Introduction
Breast cancer (BC) is the most commonly diagnosed
cancer in women globally in addition to being the leading cause of
cancer mortality in women in >100 countries, with a continuously
increasing incidence (1).
Moreover, >1/3 of patients with BC will develop distant
metastases such as lung, liver, bone and brain metastases (2-5).
Ocular metastasis (OM), an uncommon distant
metastasis, is easily neglected because of its obscure clinical
symptoms in the early stage (6).
When it develops to an advanced stage, OM causes ocular pain,
foreign body sensation, vision loss, visual field defects and other
symptoms, thus seriously affecting patients' quality of life
(7). Currently, positron emission
computed tomography/computed tomography (PET/CT), magnetic
resonance imaging (MRI) and ultrasonic testing are often used in
clinical practice for diagnosis of OM (8,9).
However, these approaches have obvious disadvantages, including
their expense and damage from high-dose radiation (10). Thus, it is important to explore
improved methods for predicting OM in breast cancer. Serum testing
for clinical parameters is a practical method to assess the
possibility of distant metastases, as it can shed light on the
progress of tumors. Among the various parameters that have been
used in clinical practice, tumor markers are considered to be
reliable indices to predict distant metastases in patients with
cancer (11).
A cancer biomarker is a substance or process that
indicates the presence of cancer in the body (12). Measured either in the tumor or in
blood, tumor biomarkers can be used to evaluate tumor condition and
thus predict the possibility of developing distant metastases
(11). Traditional cancer
biomarkers include embryonic antigens, as well as protein,
carbohydrate, enzyme and hormonal markers (13).
Cancer antigen 153 (CA153) is used to detect Mucin 1
(MUC-1), a transmembrane protein consisting of two subunits
(14). MUC-1 is expressed at the
apical plasma membrane in normal secretory epithelial cells
(15). However, it is released
into the serum when metastatic BC occurs (16). Numerous other advanced types of
cancer, including ovarian, pancreatic, gastric and lung cancer,
also result in elevated CA153 levels (17-20).
High levels of C153 can also be observed in a number of benign
diseases, including chronic active hepatitis, liver cirrhosis,
sarcoidosis and metaplastic anemia (17,21,22).
Owing to its low specificity, the use of CA153 in diagnosing early
BC has been limited, although it provides useful prognostic
information. Numerous studies have demonstrated that high
preoperative levels of CA153 are associated with shorter
disease-free and overall survival times in patients with BC
(23,24).
Other cancer biomarkers have also been used in
diagnosing tumors and metastases. In patients with colorectal
cancer and liver metastasis, higher carcinoembryonic antigen (CEA)
level is associated with shorter median progression-free survival
and median liver progression-free survival (25). In Zhao's study (26), the sensitivity and specificity of
cancer antigen 125 (CA125) for diagnosing ovarian cancer were 88.2
and 67.4%, respectively. Cancer antigen 199 (CA199) level >300
mg/ml is an independent prognostic factor for postoperative
survival in Zheng's study (hazard ratio, 3.76; 95% CI, 2.18-6.49)
(27). Alkaline phosphatase (ALP)
is used to diagnose bone metastases in BC with a high specificity
(93.96%) but a low sensitivity (65.14%) (28).
A number of studies have reported altered levels of
CA153 in patients with cancer with metastases at various sites and
investigated their diagnostic value. However, whether CA153 levels
could be used to detect OM in patients with BC remains unknown. The
present retrospective study analyzed levels of CA153 and other
common tumor biomarkers of patients with BC to determine their
value in diagnosing OM.
Materials and methods
Study design
This study was designed to determine the
associations between levels of cancer biomarkers and OM in patients
with BC. The data range of sample collection is Jan 2005-Jan 2019.
All BC were diagnosed pathologically, and the pathologist was
independent from the study. The study was conducted in accordance
with the Declaration of Helsinki, and the protocol was approved by
the Ethics Committee of the First Affiliated Hospital of Nanchang
University (approval no. CDYFY-2015-112; date, 2015-05-06;
Nanchang, China). All the methods complied with relevant guidelines
and regulations. Breast tissues from patients were resected during
surgery and the diagnosis of BC was made on the basis of
pathological biopsy. CT and MRI were used to make the OM diagnosis.
Inclusion criteria were all patients who were diagnosed with BC
based on the pathologically. Exclusion criteria were primary ocular
malignant tumor, primary ocular benign tumor and secondary BC. As
this is a retrospective study, informed consent of the patients was
waived, which was approved by the Ethics Committee at The First
Affiliated Hospital of Nanchang University.
Data collection
Age, sex, histopathological subtype and menopausal
status were recorded as basic information and analyzed for
differences among patients (Table
I). As well as CA153, axillary lymph node metastasis (ALNM),
CEA, CA125, CA199, ALP and calcium were analyzed as these
parameters are frequently used in clinical practice and reflect the
condition of tumors in patients with cancer. All clinical indices
were collected from medical records when patients were first
diagnosed with BC. Consecutive data are represented as mean ±
standard deviation.
 | Table IClinical features of patients with
breast cancer. |
Table I
Clinical features of patients with
breast cancer.
| Characteristic | OM | NOM | Whole | P-value |
|---|
| Age,
years±SDa | 45.85±7.76 | 48.31±10.58 | 48.21±10.48 | 0.241 |
| Sex, nb | | | | |
|
Women | 26 | 601 | 627 | 1.000 |
|
Men | 0 | 2 | 2 | |
| Menopausal status,
nc | | | | 0.248 |
|
Premenopausal | 19 | 373 | 392 | |
|
Postmenopausal | 7 | 230 | 237 | |
| Histopathology,
nc | | | | 0.656 |
|
Invasive
ductal carcinoma | 20 | 440 | 460 | |
|
Other
types | 6 | 163 | 169 | |
| Axillary lymph node
metastases, nc | | | | <0.001 |
|
0 | 4 | 259 | 263 | |
|
1-4 | 7 | 210 | 217 | |
|
>4 | 15 | 134 | 149 | |
Statistical analysis
First, basic clinical features including age, sex,
histopathological subtype, menopausal status and ALNM number were
compared using Fisher's exact test, χ2 test and unpaired
Student's t-test. Then, clinical parameters of the ocular
metastases (OM) group and non-ocular metastases (NOM) group were
compared across different subgroups using the Mann-Whitney U test.
Binary logistic regression was carried out to determine independent
risk factors for OM. A receiver operating characteristic (ROC)
curve was constructed with MedCalc 18.2.1 (MedCalc Software, Ltd.),
and the cutoff value, area under the curve (AUC), sensitivity and
specificity were obtained. Measurement data are presented as mean ±
standard deviation (SD). P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical features of patients with
BC
There were 629 patients with BC in total (627 female
and two male), of which 26 patients had OM and 603 had NOM. There
were no differences in age (P=0.241), sex (P=1.000), menopausal
status (P=0.248), or histopathology (P=0.656) between the OM and
NOM groups. The average age of patients in the OM group was
45.85±7.76 years, compared with 48.31±10.58 years in the NOM group.
In the OM group, 19 patients had premenopausal status and seven
were postmenopausal, whereas in the NOM group the numbers were 373
and 230, respectively. Regarding histopathology, the OM group
contained 20 cases of invasive ductal carcinoma and six cases of
other types. In the NOM group, there were 440 cases of invasive
ductal carcinoma and 163 cases of other types. A statistical
difference was revealed for ALNM (P<0.001); the OM group
contained four cases without ALNM and 22 cases with ALNM, whereas
the NOM group contained 259 and 344 cases, respectively. Details
are presented in Table I.
Distributions of patients with BC and
condition of their distant organ metastases
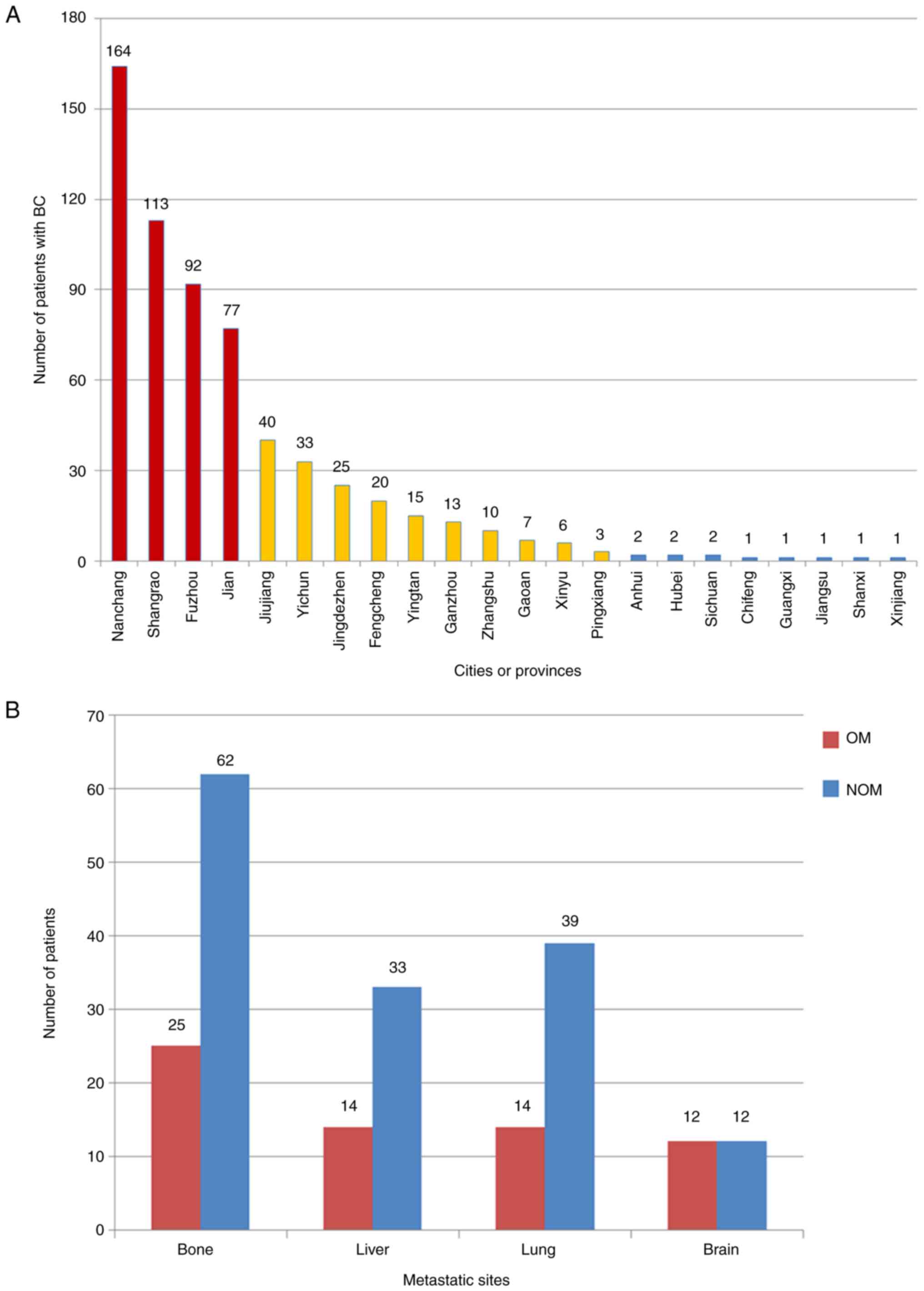
The top four cities patients came from were
Nanchang, Shangrao, Fuzhou, and Jian (marked in red in Fig. 1A). The majority of the patients
were from Jiangxi province (marked in red and yellow in Fig. 1A), but there were also 11 patients
from other provinces (marked in blue in Fig. 1A). Details of the patient
distribution are presented in Fig.
1A.
Numbers of distant organ metastases (bone, liver,
lung and brain) in the two groups were counted. Bone was the most
common metastatic site for BC, accounting for 25 and 62 cases in
the OM and NOM group, respectively (Fig. 1B).
Subgroup analysis and binary logistic
regression analysis
To exclude potential confounding by other distant
metastases and clarify the diagnostic value of CA153, data were
further divided into subgroups. Differences in CA153 levels between
the OM group and NOM group were revealed to be statistically
significant in each subgroup (Table
II). The P-values are summarized in Table III. The binary logistic
regression analysis identified CA153 as a risk factor for OM in
patients with BC (P<0.001; Table
IV).
 | Table IISubgroup analysis. |
Table II
Subgroup analysis.
| A,
Premenopausal |
|---|
| Clinical
features | OM (n=19) | NOM (n=373) | P-value |
|---|
| CEA (ng/ml) | 39.59±107.83 | 2.93±9.99 | <0.001 |
| CA125 (µ/ml) | 57.23±103.74 | 22.86±67.94 | <0.001 |
| CA153 (µ/ml) | 158.59±129.37 | 17.16±26.55 | <0.001 |
| CA199 (µ/ml) | 30.89±50.44 | 13.28±13.76 | 0.375 |
| ALP (µ/l) | 134.32±101.45 | 59.60±24.64 | <0.001 |
| Calcium
(mmol/l) | 2.30±0.42 | 2.32±0.55 | 0.688 |
| B,
Postmenopausal |
| Clinical
features | OM (n=7) | NOM (n=230) | P-value |
| CEA (ng/ml) | 43.56±72.41 | 39.45±531.00 | 0.009 |
| CA125 (µ/ml) | 138.19±203.30 | 24.63±174.47 | 0.002 |
| CA153 (µ/ml) | 147.14±72.26 | 19.55±32.91 | <0.001 |
| CA199 (µ/ml) | 8.37±4.55 | 27.50±216.90 | 0.350 |
| ALP (µ/l) | 107.29±59.49 | 82.15±42.56 | 0.391 |
| Calcium
(mmol/l) | 2.12±0.60 | 2.32±0.15 | 0.622 |
| C, NALNM |
| Clinical
features | OM (n=4) | NOM (n=259) | P-value |
| CEA (ng/ml) | 53.01±101.07 | 2.39±4.27 | 0.079 |
| CA125 (µ/ml) | 118.43±209.20 | 14.84±31.73 | 0.213 |
| CA153 (µ/ml) | 217.99±219.97 | 13.13±9.72 | <0.001 |
| CA199 (µ/ml) | 11.62±6.95 | 12.90±11.85 | 0.920 |
| ALP (µ/l) | 97.50±43.61 | 63.67±22.47 | 0.053 |
| Calcium
(mmol/l) | 2.65±0.16 | 2.31±0.13 | <0.001 |
| D, ALNM |
| Clinical
features | OM (n=22) | NOM (n=344) | P-value |
| CEA (ng/ml) | 38.41±99.88 | 27.75±434.30 | <0.001 |
| CA125 (µ/ml) | 71.87±126.60 | 30.08±156.44 | <0.001 |
| CA153 (µ/ml) | 144.15±89.88 | 21.79±37.24 | <0.001 |
| CA199 (µ/ml) | 27.22±47.59 | 23.08±177.62 | 0.646 |
| ALP (µ/l) | 132.41±97.68 | 71.61±40.89 | <0.001 |
| Calcium
(mmol/l) | 2.18±0.47 | 2.33±0.57 | 0.235 |
| E, Bone
metastases |
| Clinical
features | OM (n=25) | NOM (n=62) | P-value |
| CEA (ng/ml) | 42.14±99.88 | 141.30±1021.78 | 0.095 |
| CA125 (µ/ml) | 81.67±140.05 | 105.98±365.58 | 0.019 |
| CA153 (µ/ml) | 156.81±117.64 | 54.64±77.02 | <0.001 |
| CA199 (µ/ml) | 25.33±44.89 | 72.86±416.87 | 0.704 |
| ALP (µ/l) | 130.00±92.31 | 102.05±77.10 | 0.222 |
| Calcium
(mmol/l) | 2.26±0.48 | 2.49±1.30 | 0.980 |
| F, Liver
metastases |
| Clinical
features | OM (n=14) | NOM (n=33) | P-value |
| CEA (ng/ml) | 36.01±56.65 | 252.70±1400.68 | 0.009 |
| CA125 (µ/ml) | 53.37±109.41 | 122.59±462.49 | 0.061 |
| CA153 (µ/ml) | 140.13±127.64 | 36.24±53.15 | <0.001 |
| CA199 (µ/ml) | 31.53±55.19 | 117.97±570.94 | 0.735 |
| ALP (µ/l) | 140.36±101.37 | 93.33±81.60 | 0.036 |
| Calcium
(mmol/l) | 2.32±0.42 | 2.30±0.25 | 0.761 |
| G, Lung
metastases |
| Clinical
features | OM (n=14) | NOM (n=39) | P-value |
| CEA (ng/ml) | 53.26±131.52 | 8.04±29.49 | 0.047 |
| CA125 (µ/ml) | 78.84±148.35 | 35.70±90.15 | 0.023 |
| CA153 (µ/ml) | 131.96±125.65 | 20.37±21.18 | <0.001 |
| CA199 (µ/ml) | 37.96±57.54 | 14.28±17.47 | 0.175 |
| ALP (µ/l) | 90.50±42.53 | 70.69±20.57 | 0.102 |
| Calcium
(mmol/l) | 2.45±0.26 | 2.31±0.15 | 0.068 |
| H, Brain
metastases |
| Clinical
features | OM (n=12) | NOM (n=12) | P-value |
| CEA (ng/ml) | 20.99±31.48 | 6.06±8.68 | 0.160 |
| CA125 (µ/ml) | 77.45±131.97 | 16.19±12.78 | 0.012 |
| CA153 (µ/ml) | 185.05±151.17 | 28.40±21.97 | <0.001 |
| CA199 (µ/ml) | 44.28±60.21 | 10.18±7.11 | 0.052 |
| ALP (µ/l) | 137.25±105.89 | 64.42±22.92 | 0.045 |
| Calcium
(mmol/l) | 2.41±0.29 | 2.24±0.12 | 0.155 |
| I, Whole |
| Clinical
features | OM (n=26) | NOM (n=603) | P-value |
| CEA (ng/ml) | 40.66±98.15 | 16.86±328.08 | <0.001 |
| CA125 (µ/ml) | 79.03±137.87 | 23.53±120.14 | <0.001 |
| CA153 (µ/ml) | 155.51±115.46 | 18.07±29.14 | <0.001 |
| CA199 (µ/ml) | 24.82±44.05 | 18.71±134.39 | 0.792 |
| ALP (µ/l) | 127.04±91.70 | 68.20±34.42 | <0.001 |
| Calcium
(mmol/l) | 2.25±0.47 | 2.32±0.44 | 0.958 |
 | Table IIISummary of P-values. |
Table III
Summary of P-values.
| Clinical
features | PRE | POST | NALNM | ALNM | BM | LIM | LUM | BRM | Whole |
|---|
| CEA | <0.001 | 0.009 | 0.079 | <0.001 | 0.095 | 0.009 | 0.047 | 0.160 | <0.001 |
| CA125 | <0.001 | 0.002 | 0.213 | <0.001 | 0.019 | 0.061 | 0.023 | 0.012 | <0.001 |
| CA153 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| CA199 | 0.375 | 0.350 | 0.920 | 0.646 | 0.704 | 0.735 | 0.175 | 0.052 | 0.792 |
| ALP | <0.001 | 0.391 | 0.053 | 0.000 | 0.222 | 0.036 | 0.102 | 0.045 | <0.001 |
| Calcium | 0.688 | 0.622 | <0.001 | 0.235 | 0.980 | 0.761 | 0.068 | 0.155 | 0.958 |
 | Table IVBinary logistic regression
analysis. |
Table IV
Binary logistic regression
analysis.
| Factors | B | Exp(B) | Exp(B) 95% CI | P-value |
|---|
| CEA | -0.020 | 0.998 | 0.992-1.003 | 0.458 |
| CA125 | 0.001 | 1.001 | 0.998-1.004 | 0.464 |
| CA153 | 0.028 | 1.029 | 1.018-1.039 | <0.001 |
| CA199 | 0.002 | 1.002 | 0.989-1.015 | 0.759 |
| ALP | 0.001 | 1.001 | 0.991-1.010 | 0.892 |
| Calcium | -0.064 | 0.938 | 0.266-3.311 | 0.921 |
Sensitivity, specificity, AUC and
cutoff value for CA153
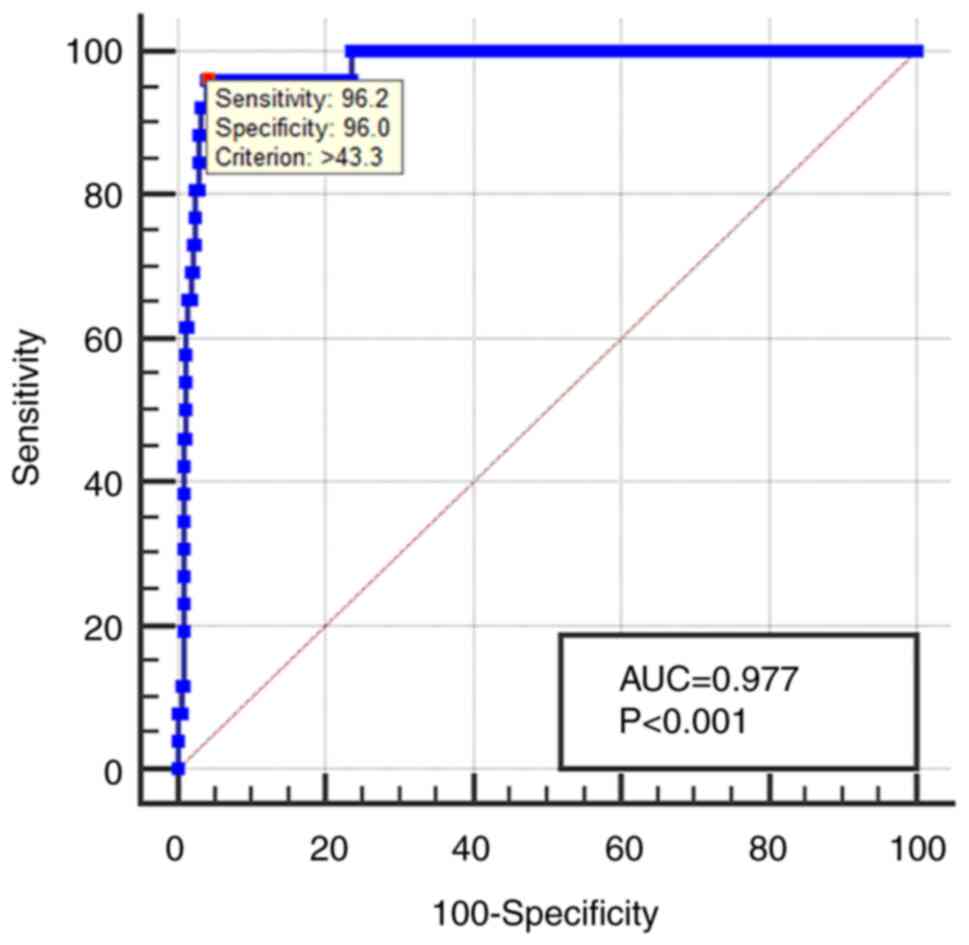
A ROC curve for CA153 was established to evaluate
its diagnostic value. The AUC for CA153 was 0.977, with a 95% CI of
0.958-0.995. The cutoff value was 43.3 µ/ml, with a corresponding
sensitivity of 96.15% and specificity of 96.02% (Fig. 2). Details are presented in Table V.
 | Table VReceiver operating characteristic
curve analysis. |
Table V
Receiver operating characteristic
curve analysis.
| Variable | Cutoff value | Sensitivity
(%) | Specificity
(%) | AUC | 95% CI | P-value |
|---|
| CA153 | 43.3 | 96.15 | 96.02 | 0.977 | 0.958-0.995 | <0.001 |
Discussion
BC has a high incidence of distant metastases and
has increased the medical cost burden of our society. Although the
widespread use of mammography, PET/CT and MRI has increased the
detection rate, there are still some disadvantages (29). Various risk factors and clinical
parameters associated with distant metastases of BC have been taken
into consideration to make up for the limitations of these
techniques (28,30-32)
(Table VI).
 | Table VILiterature summary of risk factors
for common distant metastases in patients with BC. |
Table VI
Literature summary of risk factors
for common distant metastases in patients with BC.
| Author (Refs.) | Year | Distant
metastasis | Risk factors |
|---|
| Slimane et
al (30) | 2004 | Brain | Negative hormone
receptor status |
| Cao et al
(31) | 2012 | Liver | Lactate
dehydrogenase + γ- glutamyltransferase + CA153 |
| Chen et al
(28) | 2017 | Bone | Axillary lymph node
metastases + CA125 + CA153 + alkaline phosphatase + hemoglobin |
| Hu et al
(32) | 2017 | Lung | CD44v |
Tumor marker serum tests are widely used in patients
with cancer to evaluate tumor occurrence and condition. Several
studies have already investigated the use of tumor markers in a
number of types of cancer. Brand et al (33) revealed higher levels of CA199 in
patients with pancreatic ductal carcinoma (PDC) compared with
healthy controls, indicating that CA199 can be used to diagnose
PDC. In Stojkovic's study (34),
higher CEA levels are associated with advanced stage in patients
with colorectal carcinoma (CRC); the study also demonstrates the
use of CEA as a diagnostic factor to predict the severity of CRC.
In another study (35), CA125 has
been used to diagnose ovarian cancer; the sensitivity and
specificity of CA125 according to the ROC curve were 79.6 and
82.5%, respectively. Also, in work by Tang et al (36), CA153 has been used in the diagnosis
of BC with a sensitivity of 63% and a specificity of 82%.
Notably, tumor markers are also helpful to assess a
patient with cancer's risk of distant metastasis. Yuan et al
(37) observed that CA125 can
increase A2780 and OVCAR-3 cell migration, indicating that CA125
may play an important part in tumor metastasis. Moreover, Zhou
et al (38) revealed CA125
to be an independent risk factor of bone metastases in patients
with lung cancer. Cao et al (31) revealed that CA153 is useful to
predict liver metastasis in patients with BC.
OM is often associated with poor prognosis and low
quality of life (39). The
incidence of OM in BC has varied among different studies, with
rates between 5-30% (40,41). The choroid is the most common site
of OM owing to its rich blood supply, followed by the orbit, iris,
ciliary body, optic nerve, conjunctiva and eyelid (42). When tumor cells grow in these
locations, the substances they produce and release enter the blood
and can be tested (43). Thus,
tumor markers can well reflect the stage of an OM tumor. However,
the diagnostic value of tumor markers in OM of patients with BC
remains unclear.
The present study analyzed the differences in basic
clinical features and common tumor markers between the OM group and
NOM group. Among the basic features, ALNM demonstrated significant
differences between the two groups. There were also statistically
significant differences in CA153 levels in all subgroups and in the
whole group, indicating its diagnostic value as a risk factor for
OM in patients with BC.
The present study was the first to evaluate the
diagnostic value of CA153 in patients with BC with OM. Compared
with patients with NOM, patients with OM had higher CA153 levels.
The cutoff value was 43.3 µ/ml, suggesting that patients with BC
with CA153 levels higher than this value were at risk of OM.
Moreover, the sensitivity and specificity for diagnosing OM in
patients with BC were 0.96 and 0.96, respectively. Given the high
AUC value (0.977), CA153 represents a relatively high predictive
accuracy. As an important specific marker of BC, it is widely used
in diagnosis of BC and in assessing prognosis and metastasis
(44). In a meta-analysis
performed by Tang et al (36), the sensitivity and specificity of
CA153 in breast secretions for predicting BC were 0.63 and 0.82,
respectively. Another meta-analysis conducted by Li et al
(45), demonstrated that elevated
CA153 is associated with shorter disease-free survival times in
patients with BC. In a retrospective study performed by Chen et
al (28), CA153 was revealed
to be an independent risk factor for BC bone metastases, with
sensitivity and specificity of 0.77 and 0.87, respectively.
Although CA153 is an independent risk factor for
both bone metastases and OM, there are differences between the two
conditions. First, in the present study, bone metastases were more
common, whereas OM was relatively rare. Second, OM was more likely
to occur at an advanced stage, indicating the progression of the
disease, whereas bone metastases could be found in early BC. Third,
when bone metastases occurred, elevated CA153 and ALP levels were
detected, whereas OM was not associated with high levels of ALP.
Regarding the diagnostic value of CA153, high levels of CA153 were
detected in both metastases, but with some differences. On the one
hand, the cutoff values were different. For diagnosing bone
metastases, the cutoff value was 25.42 µ/ml, whereas that for OM
was 43.3 µ/ml. On the other hand, the sensitivity and specificity
for OM were both >95%. Given these differences and clinical
symptoms, it is not difficult to distinguish bone metastases from
OM.
However, the present study had some limitations.
First, as OM is rare, the sample size was relatively small. Second,
the majority of participants were from the same province; more
studies of patients from different places are needed. Third,
although the present study indicated an association between CA153
and OM, it could not identify their causal relationship. Hence,
tests from more institutions are necessary to validate the
conclusions of the present study. It would also be appropriate to
explore the diagnostic value of CA153 in other metastases.
Furthermore, associated molecular experiments could be carried out
to explore the specific correlations.
The present study clarified CA153 as an independent
risk factor for OM in patients with BC. Patients with BC with CA153
>43.3 µ/ml were more likely to have OM. Although CA153 levels
had high predictive value for OM according to the present study,
CA153 alone was insufficient to diagnose OM in patients with BC.
Combining relevant clinical parameters with clinical symptoms would
be an improved strategy. However, the present study indicated that
physicians should be vigilant when CA153 levels are over the cutoff
value. Treatment should be performed in time to avoid severe
metastases. These results will help physicians to improve
prediction of OM in patients with BC and provide some insight into
the specific mechanisms of tumor markers in cancer metastases.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the National Natural Science
Foundation of China (grant no. 82160195), Jiangxi Double-Thousand
Plan High-Level Talent Project of Science and Technology Innovation
(grant no. jxsq2023201036) and the Key R & D Program of Jiangxi
Province (grant no. 20223BBH80014).
Data availability and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS designed the study. QG, QH and JL analyzed and
interpreted the patient data. YS and QG confirm the authenticity of
all the raw data. QG, QH, JL, QYL and YS made major contributions
writing the manuscript. YM, BL, and RL performed the statistical
analyses. WS, QL, QY, and QYL collected the clinical data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was conducted in accordance with the
Declaration of Helsinki, and the protocol was approved by the
Ethics Committee of the First Affiliated Hospital of Nanchang
University (approval no. CDYFY-2015-112). All patients provided
written consent to participate.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu
W, Giri DD, Viale A, Olshen AB, Gerald WL and Massagué J: Genes
that mediate breast cancer metastasis to lung. Nature. 436:518–524.
2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bacalbasa N, Balescu I, Ilie V, Florea R,
Sorop A, Brasoveanu V, Brezean I, Vilcu M, Dima S and Popescu I:
The impact on the long-term outcomes of hormonal status after
hepatic resection for breast cancer liver metastases. In Vivo.
32:1247–1253. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Oruç Z, Kaplan MA and Arslan Ç: An update
on the currently available and future chemotherapy for treating
bone metastases in breast cancer patients. Expert Opin
Pharmacother. 19:1305–1316. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bos PD, Zhang XH, Nadal C, Shu W, Gomis
RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA and
Massagué J: Genes that mediate breast cancer metastasis to the
brain. Nature. 459:1005–1009. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liang RB, Yu K, Wu JL, Liu JX, Lin Q, Li
B, Zhang YQ, Ge QM, Li QY, Shu HY and Shao Y: Risk factors and
their diagnostic values for ocular metastases in invasive ductal
carcinoma. Cancer Med. 10:824–832. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Georgalas I, Paraskevopoulos T,
Koutsandrea C, Kardara E, Malamos P, Ladas D and Papaconstantinou
D: Ophthalmic metastasis of breast cancer and ocular side effects
from breast cancer treatment and management: Mini review. Biomed
Res Int. 2015(574086)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Reddy S, Kurli M, Tena LB and Finger PT:
PET/CT imaging: Detection of choroidal melanoma. Br J Ophthalmol.
89:1265–1269. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Razek AA and Elkhamary S: MRI of
retinoblastoma. Br J Radiol. 84:775–784. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shao YH, Tsai K, Kim S, Wu YJ and Demissie
K: Exposure to tomographic scans and cancer risks. JNCI Cancer
Spectr. 4(pkz072)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Martin TA, Ye L, Sanders AJ, Lane J and
Jiang WG: Cancer Invasion and Metastasis: Molecular and Cellular
Perspective. In: Madame Curie Bioscience Database [Internet].
Landes Bioscience, Austin, TX, 2000-2013.
|
|
12
|
Wagner PD and Srivastava S: New paradigms
in translational science research in cancer biomarkers. Transl Res.
159:343–353. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shimetani N: Status quo of tumor markers
and important points in medical practice. Rinsho Byori.
51:1216–1220. 2003.PubMed/NCBI(In Japanese).
|
|
14
|
Kufe DW: Mucins in cancer: Function,
prognosis and therapy. Nat Rev Cancer. 9:874–885. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Duffy MJ, Evoy D and McDermott EW: CA
15-3: Uses and limitation as a biomarker for breast cancer. Clin
Chim Acta. 411:1869–1874. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rahn JJ, Dabbagh L, Pasdar M and Hugh JC:
The importance of MUC1 cellular localization in patients with
breast carcinoma: An immunohistologic study of 71 patients and
review of the literature. Cancer. 91:1973–1982. 2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hayes DF, Zurawski VR and Kufe DW:
Comparison of circulating CA15-3 and carcinoembryonic antigen
levels in patients with breast cancer. J Clin Oncol. 4:1542–1550.
1986.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Stieber P, Molina R, Chan DW, Fritsche HA,
Beyrau R, Bonfrer JM, Filella X, Gornet TG, Hoff T, Jäger W, et al:
Evaluation of the analytical and clinical performance of the
Elecsys CA 15-3 immunoassay. Clin Chem. 47:2162–2164.
2001.PubMed/NCBI
|
|
19
|
Stieber P, Molina R, Chan DW, Fritsche HA,
Beyrau R, Bonfrer JM, Filella X, Gornet TG, Hoff T, Jäger W, et al:
Clinical evaluation of the Elecsys CA 15-3 test in breast cancer
patients. Clin Lab. 49:15–24. 2003.PubMed/NCBI
|
|
20
|
Bon GG, von Mensdorff-Pouilly S, Kenemans
P, van Kamp GJ, Verstraeten RA, Hilgers J, Meijer S and Vermorken
JB: Clinical and technical evaluation of ACS BR serum assay of MUC1
gene-derived glycoprotein in breast cancer, and comparison with CA
15-3 assays. Clin Chem. 43:585–593. 1997.PubMed/NCBI
|
|
21
|
Colomer R, Ruibal A, Genollá J, Rubio D,
Del Campo JM, Bodi R and Salvador L: Circulating CA 15-3 levels in
the postsurgical follow-up of breast cancer patients and in
non-malignant diseases. Breast Cancer Res Treat. 13:123–133.
1989.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Symeonidis A, Kouraklis-Symeonidis A,
Apostolopoulos D, Arvanitopoulou E, Giannakoulas N, Vassilakos P
and Zoumbos N: Increased serum CA-15.3 levels in patients with
megaloblastic anemia due to vitamin B12 deficiency. Oncology.
67:359–367. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Molina R, Auge JM, Farrus B, Zanón G,
Pahisa J, Muñoz M, Torne A, Filella X, Escudero JM, Fernandez P and
Velasco M: Prospective evaluation of carcinoembryonic antigen (CEA)
and carbohydrate antigen 15.3 (CA 15.3) in patients with primary
locoregional breast cancer. Clin Chem. 56:1148–1157.
2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Duffy MJ, Duggan C, Keane R, Hill AD,
McDermott E, Crown J and O'Higgins N: High preoperative CA 15-3
concentrations predict adverse outcome in node-negative and
node-positive breast cancer: Study of 600 patients with
histologically confirmed breast cancer. Clin Chem. 50:559–563.
2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Peng S, Huang P, Yu H, Wen Y, Luo Y, Wang
X, Zhou J, Qin S, Li T, Chen Y, et al: Prognostic value of
carcinoembryonic antigen level in patients with colorectal cancer
liver metastasis treated with percutaneous microwave ablation under
ultrasound guidance. Medicine (Baltimore). 97(e0044)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhao T and Hu W: CA125 and HE4:
Measurement tools for ovarian cancer. Gynecol Obstet Invest.
81:430–435. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zheng BH, Yang LX, Sun QM, Fan HK, Duan M,
Shi JY, Wang XY, Zhou J, Fan J, Ma ZY and Gao Q: A new preoperative
prognostic system combining CRP and CA199 for patients with
intrahepatic cholangiocarcinoma. Clin Transl Gastroenterol.
8(e118)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen WZ, Shen JF, Zhou Y, Chen XY, Liu JM
and Liu ZL: Clinical characteristics and risk factors for
developing bone metastases in patients with breast cancer. Sci Rep.
7(11325)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kashyap D, Pal D, Sharma R, Garg VK, Goel
N, Koundal D, Zaguia A, Koundal S and Belay A: Global increase in
breast cancer incidence: Risk factors and preventive measures.
Biomed Res Int. 2022(9605439)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Slimane K, Andre F, Delaloge S, Dunant A,
Perez A, Grenier J, Massard C and Spielmann M: Risk factors for
brain relapse in patients with metastatic breast cancer. Ann Oncol.
15:1640–1644. 2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cao R and Wang LP: Serological diagnosis
of liver metastasis in patients with breast cancer. Cancer Biol
Med. 9:57–62. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hu J, Li G, Zhang P, Zhuang X and Hu G: A
CD44v subpopulation of breast cancer stem-like cells with enhanced
lung metastasis capacity. Cell Death Dis. 8(e2679)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Brand RE, Nolen BM, Zeh HJ, Allen PJ,
Eloubeidi MA, Goldberg M, Elton E, Arnoletti JP, Christein JD,
Vickers SM, et al: Serum biomarker panels for the detection of
pancreatic cancer. Clin Cancer Res. 17:805–816. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Stojkovic Lalosevic M, Stankovic S,
Stojkovic M, Markovic V, Dimitrijevic I, Lalosevic J, Petrovic J,
Brankovic M, Pavlovic Markovic A and Krivokapic Z: Can preoperative
CEA and CA19-9 serum concentrations suggest metastatic disease in
colorectal cancer patients? Hell J Nucl Med. 20:41–45.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Dayyani F, Uhlig S, Colson B, Simon K,
Rolny V, Morgenstern D and Schlumbrecht M: Diagnostic performance
of risk of ovarian malignancy algorithm against CA125 and HE4 in
connection with ovarian cancer: A meta-analysis. Int J Gynecol
Cancer. 26:1586–1593. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tang S, Wei L, Sun Y, Zhou F, Zhu S, Yang
R, Huang Y, Zhang H, Xu H and Yang J: CA153 in breast secretions as
a potential molecular marker for diagnosing breast cancer: A meta
analysis. PLoS One. 11(e0163030)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yuan Q, Song J, Yang W, Wang H, Huo Q,
Yang J, Yu X, Liu Y, Xu C and Bao H: The effect of CA125 on
metastasis of ovarian cancer: Old marker new function. Oncotarget.
8:50015–50022. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhou Y, Yu QF, Peng AF, Tong WL, Liu JM
and Liu ZL: The risk factors of bone metastases in patients with
lung cancer. Sci Rep. 7(8970)2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lin Q, Chen XY, Liu WF, Zhu PW, Shi WQ, Li
B, Yuan Q, Min YL, Liu JM and Shao Y: Diagnostic value of CA-153
and CYFRA 21-1 in predicting intraocular metastasis in patients
with metastatic lung cancer. Cancer Med. 9:1279–1286.
2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kreusel KM, Wiegel T, Stange M, Bornfeld N
and Foerster MH: Intraocular metastases of metastatic breast
carcinoma in the woman. Incidence, risk factors and therapy.
Ophthalmologe. 97:342–326. 2000.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
41
|
Nelson CC, Hertzberg BS and Klintworth GK:
A histopathologic study of 716 unselected eyes in patients with
cancer at the time of death. Am J Ophthalmol. 95:788–793.
1983.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Demirci H, Shields CL, Chao AN and Shields
JA: Uveal metastasis from breast cancer in 264 patients. Am J
Ophthalmol. 136:264–271. 2003.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Tarro G, Perna A and Esposito C: Early
diagnosis of lung cancer by detection of tumor liberated protein. J
Cell Physiol. 203:1–5. 2005.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lu F, Pan S, Qi Y, Li X and Wang J: The
clinical application value of RDW, CA153, and MPV in breast cancer.
Clin Lab. 67:2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Li X, Dai D, Chen B, Tang H, Xie X and Wei
W: Clinicopathological and prognostic significance of cancer
antigen 15-3 and carcinoembryonic antigen in breast cancer: A
Meta-analysis including 12,993 patients. Dis Markers.
2018(9863092)2018.PubMed/NCBI View Article : Google Scholar
|