). The
present study revealed that PIN1 silencing activated M5-induced
mitochondrial autophagy in HaCaT cells. To verify the function of
mitochondrial autophagy in the regulation of PIN1 silencing in
M5-induced HaCaT cells, the mitochondrial autophagy inhibitor
Mdivi-1 was applied to treat cells. The data showed that Mdivi-1
treatment reversed the effects of PIN1 silencing on M5-induced
hyperproliferation and inflammation, implying the regulatory role
of mitochondrial autophagy in M5-induced HaCaT cells. Moreover, the
present study did not perform animal and clinical studies, which
will be involved in future experiments to confirm the findings of
the present study.
In conclusion, the data demonstrated that PIN1
silencing ameliorated the hyperproliferation and inflammation in
M5-induced HaCaT cells by triggering mitochondrial autophagy, which
revealed the potential of PIN1 for the treatment of psoriasis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SX and WY designed the study, drafted and revised
the manuscript. JL and HY analyzed the data and searched the
literature. All authors performed the experiments. SX and WY
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rendon A and Schäkel K: Psoriasis
pathogenesis and treatment. Int J Mol Sci. 20(1475)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kamiya K, Kishimoto M, Sugai J, Komine M
and Ohtsuki M: Risk factors for the development of psoriasis. Int J
Mol Sci. 20(4347)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tokuyama M and Mabuchi T: New treatment
addressing the pathogenesis of psoriasis. Int J Mol Sci.
21(7488)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tashiro T and Sawada Y: Psoriasis and
systemic inflammatory disorders. Int J Mol Sci.
23(4457)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hao Y, Zhu YJ, Zou S, Zhou P, Hu YW, Zhao
QX, Gu LN, Zhang HZ, Wang Z and Li J: Metabolic syndrome and
psoriasis: Mechanisms and future directions. Front Immunol.
12(711060)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lowes MA, Russell CB, Martin DA, Towne JE
and Krueger JG: The IL-23/T17 pathogenic axis in psoriasis is
amplified by keratinocyte responses. Trends Immunol. 34:174–181.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hawkes JE, Yan BY, Chan TC and Krueger JG:
Discovery of the IL-23/IL-17 signaling pathway and the treatment of
psoriasis. J Immunol. 201:1605–1613. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Griffiths CEM, Armstrong AW, Gudjonsson JE
and Barker JNWN: Psoriasis. Lancet. 397:1301–1315. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kamata M and Tada Y: Crosstalk:
Keratinocytes and immune cells in psoriasis. Front Immunol.
14(1286344)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Montero-Vilchez T,
Segura-Fernández-Nogueras MV, Pérez-Rodríguez I, Soler-Gongora M,
Martinez-Lopez A, Fernández-González A, Molina-Leyva A and
Arias-Santiago S: Skin barrier function in psoriasis and atopic
dermatitis: Transepidermal water loss and temperature as useful
tools to assess disease severity. J Clin Med.
10(359)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Korman NJ: Management of psoriasis as a
systemic disease: What is the evidence? Br J Dermatol. 182:840–848.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Malter JS: Pin1 and Alzheimer's disease.
Transl Res. 254:24–33. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Caligiuri I, Vincenzo C, Asano T, Kumar V
and Rizzolio F: The metabolic crosstalk between PIN1 and the tumour
microenvironment. Semin Cancer Biol. 91:143–157. 2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li J, Mo C, Guo Y, Zhang B, Feng X, Si Q,
Wu X, Zhao Z, Gong L, He D and Shao J: Roles of peptidyl-prolyl
isomerase Pin1 in disease pathogenesis. Theranostics. 11:3348–3358.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cheng CW and Tse E: PIN1 in cell cycle
control and cancer. Front Pharmacol. 9(1367)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nishi M, Akutsu H, Masui S, Kondo A,
Nagashima Y, Kimura H, Perrem K, Shigeri Y, Toyoda M, Okayama A, et
al: A distinct role for Pin1 in the induction and maintenance of
pluripotency. J Biol Chem. 286:11593–11603. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nakamura K, Kosugi I, Lee DY, Hafner A,
Sinclair DA, Ryo A and Lu KP: Prolyl isomerase Pin1 regulates
neuronal differentiation via β-catenin. Mol Cell Biol.
32:2966–2978. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lee YM, Shin SY, Jue SS, Kwon IK, Cho EH,
Cho ES, Park SH and Kim EC: The role of PIN1 on odontogenic and
adipogenic differentiation in human dental pulp stem cells. Stem
Cells Dev. 23:618–630. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Luo ML, Gong C, Chen CH, Lee DY, Hu H,
Huang P, Yao Y, Guo W, Reinhardt F, Wulf G, et al: Prolyl isomerase
Pin1 acts downstream of miR200c to promote cancer stem-like cell
traits in breast cancer. Cancer Res. 74:3603–3616. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rustighi A, Zannini A, Tiberi L, Sommaggio
R, Piazza S, Sorrentino G, Nuzzo S, Tuscano A, Eterno V, Benvenuti
F, et al: Prolyl-isomerase Pin1 controls normal and cancer stem
cells of the breast. EMBO Mol Med. 6:99–119. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kanamoto M, Takahagi S, Aoyama S, Kido Y,
Nakanishi M, Naito M, Kanna M, Yamamotoya T, Tanaka A, Hide M, et
al: The expression of prolyl isomerase Pin1 is expanded in the skin
of patients with atopic dermatitis and facilitates IL-33 expression
in HaCaT cells. J Dermatol. 50:462–471. 2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xu F, Armstrong R, Urrego D, Qazzaz M,
Pehar M, Armstrong JN, Shutt T and Syed N: The mitochondrial
division inhibitor Mdivi-1 rescues mammalian neurons from
anesthetic-induced cytotoxicity. Mol Brain. 9(35)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
So EC, Hsing CH, Liang CH and Wu SN: The
actions of mdivi-1, an inhibitor of mitochondrial fission, on
rapidly activating delayed-rectifier K+ current and
membrane potential in HL-1 murine atrial cardiomyocytes. Eur J
Pharmacol. 683:1–9. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Raharja A, Mahil SK and Barker JN:
Psoriasis: A brief overview. Clin Med (Lond). 21:170–173.
2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhou X, Chen Y, Cui L, Shi Y and Guo C:
Advances in the pathogenesis of psoriasis: From keratinocyte
perspective. Cell Death Dis. 13(81)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen HL, Lo CH, Huang CC, Lu MP, Hu PY,
Chen CS, Chueh DY, Chen P, Lin TN, Lo YH, et al: Galectin-7
downregulation in lesional keratinocytes contributes to enhanced
IL-17A signaling and skin pathology in psoriasis. J Clin Invest.
131(e130740)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yu JH, Im CY and Min SH: Function of PIN1
in cancer development and its inhibitors as cancer therapeutics.
Front Cell Dev Biol. 8(120)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kim G, Bhattarai PY, Lim SC, Kim JY and
Choi HS: PIN1 facilitates ubiquitin-mediated degradation of
serine/threonine kinase 3 and promotes melanoma development via TAZ
activation. Cancer Lett. 499:164–174. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jeong HG, Pokharel YR, Lim SC, Hwang YP,
Han EH, Yoon JH, Ahn SG, Lee KY and Kang KW: Novel role of Pin1
induction in type II collagen-mediated rheumatoid arthritis. J
Immunol. 183:6689–6697. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ge ZZ, Wu YB, Xue ZY, Zhang K and Zhang
RX: The therapeutic effects of the peptidyl-prolyl cis/trans
isomerase Pin1 inhibitor juglone on animal-model experimental
autoimmune encephalomyelitis. J Physiol Pharmacol: Aug 6, 2021
(Epub ahead of print).
|
|
32
|
Wahedi HM, Park YU, Moon EY and Kim SY:
Juglone ameliorates skin wound healing by promoting skin cell
migration through Rac1/Cdc42/PAK pathway. Wound Repair Regen.
24:786–794. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Quyen BT, Choi HK and Kang KW: Pin1 is
required for ultraviolet A-stimulated cyclooxygenase-2 induction in
mouse epidermal cells. Cancer Lett. 335:31–40. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Han YC, Liu YT, Zhang H, Xu Y, Liu J, Chen
H, Song N, Qin DL and Yang S: VDR alleviates endothelial cell
injury in arteriovenous fistula through inhibition of
P66Shc-mediated mitochondrial ROS. Sci Rep.
13(11088)2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Feng D, Yao J, Wang G, Li Z, Zu G, Li Y,
Luo F, Ning S, Qasim W, Chen Z and Tian X: Inhibition of
p66Shc-mediated mitochondrial apoptosis via targeting
prolyl-isomerase Pin1 attenuates intestinal ischemia/reperfusion
injury in rats. Clin Sci (Lond). 131:759–773. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lemasters JJ: Selective mitochondrial
autophagy, or mitophagy, as a targeted defense against oxidative
stress, mitochondrial dysfunction, and aging. Rejuvenation Res.
8:3–5. 2005.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lv Z, Zhang Y, Cao H, Liu Q, Feng X, Yin H
and Wang B: PIN1 protects auditory hair cells from senescence via
autophagy. PeerJ. 10(e14267)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gholamzadeh Khoei S, Saidijam M, Amini R,
Jalali A and Najafi R: Impact of PIN1 inhibition on tumor
progression and chemotherapy sensitivity in colorectal cancer. J
Gastrointest Cancer. 53:299–310. 2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yanli M, Yu W and Yuzhen L: Elevated SIRT3
Parkin-dependently activates cell mitophagy to ameliorate
TNF-α-induced psoriasis-related phenotypes in HaCaT cells through
deacetylating FOXO3a for its activation. Arch Dermatol Res.
315:847–857. 2023.PubMed/NCBI View Article : Google Scholar
|