Introduction
Chemical peels are divided in superficial, medium
and deep; they can be applied at the whole face or they can be used
in different aesthetic facial units; at the lips, the lower
eyelids, the forehead and the temporal regions (1).
Superficial peels brighten the skin; the more acidic
the peel is, the deeper the penetration will be. The main types of
superficial peels are a-hydroxy acids such as glycolic/lactic acid
and b-hydroxy acids such as salicylic acid, while ‘Jessner's’
solutions with ethanol are universally used as peel accelerators
(2). They are safe, without severe
complications and can be applied to all skin types (3).
Medium peels are mainly trichloroacetic acid (TCA)
10-35% and phenol 80%. The TCA penetrates the upper dermis to the
stratum corneum and exfoliates the skin; it can treat actinic
keratosis improving fine wrinkles and photoaging, pigmentary
changes and/or acne scars (2).
Finally, deep peels are mainly TCA 50% and
phenol/croton oil. Phenol/croton oil peels reach the mid-reticular
dermis and can treat atrophic/pox-like acne scars, severe
dyschromias and deep rhytids (2),
solar lentigines, seborrheic keratosis, Bowen disease and
angiosarcomas; they have been used as sclerosing agents or for
chemical matrixectomy, the treatment of stable vitiligo and
alopecia areata (2). Phenol
(carbolic acid or phenolic acid) acts as a local anesthetic and
disinfectant (2). It can be
neutralized by glycerin or water and plays a key role in
Baker-Gordon's formula, together with croton oil (3). Croton oil derives from the seeds of
Croton tiglium plant (Euphorbiaceae family) and its
medical applications have been well studied even from the 19th
century. It boosts phenol's activity, coagulating the keratin
(2). By using higher
concentrations of croton oil, the concentrations of phenol can be
lowered, therefore the systemic toxicity of phenol can be reduced.
Baker et al (4)
demonstrated that a 0.2% increase of croton oil concentration
increases the phenol peel activity by 20%.
Despite its effectiveness, there are certain risks
related to phenol/croton oil use, especially in patients with dark
and thin skin.
The aim of the present study was to evaluate the
clinical success of the phenol/croton oil peel, its pathological
characteristics (at least from one patient) and the potential risks
from its use.
Patients and methods
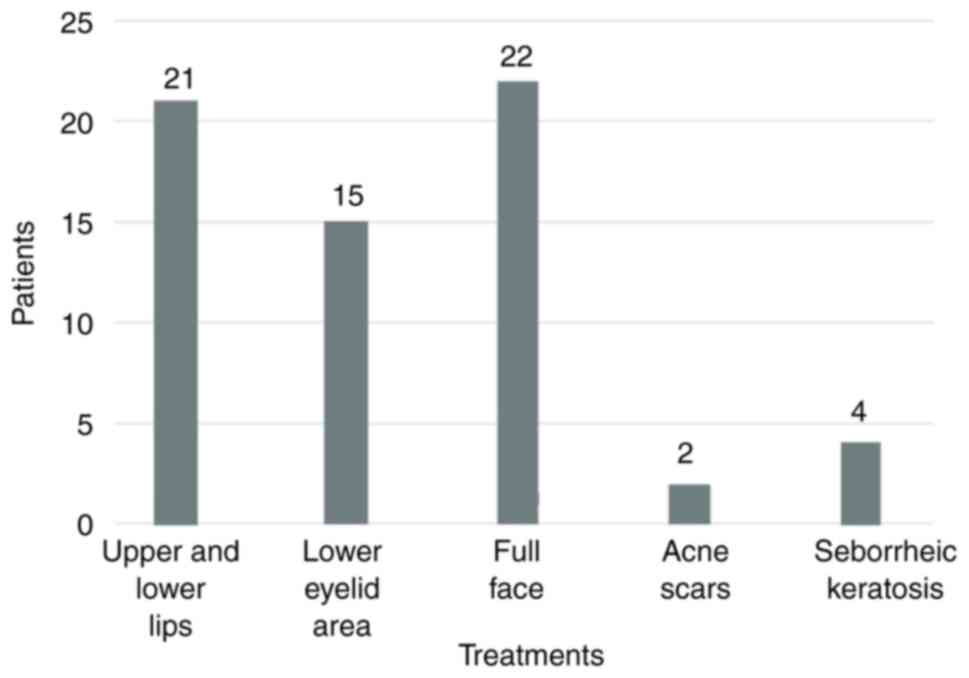
A total of 64 patients were treated with
phenol/croton oil peel between January 2014 and April 2023. In
total, 21 patients performed resurfacing at the oral area
(upper/lower lips), 15 patients at their lower eyelids, 22 patients
performed full face treatment, while 2 patients were treated for
their acne scars and an additional 4 for the multiple seborrheic
keratosis around their back and lumbar region (Fig. 1). All treatments were performed at
the Opsis Clinical Plastic Surgery Clinic (Herakion, Greece). The
patients signed an informed consent form and all procedures were
performed by the same plastic surgeon. The average age was 53 years
(range, 33-75 years), while mean follow-up was 2 years. All
patients were female except 4 male patients (1 patient with acne
scars and 3 treated at the lower eyelids) (Fig. 1).
Patients with acne, seborrheic kerastosis around
their back and lumbar region as well as aging wrinkles included
while other with heart, kidney and hepatic history or a medical
background of keloid, smoking, recent use of isotretinoin,
psychological imbalance and Fitzpatrick skin types IV-VI were
excluded from the study.
Croton oil/Phenol application
Hetter's 1.2% croton oil (Delasco) solution for
perioral peel was applied; 0.8, 0.4 and 0.1-0.2% croton oil were
used for the cheek/forehead, temporal regions, eyelids and neck,
respectively. The 0.8% croton oil solution consisted of 5.5 ml
water, 0.5 ml septisol (Delasco), 2 ml phenol 88% (Delasco) and 2
ml stock solution [containing 24 ml phenol and 1 ml croton oil;
(Delasco)] (5). Specific care was
given to the periorbital area, since the application of
concentrations higher than 1% croton oil, increases the risk of
hypopigmentation (6). The skin was
cleaned with alcohol 70% prior to application and the phenol/croton
oil peel was applied by gauze/cotton pads. Topical anesthesia was
applied, with sensory facial nerve blocks with
xylocaine/epinephrine in addition to the anesthetic properties of
the phenol solutions. All patients were fully monitored and
intravenously hydrated (7,8).
As soon as a full-face peel was applied, the face
was subdivided into 6 aesthetic units. Subsequently, the peel was
administered to the forehead, followed by the right and the left
cheek, the perioral and the periorbital area, and finally the nasal
and mental region. Assessing the endpoint of the croton oil/phenol
peel was crucial: A white frost was observed immediately after the
peel, followed by a fine gray color and, finally, by a mild pink
color at the skin; the peel penetration increased proportionally to
the number of passes, the pressure applied and the croton oil
concentration (9).
Hematoxylin and eosin staining
Biopsy was taken from the right lower eyelid of 1
patient, 6 months after phenol/croton oil peel application. The
lower eyelid was selected for pathology sections because of minimal
scarring in order to further study the peel and compare it with the
non-peel area. Tissue culture microscope was used (Leica
Microsystems); the tissue thickness was 1-µm and fixed by fresh 4%
formalin for 24 h at room temperature. The paraffin sections were
placed in xylene I and II solution each for 5 min (56˚C) for
dewaxing. Then they were placed in 100, 95, 80 and 75% ethanol
solution, each time for 3 min, rinsed by distilled water for 5 min
and dried for rehydration. The paraffin sections were stained by
hematoxylin for 10 min (30˚C), rinsed by water for 15 min and put
into 1% hydrochloric acid ethanol solution for 5-30 sec until every
slice turned red, then rinsed by water for another 15 min until
every slice turned blue. The paraffin slices were put into 75, 95,
100 and l00% ethanol solution for 5 min each, stained by eosin for
another 2 min at 40˚C and washed by tap water for 1 min to
rehydrate. The slices were placed later into 95 and 100% ethanol
solution for 5 min, then dehydrated by 95% ethanol for 2 min, 100%
ethanol for 1 min and 100% ethanol for 1 min. Finally, the paraffin
slices were put into xylene I and II solution for 5 min.
Results
Photographic evaluation
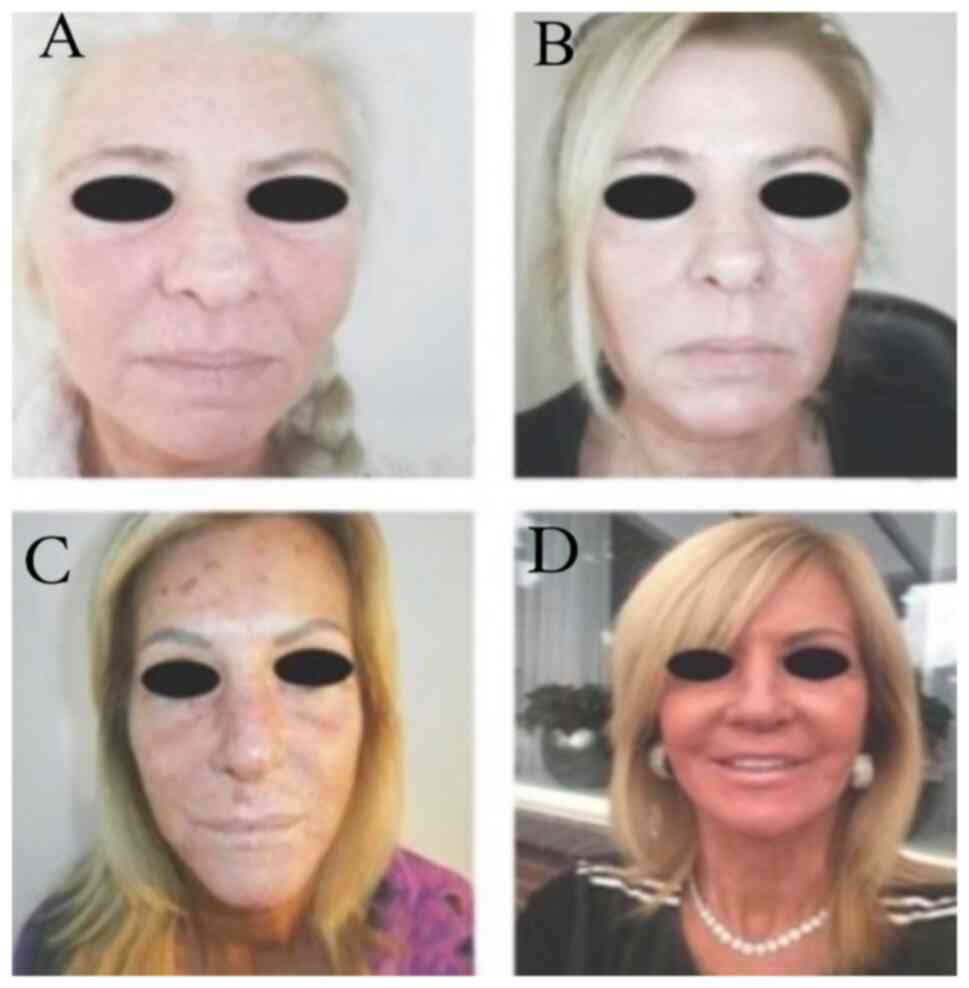
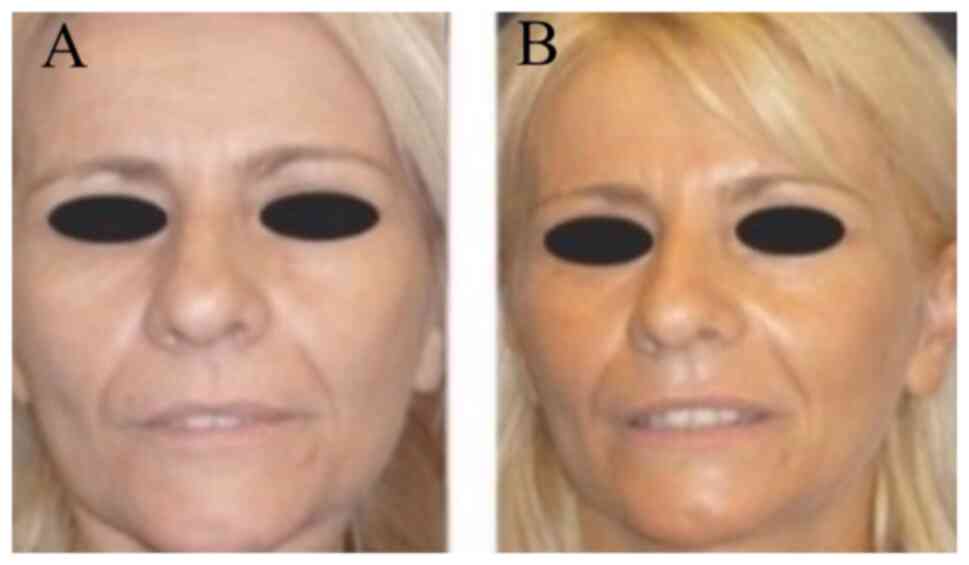
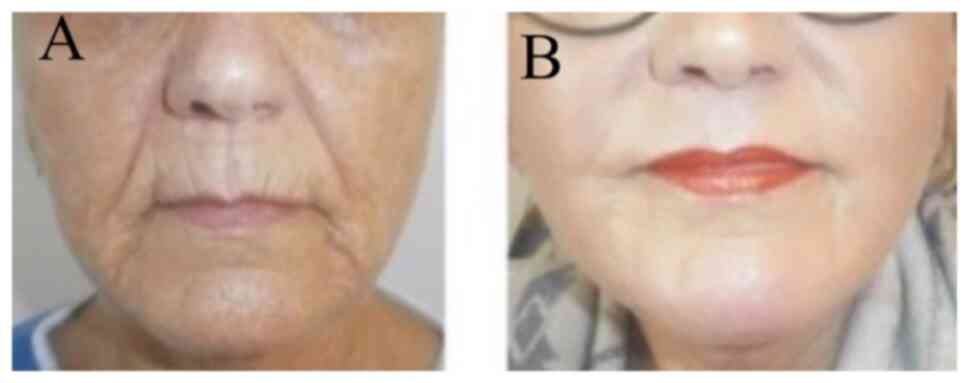
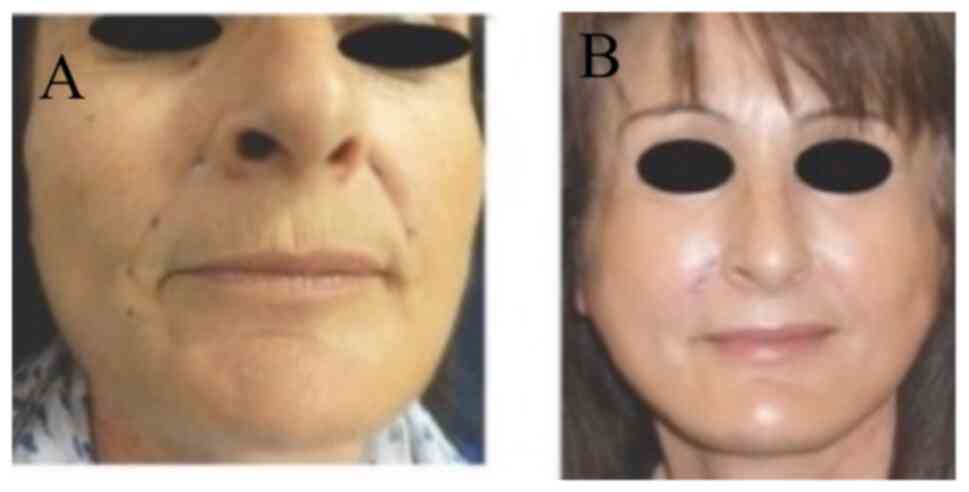
Photographic evaluation was performed on all
patients. The improvement was remarkable usually 3-8 months after
phenol/croton oil peel application at the whole face (Figs. 2 and 3), at the upper/lower lips (Fig. 4) or at the lower eyelids/upper lip
together with face lift (Fig. 5).
Different facial areas presented different penetrations; the
perioral skin was thicker than temporal and eyelid areas. Hair
growth was not compromised by phenol/croton oil, and the peel was
safely applied at the hairy areas of the beard and eyebrows and
scalp (10,11). An oral analgesic treatment
(ibuprofen tablets) was administered 3 times/day, during the first
3 days while a sulfadiazine cream was applied every 2-6 h, during
the first 8-10 days, in order to hydrate, disinfect (12) and reduce the erythema (8).
In case of herpes/viral infection history, oral
valacyclovir 500 mg was administered for 10-14 days, starting from
the first day after the peel application. Finally, following the
sulfadiazine cream application, a sun care cream was used on a
daily basis, along with a modified Kligman's formula (tretinoin
0.1% + hydroquinone 5% + hydrocortisone 0.1% + vitamin C
5%), for at least 15 days to eliminate the skin erythema
(13).
The mean re-epithelialization process was 10-15
days, while significant improvement was observed in all 64
patients; 2 patients appeared with prolonged erythema and treated
with modified Kligman's formula. Additionally, 2 more patients were
not fully satisfied with the results at the perioral area and the
peel had to be repeated in one patient for his acne scars after 6
months.
Histological findings
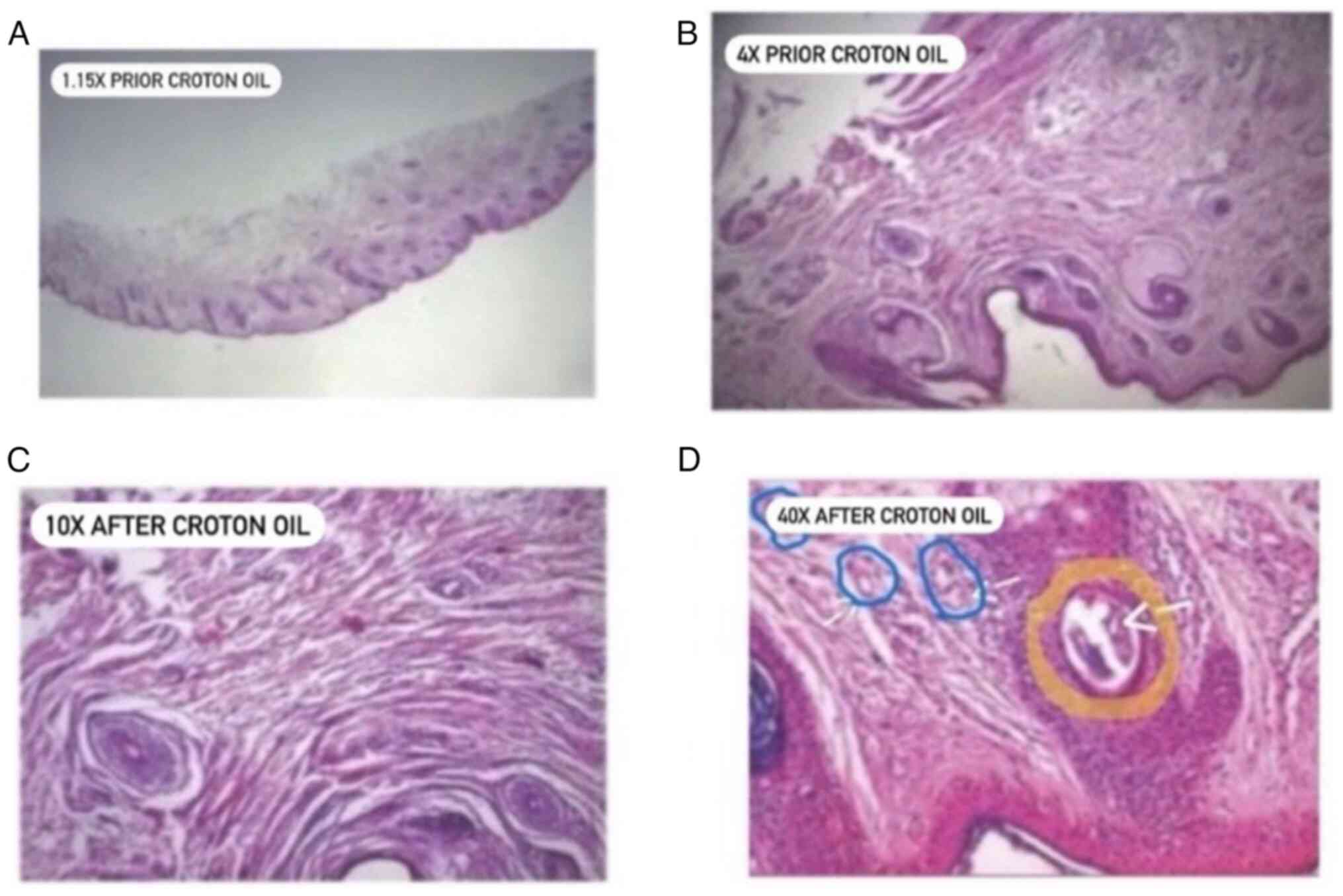
Histopathology examinations of the phenol/croton oil
peel skin (Fig. 6) showed mild
perivascular and periadnex reepithelization, lymphocytic
infiltrations and vasodilation (probably due to the regeneration
process of the peeling). Interestingly, there was evidence of a
large quantity of new collagen deposits at bands beneath the
dermis. To the best of our knowledge, this is the first
histopathology report of a patient treated with phenol/croton
oil.
Discussion
Skin peels were well known from the past and varied
from superficial methods, such as milk and honey, to more invasive
including mustards, sulfur, lemon, even fire (14). They were popularized in the early
20th century and were widely used after the 2nd World War in
plastic surgery (14).
Superficial peels are relatively safe, while medium
and deep peels should be cautiously applied, especially to
Fitzpatrick's type IV-VI patients, due to their post-inflammatory
properties (15). Smoking,
immunosuppression, cardiac/renal or hepatic history, and
isotretinoin use of ≤6-8 months before the application, are risk
factors for poor healing, while history of herpes complex requires
prophylactic treatment (16).
TCA 50% may require multiple sessions, while laser
resurfacing by CO2/Erbium-YAG can lead to scarring or
persistent erythema (especially CO2); the necessary
equipment is also expensive to obtain. Dermabrasion can be an
optimal choice for resurfacing due to its low cost and considerable
efficacy; however, it requires a long learning curve (17).
Therefore, there is a need for an alternative deep
peel strategy. The application of phenol creates a chemical burn
helping new collagen formation. It whitens the skin and eliminates
the pigmentary changes; it can treat severe acne scars,
xanthelasma, actinic/seborrheic keratosis and actinic cheilitis
(14-16).
In Baker-Gordon's formula, phenol was considered to be the main
factor for years (7) and multiple
variations had been described including different concentrations or
buffered phenol peels/glycerin use (18). Baker et al (4) proved that croton oil was the active
agent and Justo et al (15)
showed that Hetter's peel is safer than Baker-Gordon's due to its
lower post-inflammatory hyperpigmentation properties.
However, even the newest Hetter's croton/oil peel
solutions have certain risks. Following local application, phenol
is absorbed systemically and is finally excreted by the kidneys.
Potential adverse events include respiratory distress and cardiac
arrhythmias, therefore it is critical to minimize the risk by
increasing croton oil concentrations.
There were no heart or respiratory complications in
our series because of the increased croton oil concentrations, the
adequate hydration throughout the procedure, as well as the 15 min
intervals between different facial aesthetic units (18). Close follow-up by monitoring and
sufficient air exchange at the operating room were used when
phenol/croton oil peels were performed on larger areas. Patients on
antihypertensive and/or antidepressants were given short acting
beta-blockers, such as propranolol, to reduce the risk of
arrhythmias. There was a risk of eyes' irritation during
phenol/croton oil application, so eyes were kept closed during the
procedure.
Other phenol/croton oil risks involve hypertrophic
scar or keloid formation, skin dyschromias (19) as well as acne and milia, small
white cysts, contact dermatitis or infection (mainly due to
Staphylococcus spp., Streptococcus spp. and Pseudomonas
aeruginosa) (11). In order to
eliminate the aforementioned complications, ‘lighter concentration’
of croton oil/phenol was used, thus the main healing period for the
majority of the patients was 10-12 days.
The most devastating complication of the croton
oil/phenol peel is the prolonged erythema which is more likely to
occur in dark/thin skin areas, such as the eyelids or the neck and
the mandible (5). Avoiding peeling
at the neck or dark skin patients and giving a lot of attention to
the application at the eyelids/mandible were both crucial in order
to prevent scars (17); male
patients with thicker skin were safer than the other individuals
(6,10), while female patients were peeled
more cautiously. The edges of the peels were feathered 1-2 cm to
the surrounding skin in the patients, particularly at the
mandibular borders, to prevent lines of demarcation. Hydration
cream containing antibiotic was applied after the peel to minimize
possible infection/skin dehydration (16,19,20)
combined with sulfadiazine cream (with no occlusive tape) for a
better follow-up.
The present study has certain limitations. The
current study was not randomized or retrospective and there was no
comparison with other deep peels, ablative lasers, radiofrequency
applications or dermabrasion.
In conclusion, phenol/croton oil peel stimulates new
collagen formation; it can disrupt the keratin bonds and the
cellular structure, producing a new skin frame with less wrinkles.
Despite its clinical advantages, close follow-up is required at the
post-peel period due to its potential risks.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
IEL and AM performed the phenol/croton oil peels.
IEL was a major contributor in drawing up the manuscript. GKI, GPT,
GG, IK and ADM confirm the authenticity of all the raw data. ADM
made contributions to conception and design and acquisition and
interpretation of the data. GKI, AM, GPT, GG and IK made
substantial contributions to conception and design of the study.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Procedures performed to all patients complied with
all national and international regulations and were in agreement
with The Declaration of Helsinki. The present study was approved by
Opsis Clinical Plastic Surgery Ethics Committee which belongs to
Heraklion Medical Association (approval no. OPS/CLNC/16-12-13/001)
and informed consent was obtained from all participants.
Patient consent for publication
The patients provided written informed consent for
the publication of their accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fischer TC, Perosino E, Poli F, Viera MS
and Dreno B: Cosmetic dermatology european expert group. Chemical
peels in aesthetic dermatology: An update 2009. J Eur Acad Dermatol
Venereol. 24:281–292. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rullan P: Commentary on characterization
of the activity of croton tiglium oil in Hetter's very heavy
phenol-croton oil chemical peels. Dermatol Surg.
47(947)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lemes BM, da Silva Justo A, Lin EM, Capote
ACMO, Neves AKL, Machinski I, Pereira AV, Koga AY, Lipinski LC,
Beltrame FL, et al: The effects of 35% trichloroacetic acid-croton
oil and 35% glycolic acid-croton oil compared to 35% phenol-croton
oil Hetter's very heavy formula for deep chemical peel. J Am Acad
Dermatol. 87:1227–1229. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Baker TJ, Gordon HL, Mosienko P and
Seckinger DL: Long-term histological study of skin after chemical
face peeling. Plast Reconstr Surg. 53:522–525. 1974.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hetter GP: An examination of the
phenol-croton oil peel: Parts I-IV. Plast Reconstr Surg.
105:1227–1083. 2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nogueira GC, Oliveira RIFM, de Queiroz
MVR, de Medeiros ACTR, Oliveira LPS and de Oliveira GV: Static
glabellar lines can be treated using a superlocalized phenol-croton
oil peel. JAAD Int. 11:63–64. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Atiyeh B, Oneisi A and Ghieh FJ:
Medium-depth trichloroacetic acid and deep phenol-croton oil
chemical peeling for facial rejuvenation: An update. Craniofac
Surg. 32:e745–e750. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wambier CG and Beltrame FL: Air safety and
personal protective equipment for phenol-croton oil peels. Dermatol
Surg. 44:1035–1037. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Starkman SJ and Mangat DS: Chemical peels:
Deep, medium and light. Facial Plast Surg. 35:239–247.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cardoso FAMES, Moura RD, Pilar EFS, Moura
ICG, Miot HA and da Costa A: Phenol-croton oil peel enhances type-1
and type-3 collagen amounts by stimulating SIRT-6 and SIRT-7. Int J
Dermatol. 61:e71–e74. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Justo AS, Lemes BM, Nunes B, Antunes KA,
Carletto B, Koga AY, Lipinski LC, Montemor Netto MR, Campagnoli EB,
Beltrame FL and Wambier CG: Depth of injury of Hetter's
phenol-croton oil chemical peel formula using 2 different
emulsifying agents. J Am Acad Dermatol. 82:1544–1546.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cardoso FAMES, Pilar EFS, Moura ICG, de
Melo CB, Pompeu VMA, Moura RD, Ramos-E-Silva M and da Costa A:
Neocolagenesis by phenol-croton oil peel is independent from the
MMP-1/MMP-2/JunB and p53 pathways. J Cosmet Dermatol. 21:1766–1769.
2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liapakis IE, Tzouganakis AC, Paschalis EI,
Englander M, Christopoulos A, Gloustianou G and Kontoes P: Parry
Romberg syndrome treatment with fat transfer and a new bleaching
formula. J Cosmet Dermatol. 18:1424–1429. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lee JC, Daniels MA and Roth MZ:
Mesotherapy, microneedling, and chemical peels. Clin Plastic Surg.
43:583–595. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Justo AS, Lemes BM, Nunes B, Antunes KA,
Capote ACMO, Lipinski LC, Campagnoli EB, Emiliano J, Meurer EC,
Ezemma O, et al: Characterization of the activity of croton
tiglium oil in Hetter's very heavy phenol-croton oil chemical
peels. Dermatol Surg. 47:944–946. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wambier CG, Lee KC, Soon SL, Sterling JB,
Rulla PP, Landau M and Brody HJ: International Peeling Society.
Advanced chemical peels: Phenol-croton oil peel. JAAD. 2:327–336.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bensimon RH: Croton oil peels. Aesth Plast
Surg. 28:33–45. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rullan P, Landau M and Rullan J:
Emulsifiers are not necessary to make formulas for deep chemical
peeling: response to Wambier et al: Advanced chemical peels:
Phenol-croton oil peel. J Am Acad Dermatol. 90:e197–e198.
2024.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Muriel-Sanchez JM, Cohena-Jimenez M and
Montano-Jimenez P: Effect of phenol application time in the
treatment of onychocryptosis: A randomized double-blind clinical
trial. Int J Environ Res Pub Health. 18(10478)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sun HF, Lu HS, Sun LQ, Ping WD, Mao DS and
Li D: Chemical peeling with a modified phenol formula for the
treatment of facial freckles on asian skin. Aesthetic Plast Surg.
42:546–552. 2018.PubMed/NCBI View Article : Google Scholar
|