Introduction
Primary liver cancer ranks sixth in incidence among
malignant tumors and third in mortality, representing a significant
global public health issue (1).
According to statistics, it is currently the fourth most common
malignant tumor and the second leading cause of tumor-related
deaths in China, posing a serious threat to the life and health of
the Chinese population (1,2). In recent years, improvements have
been made to the surgical methods and hepatic artery infusion
chemotherapy (HAIC) regimens of liver cancer. The FOLFOX regimen,
based on oxaliplatin, has significantly improved tumor response
rates, with high disease control rate and objective response rates
(3-6).
Furthermore, the combination of HAIC and immunotherapy has been
suggested to further increase the surgical conversion rate and
objective response rate (7-11).
Patients with liver cancer often have underlying
liver cirrhosis, which is commonly associated with portal
hypertension and complications, such as esophageal varices and
high-pressure gastric diseases. In the Xiaogan hospital affiliated
to Wuhan University of Science and Technology (Hubei, China), three
cases of giant gastric ulcer complications occurred during the
process of HAIC or combined immunotherapy, with clinical
manifestations of abdominal pain and severe nausea and vomiting,
which were discovered upon re-examination by gastroscopy. In
addition, patients with tumors receiving immunotherapy may also
experience gastrointestinal adverse reactions, including nausea,
vomiting, loss of appetite, bloating, constipation, diarrhea and
abdominal pain (12-14).
A small number (<1%) of patients may develop serious
complications, such as gastrointestinal perforation (12). The cause of the adverse reactions
in this case needs to be further identified, to determine whether
it is due to HAIC or immunotherapy. The present study provides a
detailed analysis of this issue.
Case report
Case 1
A 54-year-old male patient was admitted to Xiaogan
hospital affiliated to Wuhan University of Science and Technology
(Hubei, China) in October 2021, due to multiple liver lesions found
4 years after liver cancer surgery. During a routine medical
examination in March 2017, liver MRI revealed a space-occupying
lesion, which was subsequently treated with left hepatectomy and
microwave-assisted liver tumor ablation at another hospital in
April 2017. The details of the surgery are unknown (the patient
could not provide details). The patient underwent regular follow-up
examinations and was diagnosed with a slightly hyperechoic nodule
in the right anterior lobe of the liver measuring 2.08x1.91 cm by
color Doppler ultrasound during an outpatient visit at the Xiaogan
hospital affiliated to Wuhan University of Science and Technology
(Hubei, China) in December 2020. The nodule was considered to be a
proliferative nodule, but it was not given much attention when it
was found to have grown larger in subsequent regular follow-up
examinations every 3 months as the nodules were not significantly
enlarged at the time and were considered hyperplastic, they were
not considered non-malignant. In October 2021, a color Doppler
ultrasound revealed a slightly hyperechoic nodule in the right
anterior lobe of the liver measuring 4.76x4.33 cm. A subsequent
liver CT scan with contrast enhancement showed multiple low-density
lesions in the right anterior lobe of the liver, with the largest
one measuring 5.0x4.4 cm and the smallest one measuring 2.2x2.1 cm.
The lesions were enhanced during the arterial phase and showed
decreased density during the delayed phase. The branch of the
portal vein in the right anterior lobe of the liver was poorly
displayed, and the possibility of multiple malignant tumors in the
right anterior lobe of the liver was suspected.
The patient underwent a bone scan and chest CT, but
no metastases were found. Esophagogastric gastric varices and
ulcers were not observed by gastroscopy. The patient then underwent
a CT-guided liver biopsy of the right lobe liver mass. The
pathological results of the biopsy showed liver cancer consistent
with hepatocellular carcinoma. The results of immunohistochemistry
(IHC) (Fig. S1) showed that
Alpha-fetoprotein (AFP; Fig. S1A)
was negative, Hepatocyte exhibited focal positivity (Fig. S1B), Arg-1 displayed weak
positivity (Fig. S1C), was
positive for CD34 (Fig. S1D),
negative for CK19 (Fig. S1E), p53
exhibited a mutated pattern (Fig.
S1F), and Ki-67 labeling index of ~10% (Fig. S1G). After excluding
contraindications, the patient underwent transarterial
chemoembolization (TACE). The procedure was successful, but the
patient experienced pain in the liver area, mild liver dysfunction
and gastrointestinal symptoms, such as nausea and vomiting, after
the surgery. Supportive treatment was given, and the condition of
the patient improved.
A follow-up examination with abdominal pain and
contrast-enhanced CT in December 2021 showed that the lesion had
decreased in size compared with previous examinations. The patient
underwent hepatic arterial angiography and placement of a hepatic
artery catheter after exclusion of contraindications. Subsequently,
the patient received continuous HAIC with FOLFOX4 regimen [day 1:
oxaliplatin (100 mg/m2) over 2 h, calcium folinate (200
mg/m2) over 2 h, 5-fluorouracil (5-FU; 400
mg/m2) over 15 min and 5-FU (600 mg/m2) over
22 h; day 2: calcium folinate (200 mg/m2) over 2 h, 5-FU
(400 mg/m2) over 15 min and 5-FU (600 mg/m2)
over 22 h]. The chemotherapy was well-tolerated, and the patient
did not report any significant discomfort. However, 7 days after
the procedure, the patient experienced paroxysmal upper abdominal
pain without any clear triggering factor, which did not improve
with self-administration of proton pump inhibitor (PPI) and
prokinetic drugs (specific medications unknown). After 10 days, a
follow-up abdominal CT scan showed postoperative changes in the
liver, liver cirrhosis, left hepatic duct dilation and bilateral
kidney stones.
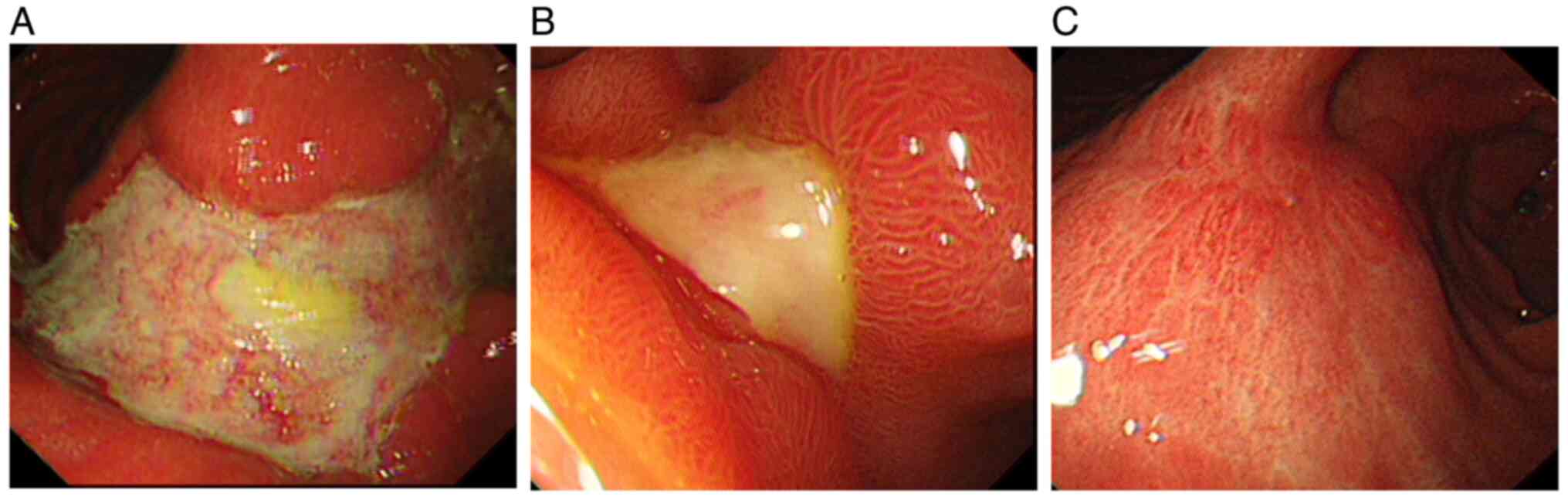
A gastroscopy performed 3 days later revealed a big
irregular ulcer of ~3.0x8.0 cm on the anterior wall of the gastric
body and angle, with a foul exudate-covered base and mucosal edema
and congestion in the gastric fundus (Fig. 1A). Multiple biopsies were taken,
and the pathological report indicated ulcerative changes. The
patient was treated with pantoprazole sodium 40 mg administered
intravenously twice daily, famotidine 20 mg intravenous infusion
before bedtime and rebamipide 0.1 g orally three times daily,
resulting in significant symptom improvement. The patient was
discharged and continued treatment with oral pantoprazole sodium
capsules 40 mg orally twice daily, famotidine 20 mg orally before
bedtime, and rebamipide sodium 0.1 g orally three times daily for
treatment. A follow-up gastroscopy conducted in February 2022,
revealed that the size of the big irregular ulcer on the anterior
wall of the gastric body and angle had decreased to ~3.0x1.5 cm
(Fig. 1B). Subsequent gastroscopy
performed in April 2022 showed that the ulcer had healed, leaving
behind a scar (Fig. 1C).
Case 2
A 58-year-old male patient initially presented to
Xiaogan hospital affiliated to Wuhan University of Science and
Technology (Hubei, China) with 1-week epigastric pain in March
2021, during which a CT scan revealed a mass with a cross-sectional
diameter of approximately 6.4x5.6 cm in the lower segment of the
right posterior lobe and caudate lobe of the liver. The mass showed
heterogeneous moderate enhancement in the arterial phase and
decreased enhancement in the delayed phase. Esophagogastric fundal
varices and ulcers were not found by gastroscopy in early March.
The patient underwent TACE followed by three rounds of
immunotherapy with camrelizumab and lenvatinib. Camrelizumab
injection, 200 mg, was administered in March, April and May, 2021.
Lenvatinib, 8 mg, was orally administered once daily as targeted
therapy. The patient was diagnosed with liver tumor with multiple
intrahepatic metastases during a laparoscopic exploration and tumor
biopsy under general anesthesia, ultrasound and endoscopy in June
2021. The postoperative pathology indicated necrosis of tumor
cells, and the patient was discharged after recovery.
Between June 2021 and November 2021 (21 days between
treatments per cycle), the patient underwent eight cycles of
targeted immunotherapy with oral lenvatinib (12 mg) and intravenous
camrelizumab (200 mg). In December 2022 the patient's tumor
progression was assessed, and they subsequently underwent a hepatic
artery angiography and left hepatic artery catheterization the next
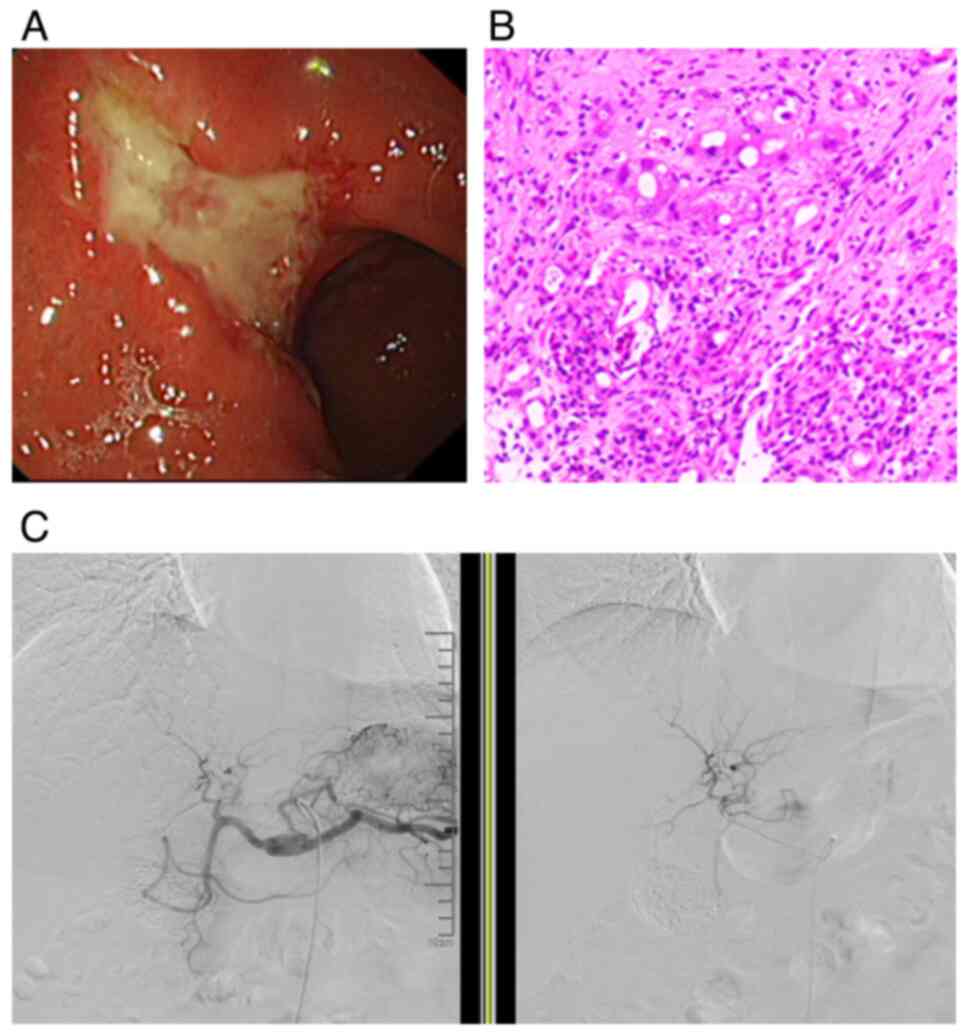
day (Fig. 2C). An endoscopy 26
days later revealed a large ulcer with uneven and edges that had
irregular, hole-like patterns (~3.0x4.0 cm) covered in pus in the
lesser curvature of the gastric antrum, with the surrounding mucosa
forming a ramp-like bulge that was hard and brittle. Eight biopsies
were taken, and pathology results showed gastric ulceration with
partially high-grade dysplasia (data not shown), indicating that
adenocarcinoma could not be excluded. Further examination via
ultrasound endoscopy in January 2022 revealed a thickened and
irregular heterogeneous hyperechoic lesion with an interrupted
mucosal layer and unclear boundaries, with the intrinsic muscle
layer at the site of the gastric lesion (Fig. 2A). The pathology revealed a gastric
ulcer with concavity and mucosal epithelial dysplasia, with some
high-grade intraepithelial neoplasia (Fig. 2B).
In January 2022, after discussion with the
multidisciplinary team and family consultation, the patient
underwent a laparoscopic distal gastrectomy and a laparoscopic
right hepatectomy under general anesthesia. The postoperative
pathology report revealed the following findings: i) Stomach:
Chronic gastric ulcer with an ulcer size of ~4.0x3.5 cm, partial
glandular atypia, and no apparent surrounding mucosal or omental
involvement. The lymph nodes had not been invaded by the tumor
cells. ii) Liver tumor: Moderately differentiated hepatocellular
carcinoma with a nodule size of ~1.8x1.8 cm. No obvious
intravascular cancer embolus or significant tumor necrosis or
hemorrhage was observed and no invasion into the liver capsule was
noted. Gastric tissue IHC (Fig.
S2) showed the following: AFP(-) (Fig. S2A), hepatocyte(+) (Fig. S2B), CK19(-) (Fig. S2C), CK8/18(+) (Fig. S2D), CD10(-) (Fig. S2E), Ki-67 labeling index (~40%)
(Fig. S2F), Galectin-3(+)
(Fig. S2G), Arg-1(focal +)
(Fig. S2H), CD31 (vascular +)
(Fig. S2I) and carcinoembryonic
antigen(-) (Fig. S2J).
Case 3
A 28-year-old male patient was found to have
elevated AFP during a physical examination at Xiaogan hospital
affiliated to Wuhan University of Science and Technology (Hubei,
China) in April 2021. Contrast-enhanced CT of the liver revealed
left lobe liver mass, with the possibility of liver cancer. The
patient's serum AFP level was 1,263 ng/ml (normal range <20
ng/ml). In November 2021, the patient underwent laparoscopic left
hepatic lobectomy under general anesthesia. The postoperative
pathological examination showed that part of the liver resected was
low-grade hepatocellular carcinoma (nodule size, 2.0x1.5 cm), with
visible vascular cancer emboli, but no obvious nerve invasion or
residual cancer at the margin of the cut (data not shown). IHC
(Fig. S3) showed the following:
AFP (+), hepatocyte (partially positive), Arg-1 (focally positive),
CD34 (positive in stromal blood vessels), CD31 (positive in
interstitial blood vessels), CK8/18 (+), Glypican-3 (+), PCK (+)
and Ki-67 (~35%). The patient's recovery after surgery was smooth.
No esophagogastric fundal varices and ulcers were observed by
gastroscopy in December 2021.
Between December 2021 and May 2022 (21 days between
treatments per cycle), the patient underwent five cycles of HAIC +
liver arterial infusion chemotherapy + sintilimab immunotherapy
(FOLFOX4). Specifically, the dose was oxaliplatin (130 mg) + 5-FU
(600 mg) x 2 days + 5-FU (1,950 mg) x 2 days + sintilimab (200 mg).
During the fifth HAIC, the patient experienced significant
gastrointestinal reactions, such as nausea and vomiting, which
improved after symptomatic treatment. On the fifth day after HAIC,
they experienced epigastric pain at home without any obvious reason
for induction, and were treated with esomeprazole 20 mg orally once
daily and famotidine 20 mg orally once daily for acid suppression,
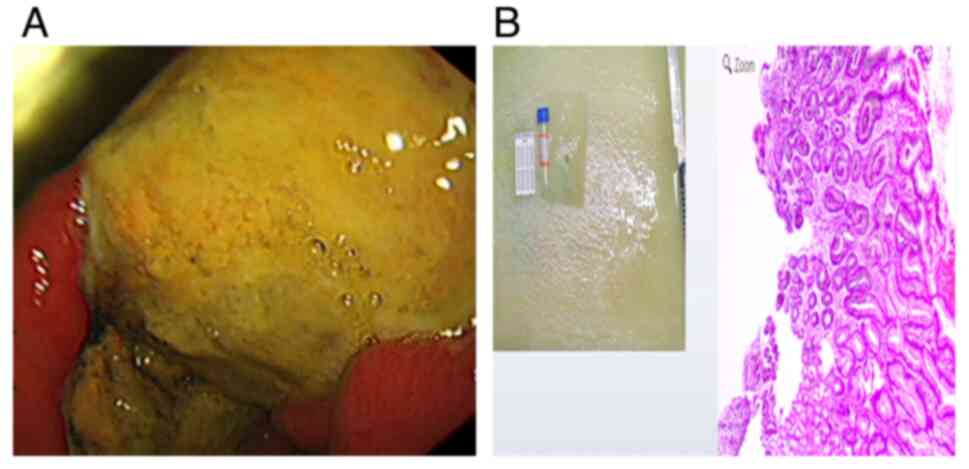
but the result was not satisfactory. Gastroscopy in May 2022
showed: mucosal congestion and edema in the lesser curvature of the
stomach, along with a large ulcer of 5.0x4.0 cm. Multiple biopsy
samples were taken. (Fig. 3A).
Endoscopic gastric biopsy pathology indicated chronic superficial
atrophic gastritis (Fig. 3B).
Gastroenterology consultation recommended the addition of
pantoprazole sodium injection 40 mg orally twice daily and
rabeprazole sodium 0.1 g orally three times daily for anti-ulcer
treatment for one week. The symptoms improved after treatment, and
the patient continued to take the above medications for 3 months
thereafter.
Histopathological review of
tissues
All IHC primary antibodies were from Beijing
Zhongshan Jinqiao Biotechnology, Co., including arginase-1 mouse
monoclonal antibody (cat. no. ZM-0328, clone number OT1IH4), CD34
mouse monoclonal antibody (cat. no. ZM-0046, clone number 10C9),
hepatocellular mouse monoclonal antibody (cat. no. ZM-0131, clone
number OCH1E5). Chromogenic reagents included improved iodine
oxidation for activating horseradish peroxidase (HRP),
diaminobenzidine (DAB) + substrate buffer, DAB + chromogenic agent
and DAB enhancer.
Paraffin sections were placed in fresh xylene and
soaked for 10 min x 3 times. Excess liquid was removed and the
sections placed in absolute ethanol and soaked for 3 min x 3 times.
Excess liquid was removed and the sections placed in 95% ethanol,
soaked for 3 min x 2 times and then soaked for 3 min x 2 times in
75% ethanol, rinsed with distilled water for 1 min and then place
in PBS buffer solution at room temperature.
The slide was placed in 0.01 M citrate buffer (PH
6.0), and microwaved at 100˚C. After natural cooling at room
temperature for ~20 min, no additional methods such as ice were
used to accelerate the cooling process. After reaching room
temperature, the slides were removed and rinsed with PBS buffer for
5 min 3 times for 10 min at room temperature.
An appropriate amount of endogenous peroxidase
blocking agent was added to the sections, which were incubated at
room temperature for 10 min and rinsed with PBS buffer solution for
3 min x 3 times.
The thickness of the tissue sections was between 4-6
µm, with a diameter of ≤2 centimeters. Then, 100 µl of primary
antibody was added and incubated at 37˚C for 60 min and rinsed with
PBS buffer solution at room temperature for 3 min 3 times.
Then 100 µl of enzyme-labeled goat anti-mouse IgG
polymer was added and incubated 37˚C for 20 min and rinsed with PBS
buffer solution at room temperature for 3 min 3 times.
An appropriate amount of freshly prepared DAB or AEC
chromogenic solution was added and incubated at room temperature
for 5-8 min.
The sections were rinsed with tap water, incubated
in hematoxylin staining solution for 20 sec, differentiated and
rinsed in running tap water until blue. The sections were
dehydrated as aforementioned, cleared and sealed
The staining results were observed and interpreted
under an optical microscope by qualified pathologists from Xiaogan
hospital affiliated to Wuhan University of Science and Technology
(Hubei, China).
Discussion
HAIC is a major treatment for advanced primary liver
cancer, with proven efficacy (15). There have been numerous reports on
the occurrence of gastric and duodenal mucosal lesions after HAIC
treatment (16); however, to the
best of our knowledge, no cases of HAIC or combined immunotherapy
leading to giant gastric ulcers have been reported. Notably, the
mortality rate of peptic ulcers in patients with cirrhosis has been
reported to be five times higher than that of the general
population (17). However, the
mechanism is not well understood. Different factors are said to be
involved, such as altered serum gastrin levels, decreased gastric
acid secretion, gastric mucosal blood flow and gastric mucosal
prostaglandin production, in addition to a correlation with
Helicobacter pylori infection. However, in all three cases,
gastric ulcers were excluded by preoperative gastroscopy.
Mucosal lesions of the gastric and duodenal mucosa
caused by simple HAIC are mostly due to vascular variations. The
gastric and duodenal arteries mainly supply blood to the greater
curvature of the stomach, while the right gastric artery supplies
blood to the lesser curvature. The affected areas are mainly
located in the pyloric region and angle of the stomach, the greater
curvature of the stomach body and the upper portion of the duodenal
bulb and descending portion, which is consistent with the blood
supply range of the gastric and duodenal arteries (18). In ~50% of the population, the
gastric and duodenal arteries originate from the midpoint between
the origin of the hepatic artery from the celiac trunk and its
division into the left and right hepatic arteries (19,20).
With this anatomical structure, placing the catheter outside the
stomach and duodenum, and entering the appropriate hepatic artery
should be the ideal choice for HAIC. In 25% of the population, the
intrahepatic artery is very short (<1.0 cm) or absent (19,20),
which makes it possible to inadvertently insert the catheter into
the right or left hepatic artery. However, this is clearly not
suitable for patients with lesions in both the left and right
liver. For example, in case 2 (Fig.
2C) it was found that the gastroduodenal artery originated from
an intermediate position between the beginning of the common
hepatic artery in the abdominal trunk and the division of the
common hepatic artery into the right and left hepatic arteries.
This anatomical variation was not emphasized, in order to perfuse
the entire hepatic tissue, and the catheter was placed in the
common hepatic artery. Consequently, during the administration of
chemotherapy drugs via the hepatic artery catheter, inadvertent
perfusion of the gastroduodenal artery occurred,. In addition,
there are variations in the length of the hepatic artery (20), making it difficult to insert the
conventional hepatic arterial catheter into the right (or left)
branch of the hepatic artery or the intrahepatic artery. During
first treatment, due to the abundant blood supply to the tumor most
of the chemotherapy drugs enter the tumor; however, during
retreatment the reduced blood supply to the tumor makes it easier
for the chemotherapy drugs to enter the gastric and duodenal
arteries or the right gastric artery, resulting in loss of drugs.
In addition to the aforementioned factors, direct damage to the
gastroduodenal mucosa by anticancer drugs is an underestimated
factor, particularly the damage caused by 5-FU and/or stress ulcers
induced by other anticancer drugs. During treatment, the
possibility of this serious complication must be highly suspected
if upper abdominal pain persists for a prolonged period, especially
if accompanied by epigastric tenderness and abdominal distension.
Acute gastroscopy examination should be performed immediately, and
abdominal CT, gastrointestinal angiography or upright abdominal
radiography and abdominal puncture should be conducted if
necessary, to exclude perforation. Routine use of
gastromucoprotective agents and H2 receptor blockers, such as
chewable aluminum magnesium carbonate tablets, famotidine,
omeprazole enteric-coated capsules or rabeprazole, is recommended
following HAIC treatment to protect the gastroduodenal mucosa.
Adequate hydration to promote the excretion of anticancer drugs is
also crucial. It is important to identify and avoid these abnormal
arteries (those that deviate from the vast majority), such as the
gastroduodenal artery, right gastric artery or pancreaticoduodenal
artery, during HAIC treatment, and to perform appropriate ligation
to prevent damage to non-target organs. Additionally, when
inserting a catheter, it is preferable to select the hepatic artery
or its branches as precisely as possible (18).
Although the colon is the most common site for
immune-related adverse events, inflammation related to immune
checkpoint inhibitors (ICIs) may also occur in the upper
gastrointestinal tract (13).
Symptoms, such as nausea, vomiting and abdominal pain, typically
coexist with gastrointestinal symptoms and are generally lacking in
specificity. Reports of upper gastrointestinal involvement take the
form of esophagitis, gastritis and duodenitis, often as case
reports (16).
Gastroscopy is an effective method to confirm the
occurrence of a gastric ulcer and evaluate its severity. When
patients experience persistent grade 1 upper abdominal pain for
>1 week, endoscopic examination should be performed. ICI-induced
gastritis exhibits a variety of endoscopic features (14) and being cautious of three
characteristic endoscopic findings is crucial: i) Antral erosions
or ulcers; ii) mucosal erythema and edema with excessive white
purulent secretion throughout the stomach; and iii) markedly
fragile mucosa. Pathological examination is the gold standard for
diagnosing gastritis. A previous study reporting characteristic
findings in the clinical pathology of 20 patients receiving ICI
treatment showed a lack of significant intraepithelial lymphocytes
and crypt rupture (21). In a case
report of acute erosive hemorrhagic gastritis induced by cindilimab
(22), a more extensive and severe
lesion was found during gastroscopy, with the entire gastric mucosa
was significantly swollen and congested with opaque mucus adhesion
and diffuse white plaque-like erosions, and active local bleeding
was widely observed. Necrotic mucosa and large areas of shedding
were found in the antrum.
Nivolumab, a monoclonal antibody against the
programmed death 1 (PD-1) receptor, has been reported to induce
gastritis in some cases (23-25).
The appearance of ICI-induced gastric ulcers on gastroscopy may
show irregular, raised or friable lesions with peripheral
inflammation. By contrast, other gastric ulcers may have different
features, such as well-defined margins or a more typical
appearance. Gastric biopsy specimens from these studies showed
marked infiltration of lymphocytes and neutrophils in the gastric
mucosa. IHC identified these lymphocytes as mainly CD3+
T cells, CD4+ helper T cells and CD8+
cytotoxic and suppressor T cells.
The exact pathogenic mechanisms of immune-related
gastrointestinal adverse events are not yet fully understood,
although several mechanisms have been proposed (26). It has been suggested that immune
checkpoint inhibition reduces the regulation of autoreactive T
cells, ultimately leading to immune-mediated adverse reactions and
damage (21,27). For example, cell and tissue damage
may be due to autoreactive CD8+ T cells. Alternatively,
self-antibodies produced by CD4+ T cells mediated by
plasma antibodies may damage cells and tissues. The lymphocyte
composition observed in the present cases were similar to previous
reports, with CD4+ and CD8+ lymphocytes being
predominant, and IHC may aid in diagnosing upper gastrointestinal
disease caused by ICIs. For patients with gastrointestinal
symptoms, accurate diagnosis should be made based on the patient's
clinical course, and endoscopic and histological findings.
In conclusion, HAIC combined with immunotherapy may
increase the risk of gastric ulcers. Therefore, attention should be
paid when selecting the appropriate placement of the HAIC catheter
by identifying vascular variations. While it is important to
actively screen for the cause of gastric ulcers, early detection of
gastric ulcers seems to also be important, as it is the basis for
early and effective treatment. In the present study, in the second
of the three cases, the gastric ulcer was directly related to
vascular degeneration, which was associated with the lack of
relevant surgical experience in the early stage of this gastric
ulcer. For the first and third cases, the causes of gastric ulcer
may be related to immunotherapy, but not vascular variations as
there was no vascular mutation in the Case 1 and Case 3 patients.
In the three cases, gastric ulcers were found to be cured after
anti-ulcer treatment, and no gastric ulcers were found after reuse
of HAIC combined with immunotherapy. In the Xiaogan hospital
affiliated to Wuhan University of Science and Technology (Hubei,
China), there have been >30 cases of HAIC combined with
immunotherapy, >10 cases of HAIC and >70 cases of simple
immunotherapy combined with targeted therapy in patients with
hepatocellular carcinoma since 2021, all of which have not yet
developed gastric ulcers.
Supplementary Material
Immunohistochemical staining results
of Case 1 (magnification, x10). (A) α-fetoprotein: Negative; (B)
hepatocyte: Focal positive; (C) Arg-1: Positive; (D) CD34:
Positive; (E) cytokeratin 19: Negative; (F) p53 (mutated):
Positive; (G) Ki-67: ~10%.
Immunohistochemical staining results
of Case 2 (magnification, x100). (A) α-fetoprotein: Negative; (B)
hepatocyte: Positive; (C) CK19: Negative; (D) CK8/18: Positive; (E)
CD10: Negative; (F) Ki-67: ~40%; (G) Galectin-3: Positive; (H)
Arg-1: Focal positive; (I) CD31: Vascular positive; (J)
carcinoembryonic antigen: Negative. CK, cytokeratin.
Immunohistochemical staining results
of Case 3 (magnification, x100). (A) α-fetoprotein: Positive; (B)
hepatocyte: Partially positive; (C) Arg-1: Focally positive; (D)
CD34 (stromal blood vessels): Positive; (E) CD31 (interstitial
blood vessels): Positive; (F) cytokeratin 8/18: Positive; (G)
Glypican-3: Positive; (H) PCK: Positive; (I) Vimentin: Negative;
(J) Ki-67: ~35%. PCK, Pan-Cytokeratin.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
DC and XL conceived the present study. SC, NZ and XL
performed the experiments. SC and NZ wrote the manuscript. DC and
XL critically reviewed the manuscript. SC and XL confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Xiaogan Hospital Affiliated to Wuhan University of
Science and Technology (approval no. XGLY2021-12-23; Xiaogan,
China).
Patient consent for publication
All patients provided their written consent for the
publication of their data and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chen W, Zheng R, Zeng H and Zhang S: The
incidence and mortality of major cancers in China, 2012. Chin J
Cancer. 35(73)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
He MK, Le Y, Li QJ, Yu ZS, Li SH, Wei W,
Guo RP and Shi M: Hepatic artery infusion chemotherapy using
mFOLFOX versus transarterial chemoembolization for massive
unresectable hepatocellular carcinoma: A prospective non-randomized
study. Chin J Cancer. 36(83)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Qin S, Bai Y, Lim HY, Thongprasert S, Chao
Y, Fan J, Yang TS, Bhudhisawasdi V, Kang WK, Zhou Y, et al:
Randomized, multicenter, open-label study of oxaliplatin plus
fluorouracil/leucovorin versus doxorubicin as palliative
chemotherapy in patients with advanced hepatocellular carcinoma
from Asia. J Clin Oncol. 31:3501–3508. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shao YY, Huang CC, Liang PC and Lin ZZ:
Hepatic arterial infusion of chemotherapy for advanced
hepatocellular carcinoma. Asia Pac J Clin Oncol. 6:80–88.
2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li QJ, He MK, Chen HW, Fang WQ, Zhou YM,
Xu L, Wei W, Zhang YJ, Guo Y, Guo RP, et al: Hepatic arterial
infusion of oxaliplatin, fluorouracil, and leucovorin versus
transarterial chemoembolization for large hepatocellular carcinoma:
A Randomized phase III trial. J Clin Oncol. 40:150–160.
2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
He MK, Liang RB, Zhao Y, Xu YJ, Chen HW,
Zhou YM, Lai ZC, Xu L, Wei W, Zhang YJ, et al: Lenvatinib,
toripalimab, plus hepatic arterial infusion chemotherapy versus
lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv
Med Oncol. 13(17588359211002720)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mai Q, Mo Z, Shi F and Chen X: Lenvatinib
plus hepatic arterial infusion of modified FOLFOX regime in
patients with advanced hepatocellular carcinoma. J Clin Oncol. 38
(Suppl 15):2020.
|
|
9
|
Gu YK, Zhang TQ, Huang ZL, Geng ZJ, Chen
C, LI FG, Xu L, Sun J, LI J, Huang ZM and Shen L: Hepatic artery
infusion chemotherapy combined with apatinib and toripalimab in
advanced hepatocellular carcinoma: Real-world data from a single
center. J Clin Oncol. 38 (Suppl 15)(e16602)2020.
|
|
10
|
Qin S, Chen Z, Liu Y, Xiong J, Ren Z, Meng
Z, Gu S, Wang L and Zou J: A phase II study of anti-PD1 antibody
camrelizumab plus FOLFOX4 or GEMOX systemic chemotherapy as
first-line therapy for advanced hepatocellular carcinoma or biliary
tract cancer. J Clini Oncol. 37 (Suppl 15)(4074)2019.
|
|
11
|
Liu BJ, Gao S, Zhu X, Guo JH, Kou FX, Liu
SX, Zhang X, Wang XD, Cao G, Chen H, et al: Real-world study of
hepatic artery infusion chemotherapy combined with anti-PD-1
immunotherapy and tyrosine kinase inhibitors for advanced
hepatocellular carcinoma. Immunotherapy. 13:1395–1405.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gupta A, De Felice KM, Loftus EV Jr and
Khanna S: Systematic review: Colitis associated with anti-CTLA-4
therapy. Aliment Pharmacol Ther. 42:406–417. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dougan M: Gastrointestinal and hepatic
complications of immunotherapy: Current management and future
perspectives. Curr Gastroenterol Rep. 22(15)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sugiyama Y, Tanabe H, Matsuya T, Kobayashi
Y, Murakami Y, Sasaki T, Kunogi T, Takahashi K, Ando K, Ueno N, et
al: Severe immune checkpoint inhibitor-associated gastritis: A case
series and literature review. Endosc Int Open. 10:E982–E989.
2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Anteby R, Kemeny NE, Kingham TP,
D'Angelica MI, Wei AC, Balachandran VP, Drebin JA, Brennan MF,
Blumgart LH and Jarnagin WR: Getting chemotherapy directly to the
liver: The historical evolution of hepatic artery chemotherapy. J
Am Coll Surg. 232:332–338. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Doria MI Jr, Doria LK, Faintuch J and
Levin B: Gastric mucosal injury after hepatic arterial infusion
chemotherapy with floxuridine. A clinical and pathologic study.
Cancer. 73:2042–2047. 1994.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Suzuki H and Ishii H: Peptic ulcer disease
complicated with liver cirrhosis. Nihon Rinsho. 62:532–540.
2004.PubMed/NCBI(In Japanese).
|
|
18
|
Hu J, Cao G, Xu L, Zheng K, Zhu X, Yang R
and Wang X and Wang X: Retrograde embolization technique of the
right gastric artery during the implantation of port-catheter
system for hepatic arterial infusion chemotherapy. J Interv Med.
4:27–31. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yamagami T, Arai Y, Matsueda K, Inaba Y,
Sueyoshi S and Takeuchi Y: The cause of nontumorous defects of
portal perfusion in the hepatic hilum revealed by CT during
arterial portography. AJR Am J Roentgenol. 172:397–402.
1999.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Imamine R, Shibata T, Shinozuka K and
Togashi K: Complications in hepatic arterial infusion chemotherapy:
Retrospective comparison of catheter tip placement in the
right/left hepatic artery vs. the gastroduodenal artery. Surg
Today. 47:851–858. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gonzalez RS, Salaria SN, Bohannon CD,
Huber AR, Feely MM and Shi C: PD-1 inhibitor gastroenterocolitis:
Case series and appraisal of ‘immunomodulatory
gastroenterocolitis’. Histopathology. 70:558–567. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ai Q, Chen W, Li Y and Li G: Upper
Gastrointestinal Tract IrAEs: A case report about
sintilimab-induced acute erosive hemorrhagic gastritis. Front
Immunol. 13(840916)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kobayashi M, Yamaguchi O, Nagata K, Nonaka
K and Ryozawa S: Acute hemorrhagic gastritis after nivolumab
treatment. Gastrointest Endosc. 86:915–916. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Onuki T, Morita E, Sakamoto N, Nagai Y,
Sata M and Hagiwara K: Severe upper gastrointestinal disorders in
pembrolizumab-treated non-small cell lung cancer patient. Respirol
Case Rep. 6(e00334)2018.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Zhang ML, Neyaz A, Patil D, Chen J, Dougan
M and Deshpande V: Immune-related adverse events in the
gastrointestinal tract: Diagnostic utility of upper
gastrointestinal biopsies. Histopathology. 76:233–243.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Patil PA and Zhang X: Pathologic
manifestations of gastrointestinal and hepatobiliary injury in
immune checkpoint inhibitor therapy. Arch Pathol Lab Med.
145:571–582. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen JH, Pezhouh MK, Lauwers GY and Masia
R: Histopathologic features of colitis due to immunotherapy with
anti-PD-1 antibodies. Am J Surg Pathol. 41:643–654. 2017.PubMed/NCBI View Article : Google Scholar
|