1. Introduction
The term ‘atypical pneumonia’ was originally used to
describe community-acquired pneumonias (CAPs) due to viruses that
differed from bacterial CAPs as regards the clinical and radiologic
features. Over time, this term has evolved to denote lower
respiratory infections caused by specific respiratory
microorganisms, including Legionella species, Mycoplasma
pneumoniae, Chlamydia pneumoniae, Chlamydia
psittaci (psittacosis), Coxiella burnetii (Q fever) or
Francisella tularensis (tularemia) (1-3).
CAPs differ from typical bacterial CAPs via several
key mechanisms. Typical CAPs are most commonly caused by pathogens,
such as Streptococcus pneumoniae and Haemophilus
influenzae, which primarily present with more acute symptoms,
such as a high fever, productive cough and localized chest pain.
These infections are typically associated with radiographical
findings of lobar consolidation and respond well to β-lactam
antibiotics, which target the bacterial cell wall. By contrast,
atypical CAPs are caused by pathogens such as Legionella
species, Mycoplasma pneumoniae, and Chlamydia
pneumoniae, which often present with a more insidious onset,
milder respiratory symptoms, and prominent extrapulmonary
manifestations, such as headache, myalgia and gastrointestinal
symptoms. Atypical pathogens generally lack cell walls or reside
intracellularly, rendering them resistant to β-lactam antibiotics.
As a result, treatment typically requires antibiotics that can
penetrate cells, such as macrolides, tetracyclines or
fluoroquinolones. Moreover, while typical CAPs usually exhibit
well-defined lobar consolidation upon imaging, atypical CAPs often
exhibit diffuse interstitial patterns or patchy infiltrates,
reflecting their distinct pathophysiology and clinical course
(1-3).
Atypical CAPs account for ~15% of all CAP cases.
Although community outbreaks linked to atypical pneumonia pathogens
exist, the majority of cases of atypical CAP are sporadic. These
atypical microorganisms can occasionally result in outbreaks of
pneumonia acquired in nursing homes or are acquired in medical
facilities. Identifying atypical pneumonia as the cause of
nosocomial infections is infrequent.
Among adults with less severe or ambulatory CAP,
atypical microorganisms are more widespread compared to typical
bacterial pathogens. Legionella notably contributes to
severe CAP cases in hospitalized patients (4,5).
Atypical pneumonias can be clinically categorized
into zoonotic transmission-based and non-zoonotic forms. Zoonotic
atypical pneumonias encompass Q fever, psittacosis and tularemia,
while non-zoonotic types involve CAPs caused by Chlamydia
pneumoniae, Mycoplasma pneumoniae and Legionella.
Both zoonotic and non-zoonotic atypical pneumonias fundamentally
differ from bacterial CAPs. Yet, the key distinguishing factor
between atypical and typical CAP pathogens lies in the presence or
absence of extrapulmonary indications. All atypical pulmonary
pathogens, irrespective of their zoonotic or non-zoonotic nature,
induce systemic infectious diseases primarily affecting the lungs
(pneumonia). By contrast, pneumonias caused by Moraxella
catarrhalis, Streptococcus pneumoniae or Haemophilus
influenzae typically manifest with clinical findings and
results from laboratory testing confined to the respiratory system.
Once this differentiation is established in CAP cases with
extrapulmonary signs, clinicians can identify the characteristic
organ involvement pattern, facilitating focused diagnostic
considerations (6,7).
Every atypical pulmonary pathogen displays a
preference for particular extrapulmonary organ systems. What sets
apart atypical pneumonias is individual clinical or laboratory
findings, but also the distinct pattern of organ engagement. For
instance, extrapulmonary organ involvement caused by
Legionella markedly differs from that caused by Chlamydia
pneumoniae or Mycoplasma pneumoniae, forming the basis
for an initial clinical assessment. Identifying these unique
extrapulmonary patterns linked to each atypical pathogen generally
enables an accurate preliminary clinical diagnosis. However, this
preliminary diagnosis is not definitive and should prompt targeted
diagnostic tests to confirm or exclude specific pathogens (8).
The majority of research has not effectively
distinguished typical from atypical pneumonias due to its focus on
comparing the individual clinical and laboratory aspects of both
pathogen types (9-12).
These studies have found minimal discernible differences in
standalone findings (9-12).
Few studies have utilized a syndromic diagnosis (9-12),
while only one study (10)
employed a weighted syndromic point system. This system
distinguishes atypical pneumonias by using a scoring system based
on the presence of specific clinical features, such as symptoms and
laboratory results, which are weighted according to their
association with atypical pathogens. The weighted system helps
clinicians to prioritize testing and treatment for atypical
pathogens when the clinical presentation aligns more closely with
the characteristics typical of atypical pneumonias, such as the
longer duration of symptoms before seeking care, the presence of
certain epidemiological factors, and the absence of findings more
common in typical bacterial pneumonias. Using this weighted
approach, considering the relative clinical specificity of
characteristic clinical findings, clinicians can effectively
differentiate between typical and atypical pneumonias, even
presumptively diagnosing Legionnaire's disease accurately (9-12).
The significance of atypical pneumonias lies not merely in their
clinical occurrence, but also in other clinical and public health
considerations, demanding distinct therapeutic approaches compared
to typical CAPs (13).
Atypical pathogens such as Mycoplasma
pneumoniae and Chlamydia pneumoniae are prevalent among
young adults with CAP in outpatient settings, surpassing typical
CAP-causing pathogens in this context. They, along with
Legionella, significantly contribute to severe CAP cases.
Unlike typical bacteria susceptible to β-lactam antimicrobial
treatment due to their vulnerable cell walls, the majority of
atypical pathogens lack these walls. Some are intracellular, such
as Legionella, while others, such as Mycoplasma
pneumoniae, use paracellular pathways for entry (14). Antimicrobials that disrupt
intracellular protein synthesis effectively combat these atypical
pathogens. Macrolides and tetracyclines impede bacterial protein
synthesis inside cells. Quinolones and recently developed ketolides
exhibit high efficacy against atypical pathogens, particularly
Legionella. Given the intracellular nature of some atypical
pathogens such as Legionella, effective antibiotic
penetration into alveolar macrophages (AMs) is crucial. Macrolides,
tetracyclines, quinolones and ketolides exhibit a tendency to
accumulate in AMs (15-18).
Atypical CAP pathogens are more commonly encountered
in outpatient cases and play a particularly crucial role in the
severity of CAP among hospitalized patients. Additionally, public
health concerns contribute to the significance of certain atypical
CAP pathogens. Chlamydia pneumoniae infection is potentially
involved in coronary artery disease and neurological diseases, such
as multiple sclerosis. Moreover, infections from Chlamydia
pneumoniae and Mycoplasma pneumoniae could complicate
asthma. Both pathogens are notable causes of nonexudative
pharyngitis (19-25).
Zoonotic atypical pneumonias have historically been pivotal in
areas endemic to these diseases. Psittacosis continues to be a key
factor in causing CAP among individuals who have contact with
psittacine birds. Q fever sporadically occurs among those in
proximity to parturient cats or in sheep-raising regions.
Endocarditis poses an infrequent yet critical issue in endemic Q
fever zones. Tularemia, with its various clinical presentations,
may coincide with pneumonia. In endemic regions, tularemia remains
a pertinent and potentially serious infectious disease (26-29).
Atypical pathogens bear greater importance due to diagnostic
challenges, susceptibility to non-β-lactam antibiotics, and the
severity of associated complications.
2. Prevalence of atypical pneumonia
According to a previous study, the detectable rates
of atypical pathogens differ across regions, with the rates being
as follows: North America at 22%, Europe at 28%, Latin America at
21% and Asia/Africa at 20% (30).
Various countries and regions exhibit distinct rates of atypical
pathogen detection.
The methods used to detect atypical pathogens, such
as Mycoplasma pneumoniae, Chlamydia pneumoniae and
Legionella species, can vary widely between regions. Some
regions may rely more heavily on serological testing, which detects
antibodies produced in response to infection, while others may use
more advanced molecular techniques, such as polymerase chain
reaction (PCR) or multiplex PCR, which directly identify the
genetic material of the pathogens. PCR is generally more sensitive
and specific but is also more costly and requires sophisticated
laboratory equipment that may not be available in all regions.
In addition, the criteria used to diagnose atypical
pneumonia can differ between regions due to variations in clinical
guidelines, healthcare practices and the experience of healthcare
providers. Some regions may adopt broader or more inclusive
criteria that capture a wider range of cases, while others may use
more stringent criteria, potentially leading to differences in
detection rates. For example, the inclusion of certain clinical
symptoms, the timing of sample collection, and the use of
confirmatory tests such as paired serology can influence the
reported prevalence of atypical pathogens (31).
The prevalence of atypical pathogens can be
influenced by local epidemiological factors, including climate,
population density and the prevalence of comorbid conditions. For
instance, Legionella infections are more common in areas
with certain environmental conditions, such as the presence of
contaminated water sources. Additionally, variations in public
health measures, vaccination rates and the presence of endemic
diseases can affect the distribution and detection of atypical
pathogens. Furthermore, regions with more advanced healthcare
systems and better access to diagnostic tools are likely to have
higher detection rates of atypical pathogens as they can employ
more sensitive and specific diagnostic tests. By contrast, regions
with limited healthcare infrastructure may have lower detection
rates due to reliance on less sensitive methods or the
unavailability of certain diagnostic technologies. Furthermore,
differences in the methods through which health data are collected,
reported and interpreted can also contribute to regional variations
in detection rates. Some regions may have more robust surveillance
systems and mandatory reporting of atypical pneumonia cases,
leading to higher reported detection rates, while others might
underreport cases due to lack of surveillance infrastructure or
different public health priorities (31).
Europe
Previously, a survey on CAP outbreaks encompassing
3,523 patients (15% outpatients, 85% inpatients) between November,
1996 and July, 2008 revealed 1,463 patients with identifiable
causes. Streptococcus pneumoniae emerged as the primary
cause in Europe, accounting for 42% of the detectable rate, while
atypical pathogens and mixed infections also played significant
roles at 18 and 14%, respectively (32). In Spain, Capelastegui et al
(33) noted a 50% detectable rate
in their prospective study, where atypical pathogens were more
prevalent among outpatients (67%) than inpatients (30.6%). In
addition, two studies in The Netherlands highlighted
Streptococcus pneumoniae as the primary cause of CAP, with
varying detectable rates for atypical pathogens (9 and 20%)
(34,35).
Israel
Conversely, a study in northern Israel showcased a
52.4% detectable rate for atypical pathogens (Chlamydia
pneumoniae, 20.6%, Mycoplasma pneumoniae, 18.3%,
Legionella pneumophila 7.1%, and others) (36).
China
An extensive epidemiological survey conducted in
China revealed results that differed from those in European
countries (37). In that study,
atypical pathogens were the primary cause of CAP. Mycoplasma
pneumoniae was the most common pathogen, with a prevalence of
20.7%, followed by Streptococcus pneumoniae at 10.3%
(37). Co-infections, particularly
with bacteria and atypical pathogens, were prominent in community
respiratory infections (37). In
two national CAP surveys in performed China (38), Mycoplasma pneumoniae
surpassed Streptococcus pneumoniae as the most common cause
among adults, with rates of 38.9 and 32.6%, respectively. Chen
et al (39) reported
Mycoplasma pneumoniae as the predominant pathogen, with a
positive percentage of 40.78%, exhibiting a significant association
with seasons, particularly prevalent in late summer and autumn.
Chile
In Chile, among 356 patients, Streptococcus
pneumoniae and viruses were predominant, with atypical
pathogens contributing to 22% of the infections (40). In a clinical study conducted in
Santiago, Chile, focusing on 104 patients with severe CAP between
2005 and 2006, the top seven identified etiological agents were
observed. Streptococcus pneumoniae accounted for 26%, while
Legionella pneumophila followed closely at 8.6%. Other
pathogens included Mycoplasma pneumoniae (6%), Chlamydia
pneumoniae (4%), Gram-negative bacillus (3%), influenza A virus
(3%) and Staphylococcus aureus (3%). Notably, Legionella
pneumophila ranks as the second etiological agent in severe CAP
cases, following Streptococcus pneumoniae. The global
mortality at 28 days in severe CAP was 25%, with Legionella
pneumophila exhibiting a mortality rate of 33.3% (three out of
nine cases); however, this difference was not significant when
compared to non-Legionella severe CAP mortality (33 vs.
24.5%) (41).
USA and other regions
The incidence of Legionella pneumophila in
CAP is relatively high worldwide, particularly in the USA (14%)
(42) and Spain (12.5%) (43). Even in Asia, the incidence stands
relatively high at 6.6% (43).
According to a previous study, the general
occurrence of atypical pathogens such as Chlamydia
pneumoniae, Mycoplasma pneumoniae and Legionella
among individuals experiencing severe pneumonia stood at 8.1%,
varying widely from 0 to 48.1%. Notably, the prevalence in adults
was slightly lower than that described in children. Notably, the
combined group that did not differentiate between adults and
children exhibited a prevalence of 12.1%, significantly influencing
the overall prevalence rates (44).
3. Diagnostic approach
Clinical presentation
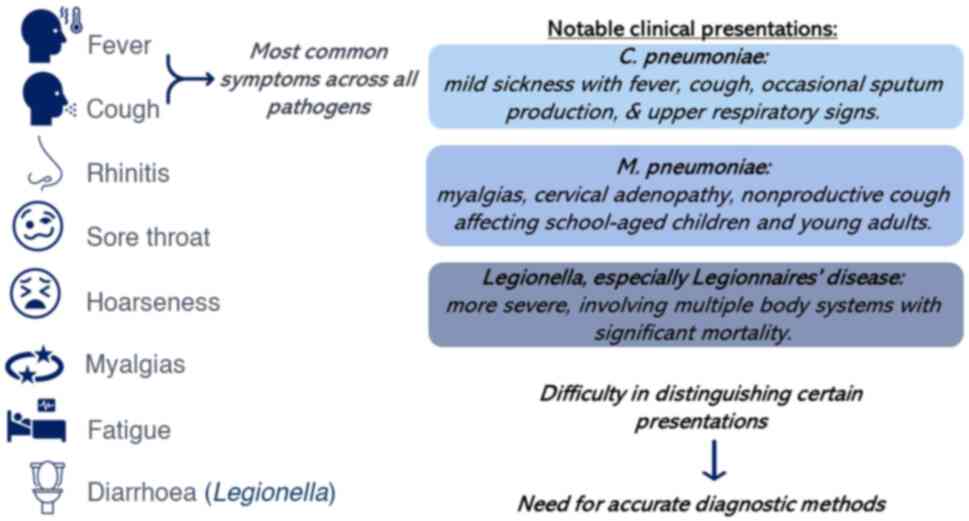
A schematic illustration of the key symptoms and
clinical presentations of pathogens is presented in Fig. 1. Pneumonia caused by Chlamydia
pneumoniae manifests as a mild illness, primarily characterized
by fever and cough, often followed by upper respiratory signs, such
as rhinitis and a sore throat. In the study in 2013 by Conklin
et al (45), the duration
of cough ranged from 1 to 64 days, averaging ~21 days. While a
non-productive cough is typically associated with this condition,
~70% of patients exhibited sputum production during Chlamydia
pneumoniae outbreaks in 2006 and 2013(45). There are difficulties in
distinguishing this presentation from Mycoplasma pneumoniae
or pneumonia caused by respiratory viruses. Despite earlier notions
suggesting that hoarseness and laryngitis were more prevalent in
Chlamydia pneumoniae infection than in Mycoplasma
pneumoniae infection, previous comparisons of clinical
characteristics have indicated the opposite (46,47).
It has been reported that rhinitis, cough and
hoarseness were notably more prevalent in Mycoplasma
pneumoniae infection compared with Chlamydia pneumoniae
infection (47). The same
researchers observed that C-reactive protein (CRP) and aspartate
aminotransferase levels were substantially higher in Chlamydia
pneumoniae infection than in Mycoplasma pneumoniae
infection. However, other clinical symptoms and laboratory findings
between the two pathogens did not exhibit significant differences
(47) according to an earlier
study, patients with pneumonia caused by both Chlamydia
pneumoniae and Mycoplasma pneumoniae have notably lower
CRP and white blood cell values than in those with pneumonia caused
by Streptococcus pneumonia (46).
No specific symptom, laboratory marker, or
combination of findings reliably distinguishes C.
pneumoniae-induced pneumonia from that caused by other respiratory
pathogens. Additionally, concurrent infection with other pathogens
alongside Chlamydia pneumoniae can affect the clinical
presentation (45).
Pneumonia stemming from Mycoplasma pneumoniae
often presents a challenging clinical scenario due to its mild and
ambiguous symptoms, such as myalgias, cervical adenopathy,
nonproductive cough and fatigue, rendering differentiation from
other viral upper respiratory infections and atypical bacterial
infections difficult (32,48,49).
Mycoplasma pneumoniae commonly affects
children attending school and young adults, often causing outbreaks
during the autumn season (32,48-50).
These outbreaks typically affect individuals in close contact with
infected patients within households or confined spaces (51). Apart from its unconventional
symptoms, the manifestations of Mycoplasma pneumoniae can
differ markedly, spanning from mild upper respiratory symptoms to
pneumonia and various manifestations unrelated to the lungs. These
include cardiovascular, dermatological and central nervous system
symptoms, even without the presence of pneumonia (52).
Legionella infections manifest primarily in
two forms: i) Legionnaires' disease, a severe pneumonia resulting
from Legionella infection. It often involves multiple body
systems, notably the lungs and gastrointestinal tract, with
associated significant mortality rates (53). ii) Pontiac fever is a mild,
self-limiting flu-like illness. Pontiac fever is characterized by
mild fever, chills, myalgia, and headaches lasting 2-5 days,
typically resolving without substantial mortality (54).
While Legionella primarily affects
individuals aged ≥50 years, instances have been documented in
infants and neonates (55).
Distinguishing Legionnaires' disease from pneumonia caused by other
pathogens can be challenging due to similar clinical symptoms;
however, the presence of diarrhea and heightened creatinine kinase
levels may signal a Legionella infection (10). Legionella-induced pneumonia
often occurs in clusters, but not through person-to-person
transmission, typically stemming from exposure to the same
infection source. Contaminated water or soil largely account for
Legionella infections. Risk factors include rainfall, high
humidity, and working in gardens with compost (56-58).
Although the majority of cases of Legionnaires' disease are
associated with Legionella pneumophila, several other
bacterial species have been identified as causative agents of
Legionella lung infections (58,59).
Zoonotic atypical CAPs stemming from Q fever,
psittacosis or tularaemia typically manifest following exposure to
their respective carriers. Notably, psittacosis stands as an
outlier, potentially transmissible through contact with healthy or
ailing psittacine birds. By contrast, incidences of tularemia and Q
fever CAP are not arbitrary; establishing a recent epidemiological
background is imperative before suspecting these diagnoses. Should
a patient displaying atypical pneumonia lack a recent contact
history associated with psittacosis, Q fever, or tularaemia, the
likelihood of a zoonotic atypical CAP is exceedingly low (19-22).
Thus, it can reasonably be inferred that the patient is
experiencing a non-zoonotic atypical pneumonia linked to
Legionella, Mycoplasma pneumoniae, or Chlamydia
pneumonia (59).
Collectively, pneumonia caused by Chlamydia
pneumoniae, Mycoplasma pneumoniae and Legionella species
presents with distinct clinical features. Chlamydia
pneumoniae typically leads to a milder, more insidious onset of
symptoms, including a prolonged cough, low-grade fever, and common
respiratory symptoms, such as a sore throat and hoarseness.
Mycoplasma pneumoniae often affects younger populations,
with a gradual onset characterized by a dry cough, fever and
extrapulmonary manifestations, such as skin rashes and neurological
symptoms. By contrast, Legionella infections, particularly
Legionella pneumophila, cause a more severe form of
pneumonia known as Legionnaires' disease. This presents with high
fever, chills, myalgia and prominent gastrointestinal symptoms,
such as diarrhea, often accompanied by neurological signs, such as
confusion. Legionella pneumophila progresses rapidly and can
lead to severe, potentially life-threatening outcomes, particularly
in older adults and individuals with underlying health conditions
(60).
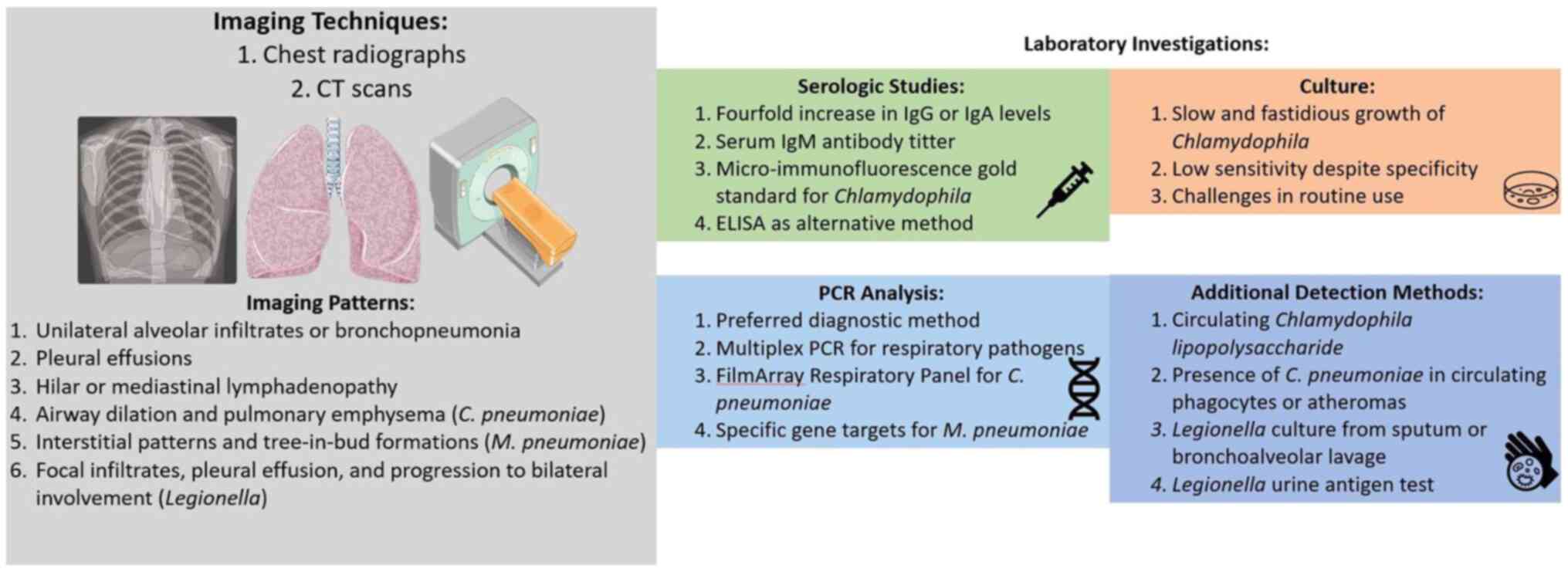
Imaging
An illustration of the imaging techniques and
characteristics, and comparison of laboratory investigations for
the detection of infection is presented in Fig. 2. As regards Chlamydia
pneumoniae, initially, chest radiographs typically reveal a
unilateral pattern of alveolar infiltrates or consolidation, often
limited to a single lobe. The lower lobe involvement is more
frequent than detecting lesions in the middle or upper lobe
(60-63).
Instances of interstitial pneumonia manifest relatively
infrequently. Approximately a quarter of patients may exhibit small
to moderate pleural effusions, while findings, such as hilar or
mediastinal lymphadenopathy are less commonly observed in chest
radiographs. Variations in findings may hinge on the timing of
imaging during the illness, the diagnostic method used, and the
exclusion of concomitant respiratory pathogens. In a previous study
involving 55 patients classified with primary infection, initial
chest radiographs depicted predominantly unilateral findings, while
subsequent radiographs taken around 3.8 days later revealed
predominantly bilateral findings (61).
In a previous retrospective analysis of thin-section
CT scans from 24 patients with serologically diagnosed with CAP
caused by Chlamydia pneumoniae, Nambu et al (64) observed a marked increase in airway
dilation compared to Streptococcus pneumoniae or
Mycoplasma pneumoniae-related pneumonia cases, along with a
higher incidence of pulmonary emphysema compared to Mycoplasma
pneumoniae cases, but not Streptococcus pneumoniae
cases. Their study suggested that the elevated airway dilation and
pulmonary emphysema may stem from pre-existing obstructive lung
disease rather than the infection itself (64). Despite significant findings in
pulmonary emphysema and airway dilation, neither these nor other CT
scan observations were able to reliably distinguish Chlamydia
pneumoniae-related pneumonia from that caused by other
pathogens (64). Overall, CT scan
or radiograph results in C. pneumoniae cases exhibit broad
variability and lack specificity for identifying the pathogen as
the cause of pneumonia (61-64).
The imaging characteristics of Mycoplasma
pneumoniae infections mirror their elusive nature. Chest
radiographs commonly reveal diffuse interstitial patterns,
occasionally disproportionate to the physical symptoms of patients.
On chest CT scans, the interstitial alterations apparent in the
radiographs manifest as tree-in-bud formations (65). In a 2016 prospective study by Gong
et al (65) involving 1,280
pediatric cases of pneumonia caused by Mycoplasma pneumoniae
between 2010 and 2014, a substantial proportion of patients
exhibited extensive patchy infiltrates, both unilateral and
bilateral, suggesting that the diagnosis of pneumonia could not be
solely determined based on imaging characteristics.
Legionellosis chest radiographs have been described
in multiple reports (66,67). While some attempts have been made
to outline specific patterns indicative of Legionella, the
radiographic findings in Legionella infection demonstrate
significant variability, predominantly influenced by the timing of
the radiograph in the course of the illness. Certain temporal
features, however, can augment the probability of diagnosing
Legionella pneumonitis. Initially, poorly defined focal
infiltrates are common, with around 10% concurrent with pleural
effusion. These infiltrates tend to progress to adjacent lobes,
eventually becoming bilateral, with pleural effusions occurring in
about 35% of cases. This progression often persists despite
appropriate antimicrobial treatment and even in the presence of
clinical improvement. Immunocompromised individuals exhibit a
similar pattern, often displaying a high incidence of cavitation
and hilar adenopathy. A lengthy resolution phase, lasting up to 6
months, frequently occurs, occasionally resulting in residual
densities. Attempts to associate radiographic characteristics with
disease severity and mortality have had limited success (68).
Collectively, imaging studies reveal distinct
patterns for each type of pneumonia. Chlamydia pneumoniae
typically presents on a chest X-ray with diffuse interstitial
infiltrates, often patchy or involving the lower lobes, with
occasional segmental consolidation. Mycoplasma pneumoniae is
usually associated with reticulonodular patterns or patchy
consolidations on an X-ray, predominantly in the lower lobes, and
occasionally, hilar lymphadenopathy. CT scans may reveal bronchial
wall thickening and centrilobular nodules. By contrast, pneumonia
caused by Legionella is characterized by rapidly progressing
lobar consolidation, often with bilateral involvement on chest
X-ray. CT imaging may reveal dense consolidations, nodular
opacities, ground-glass changes and sometimes small pleural
effusions, reflecting the more aggressive and widespread nature of
this infection (69).
Laboratory investigations
Established methods to detect Chlamydia
pneumoniae infection involve serological studies and the
culture or PCR analysis of respiratory tract samples. An organized
discussion of the different testing methods is provided below:
Serological tests
Traditionally, the diagnosis of Chlamydia
pneumoniae infection has hinged on serology, necessitating a
4-fold increase in IgG or IgA levels between acute and convalescent
serum samples. Serological approaches are generally intricate as
patients must return after 4 to 6 weeks from the initial
presentation to confirm the diagnosis retrospectively. Moreover,
this retrospective nature renders serological outcomes minimally
impactful on treatment decisions. Different serological criteria
used for diagnosis upon initial presentation, such as a serum IgM
antibody titer of 1:16 or higher, strongly depend on when the
sample was collected. This is due to the potential absence of a
titer rise early in acute infection or reinfection. Depending
entirely on initial serologic samples for diagnosis, without
confirming retrospectively using convalescent serum samples, poses
the risk of overlooking 25 to 33% of infections. The initial
serological testing could require several days to produce results,
further limiting their utility in making initial management
decisions. Possible cross-reactivity between Chlamydia
pneumoniae antigens and antigens from other Chlamydia
species limits the specificity of serological techniques.
Microimmunofluorescence is considered the gold standard for
serological diagnosis (70,71).
ELISA, an alternative method, may be less intricate
and more objectively interpretable than microimmunofluorescence
(69). However, complement
fixation is not recommended for diagnosis due to its limited
sensitivity and specificity (70,72).
PCR technology
Considering the constraints of serology and culture,
the PCR analysis of respiratory samples has become the preferred
diagnostic method. Multiplex PCR can assess multiple potential
respiratory pathogens without a significant decrease in sensitivity
compared to singleplex PCR testing (73). In 2012, the FDA sanctioned the
FilmArray Respiratory Panel, employing multiplex PCR to identify
Chlamydia pneumoniae and other microorganisms from
nasopharyngeal swabs (74). PCR,
however, faces specificity limitations due to asymptomatic carriage
and persistent identification of Chlamydia pneumoniae on
respiratory swabs even after clinical symptom resolution, possibly
extending for several weeks to months following antibiotic therapy
(75,76). This persistence complicates
definitively attributing positive PCR results to persistent
infection, reinfection, or ongoing asymptomatic carriage,
potentially involving other pathogens (76). Moreover, Chlamydia
pneumoniae detection in respiratory samples does not exclude
coinfection with other pathogens, affecting clinical presentation
as observed in multiple studies (72,76).
Other detection methods include identifying Chlamydia
lipopolysaccharide in circulation or the presence of Chlamydia
pneumoniae in circulating phagocytes or atheromas. However,
these approaches are technically complex and presently restricted
to research settings (70).
Traditionally, the diagnosis of Mycoplasma
pneumoniae relied on cultures and serology, with culture
isolation once deemed the gold standard. However, due to the slow
and inconsistent growth of Mycoplasma pneumoniae, routine
culturing is no longer common and offers limited clinical utility
(48,50). Other diagnostic avenues include
serologic studies using ELISA to quantify bacterial antibody
expression, microparticle agglutination and complement fixation
assays. Definitive diagnosis in serologic studies required paired
sera demonstrating a significant 4-fold increase in IgG or
subsequent seroconversion 3-4 weeks later (77-80).
Yet, due to delayed antibody production and seroconversion, these
tests hold limited utility for the diagnosis of acute Mycoplasma
pneumoniae infections in clinical settings and are more
retrospective for epidemiological studies (50,77-79).
As culture and serology have shortcomings in the
diagnosis of Mycoplasma pneumoniae, diagnostic methods are
shifting toward faster molecular techniques, such as nucleic acid
amplification. Molecular diagnostics enable the timely detection of
Mycoplasma pneumoniae infections and are increasingly
pivotal in clinical diagnosis. An array of laboratory techniques,
such as nucleic acid amplification, multilocus variable number
tandem-repeat analysis, and multilocus sequence typing, are
becoming prominent (50). These
tests deliver rapid, highly specific, and sensitive results
(50,77). Several tests employ real-time PCR
to target specific gene regions of Mycoplasma pneumoniae,
including those encoding the P1 gene, 16S ribosomal RNA, the ATPase
operon, and the community-acquired respiratory distress syndrome
toxin (50,77-80).
This technology has led to multiplex PCR development, allowing for
the detection of various atypical pathogens, including Chlamydia
pneumoniae, Chlamydia psittaci, Legionella
species and other respiratory viruses (50,72).
Nonetheless, debate persists over which sample types provide the
best sensitivity and specificity for these assays. Studies have
suggested that sputum samples yield more positive results compared
to nasopharyngeal aspirates, nasopharyngeal swabs, or oropharyngeal
swabs (79,81).
Since numerous aspects of Legionella closely
resemble both typical and atypical pneumonias, relying on clinical
symptoms or radiological evidence offers limited diagnostic value.
The CDC indeed relies on several methods to confirm
Legionella infections. These include culturing
Legionella bacteria from respiratory samples, such as sputum
or bronchoalveolar lavage, detecting the Legionella antigen
in urine, or observing a significant increase (≥4-fold) in
Legionella-specific antibodies in the blood serum of
patients when comparing acute and convalescent samples (82). PCR-based diagnostic tests, although
demonstrating specificity and sensitivity in ongoing assessments,
are pending approval by the FDA. Other methods, such as direct
immunostaining, are being utilized to identify the bacterium, but
often necessitate invasive procedures to procure tissue for testing
(83).
Culture methods
Culture, although considered specific due to a low
asymptomatic carriage rate, has limited sensitivity due to the slow
and fastidious growth of Chlamydia species, often requiring
weeks (68,84,85).
Previous studies indicate a minimal frequency of growth in culture,
even when infection is identified through serology or PCR (68). Some researchers in 2010(84) discouraged routine culture use due
to the inability to detect any positive results among 6,981
respiratory specimens, despite Chlamydia species accounting
for 5 to 22% of CAP and other respiratory infections.
Due to the challenging nature of isolating
Chlamydia psittaci, its diagnosis relies entirely on
serological methods. In individuals lacking immunity or prior
exposure, heightened tube agglutination tests for Chlamydia
psittaci serve as a definitive diagnostic tool. Similarly, the
diagnosis of tularemia and Q fever relies on serology due to the
highly infectious, perilous and elusive nature of these organisms.
In individuals lacking immunity or previous exposure, acute
increases in Francisella tularensis IgM/IgG levels serve as
diagnostic indicators. As regards Q fever or tularemia, apart from
initially elevated acute titers, the diagnosis of these zoonotic
CAPs is contingent upon a 4-fold increase in titers between acute
and convalescent samples taken 4-8 weeks apart (83).
Collectively, the diagnosis of atypical pneumonia
can be achieved through several methods, each with distinct
advantages and limitations. Serologic testing, while widely
available and cost-effective, often suffers from delayed diagnosis
due to the need for paired sera to detect rising antibody titers,
and it may produce false positives due to cross-reactivity with
other pathogens (6,71). Culture methods offer high
specificity and allow for direct pathogen identification and
susceptibility testing; however, they are time-consuming, have a
low sensitivity and require specialized media, rendering them less
practical for routine diagnostics (72,83).
PCR assays provide a highly sensitive and specific method for early
pathogen detection, delivering rapid results that can significantly
impact patient management. However, PCR is more costly, requires
specialized equipment and may detect non-viable organisms,
complicating the interpretation of positive results (60,75).
Combining these methods can enhance diagnostic accuracy,
particularly in complex cases of atypical pneumonia.
4. Treatment
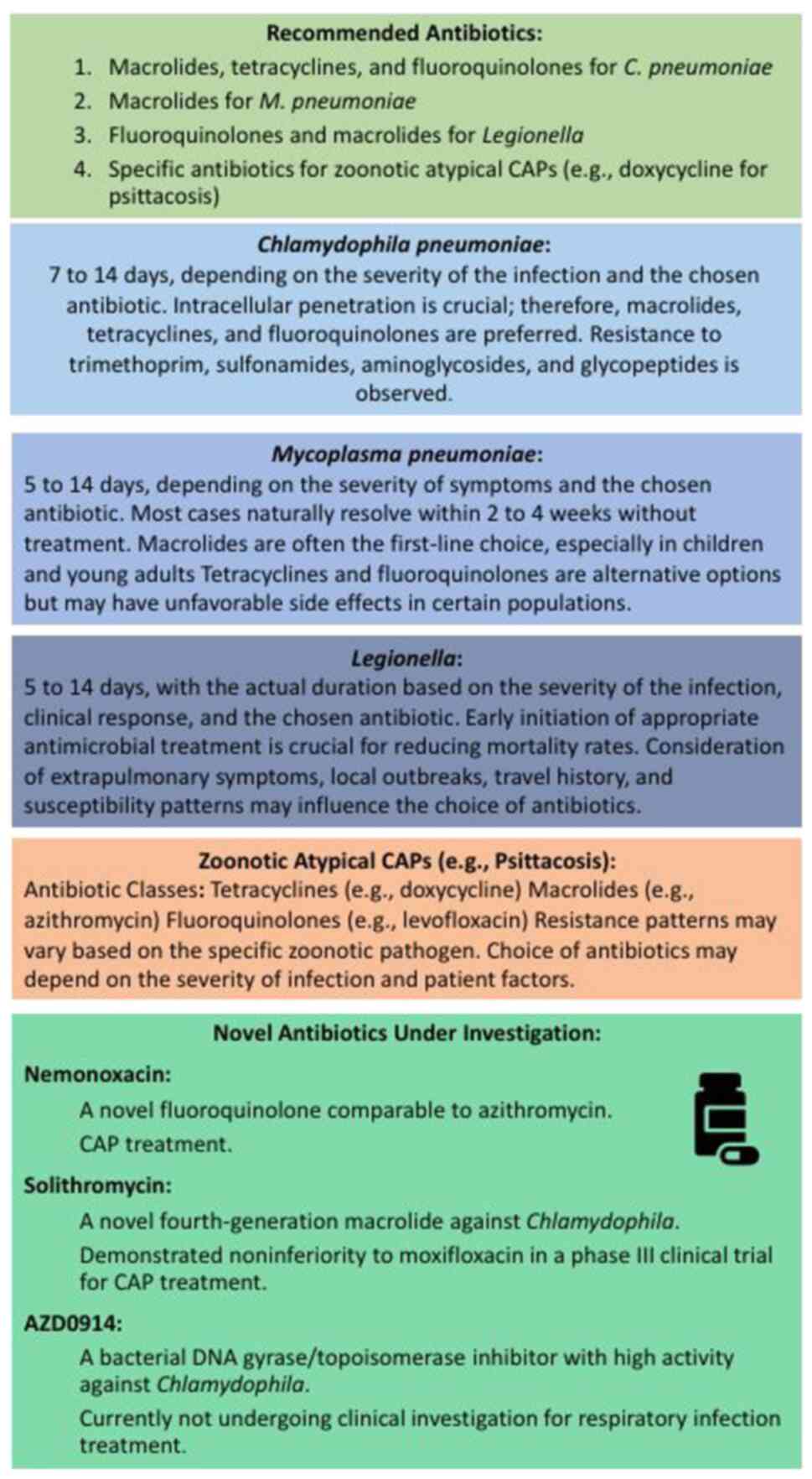
The antibiotic treatment recommendations (Fig. 3) for Chlamydia pneumoniae
face limitations due to the absence of standardized diagnostic
criteria and reliance on serology alone in most past studies. The
2007 guidelines from the Infectious Diseases Society of America
(IDSA) acknowledge a lack of robust evidence supporting specific
antibiotic therapies for this pathogen (85). Consequently, treatment suggestions
are still largely based on expert opinions. In cases where symptoms
reappear after a standard antibiotic course, experts recommend
prolonged treatment upon identification of Chlamydia species
(70).
Effective antibiotic therapy against Chlamydia
pneumoniae necessitates intracellular penetration due to its
nature as an obligate intracellular microorganism. Antibiotic
classes, such as macrolides, tetracyclines and fluoroquinolones,
which disrupt DNA and protein synthesis, display in vitro
activity against this pathogen, thus becoming the recommended drugs
for clinical treatment (86).
Macrolide and tetracycline antibiotics are
effective against atypical pathogens, such as Chlamydia
pneumoniae, Mycoplasma pneumoniae and Legionella
species, primarily due to their ability to penetrate and act within
host cells, targeting intracellular processes. These atypical
pathogens are often intracellular or lack the typical cell wall
structure, which renders them inherently resistant to β-lactam
antibiotics such as penicillin (4).
Among fluoroquinolones, ciprofloxacin exhibits a
higher minimum inhibitory concentration compared to others in this
class, potentially reducing its efficacy. Notably, Chlamydia
pneumoniae exhibits resistance to trimethoprim, sulfonamides,
aminoglycosides and glycopeptides. While penicillin and amoxicillin
display in vitro activity against Chlamydia species,
they are not recommended as routine therapies for Chlamydia
pneumoniae. Resistance to the recommended treatments is
infrequent and does not appear to contribute to treatment
ineffectiveness or the persistence of Chlamydia pneumoniae
identified in respiratory samples following the completion of
therapy. This is evidenced by isolates obtained from patients
following appropriate therapy, displaying in vitro
sensitivity (86).
Three new antimicrobial agents, solithromycin
nemonoxacin and AZD0914, have exhibited in vitro activity
against Chlamydia species but are currently undergoing trial
phases and await FDA approval for treatment (87-89).
Nemonoxacin, a new fluoroquinolone, demonstrates in vitro
effectiveness similar to that of azithromycin, doxycycline and
levofloxacin (88). Clinical
trials involving 256 and 192 patients with mild to moderately
severe CAP have demonstrated the effectiveness of nemonoxacin in
treating all identified patients with Chlamydia pneumoniae,
albeit a total of only 9 patients between both trials (90-91).
Solithromycin a novel fourth-generation macrolide,
has been shown to exhibit in vitro activity against
Chlamydia species and has demonstrated non-inferiority to
moxifloxacin in a phase III clinical trial for CAP treatment;
however, that study did not specifically identify patients with
Chlamydia infection (92).
AZD0914 exhibits potent activity against Chlamydia species
and various other respiratory pathogens in vitro as a
bacterial DNA gyrase/topoisomerase inhibitor. Nevertheless, it is
not currently undergoing clinical investigation for respiratory
infection treatment (88).
Infection caused by Mycoplasma pneumoniae
often goes undetected, as patients tend to forgo seeking treatment
due to the gradual onset of symptoms (32,48,49).
The bacterium has an extended incubation period of ~3 weeks, and
symptomatic shedding can persist for up to 4 months; however, the
majority of cases naturally resolve within 2 to 4 weeks without
treatment (32,48,77).
When patients seek clinical care, their treatment
is commonly directed by the IDSA guidelines for CAP, considering
the symptoms of the patient and imaging outcomes (93). Mycoplasma pneumoniae, being
a small bacterium lacking a cell wall, inherently resists β-lactam
antimicrobials. Despite this, it is usually treated in empirical
CAP treatment with macrolide, often in the absence of a confirmed
laboratory diagnosis. This antimicrobial treatment has the
potential to reduce the duration of the illness, requiring a course
of antibiotics ranging from 5 days to 2 weeks, depending on the
selected antibiotic for individuals affected by the infection
(94,95). Due to its prevalence among children
and young adults, macrolides have become the preferred treatment
choice. Tetracyclines and fluoroquinolones, while effective, are
associated with unfavorable side-effects that are more problematic
in younger patients, such as dentition discoloration with
tetracyclines and tendinitis with fluoroquinolones (95).
Managing extrapulmonary symptoms or complex cases
of Mycoplasma pneumoniae infection beyond antibiotic
treatment remains uncertain in terms of specific treatment
protocols. For patients with Mycoplasma
pneumoniae-associated extrapulmonary conditions, understanding
the inflammatory nature of the bacteria is crucial (96). Through pathways linked to Toll-like
receptor 2, the bacteria can prompt pro-inflammatory cytokine
production and inflammasome activity. This could clarify why
symptoms are more common among young adults, as they typically have
a stronger immune response compared to infants or elderly patients
who may not generate the same level of response (97). In patients with central nervous
system complications or severe pneumonia caused by Mycoplasma
pneumoniae, there have been reports suggesting the potential
benefits of steroids and immunoglobulin therapy, although these
findings have not been validated in clinical trials (56,98).
Additionally, for severe pneumonia leading to acute respiratory
distress syndrome, reports indicate potential benefits from
extracorporeal membrane oxygenation and the use of steroids
(56,79,81).
The primary treatment for pneumonia due to
Legionella involves antibiotics. Failure to administer
appropriate antimicrobial treatment at an early stage is linked to
high mortality rates (99,100). Selecting the right antibiotic is
not solely based on its in vitro ability to kill or inhibit
bacteria, but also on its capacity to penetrate host tissue cell
membranes, where Legionella resides. Among the most commonly
used and highly effective antibiotics for treating Legionnaires'
disease are fluoroquinolones and macrolides. Including these agents
in the initial treatment plan is advisable when Legionella
infection is suspected due to local outbreaks, travel history or
extrapulmonary symptoms (86).
Early reports from the initial outbreak of
Legionnaires' disease found that tetracycline and erythromycin were
more effective than other antibiotics, such as β-lactams, while the
use of steroids was linked to unfavorable outcomes (53). Erythromycin, a historically
preferred antibiotic, has exhibited high effectiveness against
Legionnaires' disease, but may cause notable side-effects,
particularly when administered intravenously (100-103).
Azithromycin, another macrolide, has demonstrated high efficacy
with fewer side-effects in treating Legionella infection, often
used when erythromycin does not yield results (104,105).
Clarithromycin, rifampin, ciprofloxacin and
doxycycline are other effective antibiotics against
Legionella, either used individually or in combination with
erythromycin (98). Research
findings suggest that fluoroquinolones demonstrate effectiveness
comparable to, or even greater than, erythromycin in treating
Legionnaires' disease. Levofloxacin has exhibited a high efficacy,
with shorter periods of hospitalization and early clinical
responses, becoming a favored antibiotic for this condition
(40,106-108).
While the majority of antibiotic therapies span 5
to 10 days and effectively treat Legionella infection,
immunocompromised patients may require longer durations, up to 3
weeks. Administration routes vary based on infection severity, with
parenteral therapy preferred for severe cases, transitioning to
oral treatment once a positive response is observed (101).
Antibiotic resistance in Legionella species
is rarely reported in clinical settings, although in vitro
resistance has been observed. Previous reports have highlighted
instances of fluoroquinolone resistance in patients undergoing
treatment, emphasizing the need for close monitoring during ongoing
antibiotic therapy (109,110). Table
I summarizes the effective therapies for atypical pneumonia
microorganisms.
 | Table IMost effective therapies for atypical
pneumonia. |
Table I
Most effective therapies for atypical
pneumonia.
| Microorganism | Effective
therapies |
|---|
| Chlamydia
pneumoniae | Macrolides,
tetracyclines, fluoroquinolones |
| Mycoplasma
pneumoniae | Macrolides,
tetracyclines, fluoroquinolones |
| Legionella
species | Fluoroquinolones,
macrolides |
| Chlamydia
psittaci (psittacosis) | Tetracyclines
(e.g., doxycycline), macrolides (e.g., azithromycin),
fluoroquinolones |
| Coxiella
burnetii (Q fever) | Tetracyclines
(e.g., doxycycline), fluoroquinolones |
| Francisella
tularensis (tularemia) | Aminoglycosides
(e.g., streptomycin, gentamicin), tetracyclines (e.g.,
doxycycline), fluoroquinolones |
Increased antibiotic resistance in the treatment of
atypical pneumonia, caused by pathogens such as Mycoplasma
pneumoniae, Chlamydia pneumoniae and Legionella
species, can significantly affect treatment outcomes and disease
progression. When atypical pneumonia pathogens, particularly
Mycoplasma pneumoniae, develop resistance to commonly used
antibiotics, such as macrolides (e.g., azithromycin,
clarithromycin), patients may experience delayed a clinical
improvement. For instance, macrolide-resistant Mycoplasma
pneumoniae has been increasingly reported, particularly in
Asia. In cases where macrolide resistance is present, the initial
antibiotic therapy may fail, leading to prolonged symptoms, such as
persistent cough, fever and malaise, and necessitating the use of
alternative antibiotics such as fluoroquinolones or tetracyclines,
which may have a broader side-effect profile. Antibiotic resistance
can lead to more severe disease progression due to ineffective
initial treatment. For example, in Legionella infections,
delayed or inappropriate antibiotic therapy due to resistance can
result in a higher risk of complications, such as acute respiratory
distress syndrome (ARDS), multi-organ failure, or even death,
particularly in vulnerable populations, such as the elderly or
immunocompromised patients. The timely administration of effective
antibiotics is critical in treating Legionnaires' disease, and
resistance can undermine this, leading to more aggressive disease
progression.
Resistance to first-line antibiotics often requires
switching to second- or third-line treatments, which may be less
effective, more toxic, or more expensive. For instance, patients
with macrolide-resistant Chlamydia pneumoniae may require
alternative treatments, such as doxycycline or fluoroquinolones,
which could extend the duration of therapy and hospitalization.
This not only increases healthcare costs, but also places patients
at higher risk of hospital-acquired infections and other
complications associated with prolonged hospital stays. In some
cases, antibiotic resistance can lead to the failure to completely
eradicate the infection, resulting in chronic or recurrent
pneumonia. This is particularly concerning in Chlamydia
pneumoniae infections, where resistance can lead to a chronic,
low-grade infection that persists despite treatment, potentially
contributing to the chronic inflammatory state and associated
complications, such as chronic bronchitis or worsening of chronic
obstructive pulmonary disease (COPD).
When antibiotic-resistant atypical pneumonia is not
adequately treated, there is a higher risk of ongoing transmission,
particularly in community or healthcare settings. For example,
patients with macrolide-resistant Mycoplasma pneumoniae may
remain infectious for a longer period of time, leading to outbreaks
in settings, such as schools, military barracks, or long-term care
facilities, where close contact facilitates the spread of infection
(111-113).
5. Prognosis
Pneumonia caused by atypical pathogens typically
presents as mild or moderate, although its progression to severe
pneumonia often results in a fatal outcome (6). A previous retrospective study
revealed that among patients with pneumonia infected with
Chlamydia pneumoniae, ARDS developed in 6 out of 11 cases
(114). The mortality rate was
notably high, reaching 83% among those with APACHE II scores ≥12
and 100% among those with CURB-65 scores ≥2(114). Detecting multi-lobar involvement
at an earlier stage is crucial. In Europe, a previous study
involving patients with pneumonia averaging 66 years of age
highlighted a worse prognosis among elderly patients with
Legionella pneumophila infection (115). That study reported an overall
mortality rate as high as 23%, with a majority of fatalities
attributed to UK community-acquired Legionella pneumophila
infections (115). Complications
arising from atypical pathogen infections extend beyond the
respiratory system, leading to a poorer prognosis. These
complications include damage to various organs such as the heart,
liver, kidneys, blood system and mucous membranes. Atypical
pathogen infections can exacerbate conditions, such as COPD, induce
bronchial asthma, progress to ARDS and potentially increase the
risk of lung cancer. In cases of the acute exacerbation of COPD,
atypical pathogens, predominantly Mycoplasma pneumoniae and
Chlamydia pneumoniae, account for 5-10% of cases, with as
many as 14% associated with Mycoplasma pneumoniae and
5.0-8.9% with Chlamydia pneumoniae infections (116). Interaction between Chlamydia
pneumoniae infection and allergic inflammation may exacerbate
the symptoms of asthma (117,118). Legionella pneumophila
pneumonia tends to progress to ARDS more frequently compared to
other pathogens (41). While the
association between Chlamydia pneumoniae infection and lung
cancer remains debatable, studies suggest a potential link
(119-122).
Complications in the cardiovascular system induced by atypical
pathogen infections include coronary artery disease, myocardial
infarction, unstable angina, atherosclerosis and cerebral
infarction. Studies have shown a higher incidence of Chlamydia
pneumoniae infections among patients with coronary artery
disease (CAD), with implications for myocardial infarction and the
occurrence of more extensive vessel lesions. Antibiotic treatment,
particularly with azithromycin, has exhibited positive correlations
with the secondary prevention of CAD. Additionally, Chlamydia
pneumoniae infection has been significantly associated with an
increased risk of cerebral infarction (123-125).
Extrapulmonary complications, such as hepatic function
insufficiency and septic shock, also arise. Severe-atypical CAP has
been shown to present significantly in Vietnamese children, with
various factors such as age, co-infection with bacteria and
viruses, and respiratory/cardiac system malformations significantly
associated with its severity (126). Increasing antibiotic resistance
poses a critical factor affecting prognosis. The widespread use of
antibiotics has prompted atypical pathogens to alter their form,
structure and metabolism, complicating antibiotic treatment.
Reports from Japan, Germany, France and China have highlighted
increasing macrolide resistance rates in Mycoplasma
pneumoniae strains, necessitating longer antibiotic therapy
durations and delayed fever resolution in macrolide-resistant
cases. Alternative therapies with moxifloxacin or levofloxacin have
been employed for macrolide-resistant strains (95,127-129).
Patients infected with macrolide-resistant Mycoplasma
pneumoniae have experienced more persistent symptoms, leading
to therapeutic changes from macrolides to tetracycline or
fluoroquinolone for a more rapid clinical improvement.
Macrolide-resistant groups have exhibited a higher incidence of
extrapulmonary complications, such as liver function abnormalities,
myocarditis, rash and encephalitis, along with more severe
radiological findings compared to macrolide-sensitive groups. The
interplay between drug resistance and complications contributes to
severe clinical symptoms, prolonged illnesses and a worse prognosis
(130,131). The treatment of pneumonia caused
by Chlamydia psittaci typically involves antibiotics.
Tetracyclines, such as doxycycline or tetracycline itself, are
often considered the first-line treatment for psittacosis.
Macrolides, such as azithromycin, and fluoroquinolones can also be
effective alternatives for treating this type of pneumonia. The
duration of antibiotic treatment and specific medication choice may
vary based on the severity of the infection, the overall health of
the patient and any existing medical conditions (132,133).
In the case that a patient does not respond to
treatment for atypical pneumonia, it is important to consider
alternative diagnoses, including lung adenocarcinoma, particularly
in the case that symptoms persist or worsen. The key difference is
that while atypical pneumonia is an infectious disease that
typically responds to antibiotics or antiviral treatments, lung
adenocarcinoma is a type of cancer that may present with similar
respiratory symptoms, such as cough and chest discomfort, but will
not improve with antimicrobial therapy. Instead, lung
adenocarcinoma often requires further analyses through imaging
studies, such as a CT scan, and possibly a biopsy to confirm the
diagnosis and guide appropriate oncological treatment. Therefore,
in the case that there is no clinical improvement with standard
pneumonia treatments, lung adenocarcinoma should be considered as a
potential underlying cause, prompting further diagnostic evaluation
(134).
6. Conclusions and future perspectives
In conclusion, atypical pneumonia, caused by
diverse pathogens, such as Chlamydia pneumoniae,
Mycoplasma pneumoniae and Legionella species,
presents diagnostic challenges due to its varied symptoms and
systemic impact. Despite this complexity, antibiotics targeting
intracellular processes have proven effective, though antibiotic
resistance poses a growing concern. While Streptococcus
pneumoniae remains a primary cause, atypical pathogens
significantly contribute to cases, particularly among young adults
and in outpatient settings. Diagnosis methods, while valuable, have
limitations in accuracy. The prognosis of atypical pneumonia varies
widely, potentially leading to severe complications beyond the
respiratory system and impacting overall health. Managing this
condition demands a nuanced approach considering the diverse
pathogens involved and their varied clinical impacts.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
DAS and VEG conceptualized the study. IGL, KT, PS,
NT, VEG and DAS made a substantial contribution to the
interpretation and analysis of the data from the literature to be
included in the review, and wrote and prepared the draft of the
manuscript. DAS and VEG analyzed the data from the literature to be
included in the review and provided critical revisions. All authors
contributed to manuscript revision, and all authors have read and
approved the final version of the manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tool Chat
GPT was used to improve the readability and language of the
manuscript, and subsequently, the authors revised and edited the
content produced by the AI tool as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Murray HW and Tuazon C: Atypical
pneumonias. Med Clin North Am. 64:507–527. 1980.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Martin RE and Bates JH: Atypical
pneumonia. Infect Dis Clin North Am. 5:585–601. 1991.PubMed/NCBI
|
|
3
|
Blasi F: Atypical pathogens and
respiratory tract infections. Eur Respir J. 24:171–181.
2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Garin N, Marti C, Skali Lami A and Prendki
V: Atypical pathogens in adult community-acquired pneumonia and
implications for empiric antibiotic treatment: A narrative review.
Microorganisms. 10(2326)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gong C, Zhang T, Luo M, Li A, Dong M, Li
M, Wang Y and Huang F: Distribution of the atypical pathogens of
community-acquired pneumonia to disease severity. J Thorac Dis.
10:5991–6001. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cunha BA: The atypical pneumonias:
Clinical diagnosis and importance. Clin Microbiol Infect. 12 (Suppl
3):S12–S24. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cheng YJ, Lin KY, Chen CC, Huang YL, Liu
CE and Li SY: Zoonotic atypical pneumonia due to Chlamydophila
psittaci: First reported psittacosis case in Taiwan. J Formos Med
Assoc. 112:430–433. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lynch JP III and Clark NM: Pneumonia |
Atypical. Encyclopedia of Respiratory Medicine. 2006:410–417.
2006.
|
|
9
|
Woodhead MA and Macfarlane JT: Comparative
clinical and laboratory features of legionella with pneumococcal
and mycoplasma pneumonias. Br J Dis Chest. 81:133–139.
1987.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sopena N, Sabrià-Leal M, Pedro-Botet ML,
Padilla E, Dominguez J, Morera J and Tudela P: Comparative study of
the clinical presentation of Legionella pneumonia and other
community-acquired pneumonias. Chest. 113:1195–1200.
1998.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mulazimoglu L and Yu VL: Can Legionnaires
disease be diagnosed by clinical criteria? A critical review.
Chest. 120:1049–1053. 2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fernández-Sabé N, Rosón B, Carratalà J,
Dorca J, Manresa F and Gudiol F: Clinical diagnosis of Legionella
pneumonia revisited: Evaluation of the Community-Based Pneumonia
Incidence Study Group scoring system. Clin Infect Dis. 37:483–489.
2003.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Gupta SK and Sarosi GA: The role of
atypical pathogens in community-acquired pneumonia. Med Clin North
Am. 85:1349–1365, vii. 2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gramegna A, Sotgiu G, Di Pasquale M,
Radovanovic D, Terraneo S, Reyes LF, Vendrell E, Neves J, Menzella
F, Blasi F, et al: Atypical pathogens in hospitalized patients with
community-acquired pneumonia: A worldwide perspective. BMC Infect
Dis. 18(677)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cunha BA: Antibiotic pharmacokinetic
considerations in pulmonary infections. Semin Respir Infect.
6:168–182. 1991.PubMed/NCBI
|
|
16
|
Schüler P, Zemper K, Borner K, Koeppe P,
Schaberg T and Lode H: Penetration of sparfloxacin and
ciprofloxacin into alveolar macrophages, epithelial lining fluid,
and polymorphonuclear leucocytes. Eur Respir J. 10:1130–1136.
1997.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Honeybourne D: Antibiotic penetration in
the respiratory tract and implications for the selection of
antimicrobial therapy. Curr Opin Pulm Med. 3:170–174.
1997.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Muller-Serieys C, Soler P, Cantalloube C,
Lemaitre F, Gia HP, Brunner F and Andremont A: Bronchopulmonary
disposition of the ketolide telithromycin (HMR 3647). Antimicrob
Agents Chemother. 45:3104–3108. 2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Esposito S and Principi N: Asthma in
children: Are chlamydia or mycoplasma involved? Paediatr Drugs.
3:159–168. 2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Daian CM, Wolff AH and Bielory L: The role
of atypical organisms in asthma. Allergy Asthma Proc. 21:107–111.
2000.PubMed/NCBI View Article : Google Scholar
|
|
21
|
MacDowell AL and Bacharier LB: Infectious
triggers of asthma. Immunol Allergy Clin North Am. 25:45–66.
2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Micillo E, Bianco A, D'Auria D, Mazzarella
G and Abbate GF: Respiratory infections and asthma. Allergy. 55
(Suppl 61):S42–S45. 2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sessa R, Di Pietro M, Santino I, del Piano
M, Varveri A, Dagianti A and Penco M: Chlamydia pneumoniae
infection and atherosclerotic coronary disease. Am Heart J.
137:1116–1119. 1999.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ericson K, Saldeen TG, Lindquist O,
Pâhlson C and Mehta JL: Relationship of Chlamydia pneumoniae
infection to severity of human coronary atherosclerosis.
Circulation. 101:2568–2571. 2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zamorano J, García-Tejada J, Suárez A,
Culebras E, Castañón J, Moreno R, Reguillo F, Gil M, Picazo J and
Sánchez-Harguindey L: Chlamydia pneumoniae in the atherosclerotic
plaques of patients with unstable angina undergoing coronary artery
bypass grafting: Does it have prognostic implications? Int J
Cardiol. 90:297–302. 2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Weinberg AN: Respiratory infections
transmitted from animals. Infect Dis Clin North Am. 5:649–661.
1991.PubMed/NCBI
|
|
27
|
Gill V and Cunha BA: Tularemia pneumonia.
Semin Respir Infect. 12:61–67. 1997.PubMed/NCBI
|
|
28
|
Stubbs R, Dralle W and Williams J:
Psittacosis pneumonia. J Tenn Med Assoc. 82:189–190.
1989.PubMed/NCBI
|
|
29
|
Cotton EM, Strampfer MJ and Cunha BA:
Legionella and mycoplasma pneumonia-a community hospital experience
with atypical pneumonias. Clin Chest Med. 8:441–453.
1987.PubMed/NCBI
|
|
30
|
Arnold FW, Summersgill JT, Lajoie AS,
Peyrani P, Marrie TJ, Rossi P, Blasi F, Fernandez P, File TM Jr,
Rello J, et al: A worldwide perspective of atypical pathogens in
community-acquired pneumonia. Am J Respir Crit Care Med.
175:1086–1093. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang S, Tang J, Tan Y, Song Z and Qin L:
Prevalence of atypical pathogens in patients with severe pneumonia:
A systematic review and meta-analysis. BMJ Open.
13(e066721)2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cillóniz C, Ewig S, Polverino E, Marcos
MA, Esquinas C, Gabarrús A, Mensa J and Torres A: Microbial
aetiology of community-acquired pneumonia and its relation to
severity. Thorax. 66:340–346. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Capelastegui A, España PP, Bilbao A,
Gamazo J, Medel F, Salgado J, Gorostiaga I, Lopez de Goicoechea MJ,
Gorordo I, Esteban C, et al: Etiology of community-acquired
pneumonia in a population-based study: Link between etiology and
patients characteristics, process-of-care, clinical evolution and
outcomes. BMC Infect Dis. 12(134)2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Spoorenberg SM, Bos WJ, Heijligenberg R,
Voorn PG, Grutters JC, Rijkers GT and van de Garde EM: Microbial
aetiology, outcomes, and costs of hospitalisation for
community-acquired pneumonia; an observational analysis. BMC Infect
Dis. 14(335)2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Van Gageldonk-Lafeber AB, Wever PC, van
der Lubben IM, de Jager CP, Meijer A, de Vries MC, Elberse K, van
der Sande MA and van der Hoek W: The aetiology of
community-acquired pneumonia and implications for patient
management. Neth J Med. 71:418–425. 2013.PubMed/NCBI
|
|
36
|
Shibli F, Chazan B, Nitzan O, Flatau E,
Edelstein H, Blondheim O, Raz R and Colodner R: Etiology of
community-acquired pneumonia in hospitalized patients in northern
Israel. Isr Med Assoc J. 12:477–482. 2010.PubMed/NCBI
|
|
37
|
Liu Y, Chen M, Zhao T, Wang H, Wang R, Cai
B, Cao B, Sun T, Hu Y, Xiu Q, et al: Causative agent distribution
and antibiotic therapy assessment among adult patients with
community acquired pneumonia in Chinese urban population. BMC
Infect Dis. 9(31)2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tao LL, Hu BJ, He LX, Wei L, Xie HM, Wang
BQ, Li HY, Chen XH, Zhou CM and Deng WW: Etiology and antimicrobial
resistance of community-acquired pneumonia in adult patients in
China. Chin Med J (Engl). 125:2967–2972. 2012.PubMed/NCBI
|
|
39
|
Chen K, Jia R, Li L, Yang C and Shi Y: The
aetiology of community associated pneumonia in children in Nanjing,
China and aetiological patterns associated with age and season. BMC
Public Health. 15(113)2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Arancibia F, Cortes CP, Valdés M, Cerda J,
Hernández A, Soto L and Torres A: Importance of Legionella
pneumophila in the etiology of severe community-acquired pneumonia
in Santiago, Chile. Chest. 145:290–296. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Luchsinger V, Ruiz M, Zunino E, Martínez
MA, Machado C, Piedra PA, Fasce R, Ulloa MT, Fink MC, Lara P, et
al: Community-acquired pneumonia in Chile: The clinical relevance
in the detection of viruses and atypical bacteria. Thorax.
68:1000–1006. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Vergis EN, Indorf A, File TM Jr, Phillips
J, Bates J, Tan J, Sarosi GA, Grayston JT, Summersgill J and YU VL:
Azithromycin vs cefuroxime plus erythromycin for empirical
treatment of community-acquired pneumonia in hospitalized patients:
A prospective, randomized, multicenter trial. Arch Intern Med.
160:1294–1300. 2000.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Sopena N, Sabrià M, Pedro-Botet ML,
Manterola JM, Matas L, Domínguez J, Modol JM, Tudela P, Ausina V
and Foz M: Prospective study of community-acquired pneumonia of
bacterial etiology in adults. Eur J Clin Microbiol Infect Dis.
18:852–858. 1999.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Viasus D, Di Yacovo S, Garcia-Vidal C,
Verdaguer R, Manresa F, Dorca J, Gudiol F and Carratalà J:
Community-acquired Legionella pneumophila pneumonia: A
single-center experience with 214 hospitalized sporadic cases over
15 years. Medicine (Baltimore). 92:51–60. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Conklin L, Adjemian J, Loo J, Mandal S,
Davis C, Parks S, Parsons T, McDonough B, Partida J, Thurman K, et
al: Investigation of a Chlamydia pneumoniae outbreak in a Federal
correctional facility in Texas. Clin Infect Dis. 57:639–647.
2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Miyashita N, Fukano H, Okimoto N, Hara H,
Yoshida K, Niki Y and Matsushima T: Clinical presentation of
community-acquired Chlamydia pneumoniae pneumonia in adults. Chest.
121:1776–1781. 2002.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Puljiz I, Kuzman I, Dakovic-Rode O,
Schönwald N and Mise B: Chlamydia pneumoniae and Mycoplasma
pneumoniae pneumonia: Comparison of clinical, epidemiological
characteristics and laboratory profiles. Epidemiol Infect.
134:548–555. 2006.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yu Y and Fei A: Atypical pathogen
infection in community-acquired pneumonia. Biosci Trends. 10:7–13.
2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Reinton N, Manley L, Tjade T and Moghaddam
A: Respiratory tract infections during the 2011 Mycoplasma
pneumoniae epidemic. Eur J Clin Microbiol Infect Dis. 32:835–840.
2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Diaz MH and Winchell JM: The evolution of
advanced molecular diagnostics for the detection and
characterization of mycoplasma pneumoniae. Front Microbiol.
7(232)2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Brown RJ, Nguipdop-Djomo P, Zhao H,
Stanford E, Spiller OB and Chalker VJ: Mycoplasma pneumoniae
Epidemiology in England and Wales: A National Perspective. Front
Microbiol. 7(157)2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Narita M: Classification of extrapulmonary
manifestations due to mycoplasma pneumoniae infection on the basis
of possible pathogenesis. Front Microbiol. 7(23)2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Fraser DW, Tsai TR, Orenstein W, Parkin
WE, Beecham HJ, Sharrar RG, Harris J, Mallison GF, Martin SM,
McDade JE, et al: Legionnaires' disease: Description of an epidemic
of pneumonia. N Engl J Med. 297:1189–1197. 1977.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Glick TH, Gregg MB, Berman B, Mallison G,
Rhodes WW Jr and Kassanoff I: Pontiac fever. An epidemic of unknown
etiology in a health department: I. Clinical and epidemiologic
aspects. Am J Epidemiol. 107:149–160. 1978.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Levy I and Rubin LG: Legionella pneumonia
in neonates: A literature review. J Perinatol. 18:287–290.
1998.PubMed/NCBI
|
|
56
|
Garcia AV, Fingeret AL, Thirumoorthi AS,
Kadenhe-Chiweshe A and Kandel JJ: Severe Mycoplasma pneumoniae
infection requiring extracorporeal membrane oxygenation with
concomitant ischemic stroke in a child. Pediatr Pulmonol.
48:98–101. 2013.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Fisman DN, Lim S, Wellenius GA, Johnson C,
Britz P, Gaskins M, Maher J, Mittleman MA, Spain CV, Haas CN and
Newbern C: It's not the heat, it's the humidity: Wet weather
increases legionellosis risk in the greater Philadelphia
metropolitan area. J Infect Dis. 192:2066–2073. 2005.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Graham FF, White PS, Harte DJ and Kingham
SP: Changing epidemiological trends of legionellosis in New
Zealand, 1979-2009. Epidemiol Infect. 140:1481–1496.
2012.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Johnson DH and Cunha BA: Atypical
pneumonias. Clinical and extrapulmonary features of Chlamydia,
Mycoplasma, and Legionella infections. Postgrad Med. 93:69–72,
75-76, 79-82. 1993.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Sharma L, Losier A, Tolbert T, Dela Cruz
CS and Marion CR: Atypical Pneumonia: Updates on legionella,
chlamydophila, and mycoplasma pneumonia. Clin Chest Med. 38:45–58.
2017.PubMed/NCBI View Article : Google Scholar
|
|
61
|
McConnell CT Jr, Plouffe JF, File TM,
Mueller CF, Wong KH, Skelton SK, Marston BJ and Breiman RF:
Radiographic appearance of Chlamydia pneumoniae (TWAR strain)
respiratory infections. CBPIS Study Group. Community-based
Pneumonia Incidence Study. Radiology. 192:819–824. 1994.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Kauppinen MT, Lähde S and Syrjälä H:
Roentgenographic findings of pneumonia caused by Chlamydia
pneumoniae. A comparison with streptococcus pneumonia. Arch Intern
Med. 156:1851–1856. 1996.PubMed/NCBI
|
|
63
|
Boersma WG, Daniels JM, Löwenberg A, Boeve
WJ and van de Jagt EJ: Reliability of radiographic findings and the
relation to etiologic agents in community-acquired pneumonia.
Respir Med. 100:926–932. 2006.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Nambu A, Saito A, Araki T, Ozawa K,
Hiejima Y, Akao M, Ohki Z and Yamaguchi H: Chlamydia pneumoniae:
Comparison with findings of Mycoplasma pneumoniae and Streptococcus
pneumoniae at thin-section CT. Radiology. 238:330–338.
2006.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Gong L, Zhang CL and Zhen Q: Analysis of
clinical value of CT in the diagnosis of pediatric pneumonia and
mycoplasma pneumonia. Exp Ther Med. 11:1271–1274. 2016.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Mittal S, Singh A, Gold M, Leung AN,
Haramati LB and Katz DS: Thoracic imaging features of Legionnaire's
disease. Infect Dis Clin North Am. 31:43–54. 2017.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Evans AF, Oakley RH and Whitehouse GH:
Analysis of the chest radiograph in Legionnaires' disease. Clin
Radiol. 32:361–365. 1981.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Tan MJ, Tan JS, Hamor RH, File TM Jr and
Breiman RF: The radiologic manifestations of Legionnaire's disease.
The ohio community-based pneumonia incidence study group. Chest.
117:398–403. 2000.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Garg M, Prabhakar N, Gulati A, Agarwal R
and Dhooria S: Spectrum of imaging findings in pulmonary
infections. Part 1: Bacterial and viral. Pol J Radiol.
84:e205–e213. 2019.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Burillo A and Bouza E: Chlamydophila
pneumoniae. Infect Dis Clin North Am. 24:61–71. 2010.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Puolakkainen M: Laboratory diagnosis of
persistent human chlamydial infection. Front Cell Infect Microbiol.
3(99)2013.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Verkooyen RP, Willemse D, Hiep-van
Casteren SC, Joulandan SA, Snijder RJ, van den Bosch JM, van Helden
HP, Peeters MF and Verbrugh HA: Evaluation of PCR, culture, and
serology for diagnosis of Chlamydia pneumoniae respiratory
infections. J Clin Microbiol. 36:2301–2307. 1998.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Thurman KA, Warner AK, Cowart KC, Benitez
AJ and Winchell JM: Detection of Mycoplasma pneumoniae, Chlamydia
pneumoniae, and Legionella spp. in clinical specimens using a
single-tube multiplex real-time PCR assay. Diagn Microbiol Infect
Dis. 70:1–9. 2011.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Hammerschlag MR, Chirgwin K, Roblin PM,
Gelling M, Dumornay W, Mandel L, Smith P and Schachter J:
Persistent infection with Chlamydia pneumoniae following acute
respiratory illness. Clin Infect Dis. 14:178–182. 1992.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Jain S, Self WH, Wunderink RG, Fakhran S,
Balk R, Bramley AM, Reed C, Grijalva CG, Anderson EJ, Courtney DM,
et al: Community-Acquired Pneumonia Requiring Hospitalization among
U.S. Adults. N Engl J Med. 373:415–427. 2015.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Loens K and Ieven M: Mycoplasma
pneumoniae: Current knowledge on nucleic acid amplification
techniques and serological diagnostics. Front Microbiol.
7(448)2016.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Youn YS and Lee KY: Mycoplasma pneumoniae
pneumonia in children. Korean J Pediatr. 55:42–47. 2012.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Loens K, Ursi D, Goossens H and Ieven M:
Molecular diagnosis of Mycoplasma pneumoniae respiratory tract
infections. J Clin Microbiol. 41:4915–4923. 2003.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Herrera M, Aguilar YA, Rueda ZV, Muskus C
and Vélez LA: Comparison of serological methods with PCR-based
methods for the diagnosis of community-acquired pneumonia caused by
atypical bacteria. J Negat Results Biomed. 15(3)2016.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Loens K, Van Heirstraeten L,
Malhotra-Kumar S, Goossens H and Ieven M: Optimal sampling sites
and methods for detection of pathogens possibly causing
community-acquired lower respiratory tract infections. J Clin
Microbiol. 47:21–31. 2009.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Phin N, Parry-Ford F, Harrison T, Stagg
HR, Zhang N, Kumar K, Lortholary O, Zumla A and Abubakar I:
Epidemiology and clinical management of Legionnaires' disease.
Lancet Infect Dis. 14:1011–1021. 2014.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Avni T, Bieber A, Green H, Steinmetz T,
Leibovici L and Paul M: Diagnostic Accuracy of PCR Alone and
compared to urinary antigen testing for detection of legionella
spp: A systematic review. J Clin Microbiol. 54:401–411.
2016.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Nieuwenhuizen AA, Dijkstra F, Notermans DW
and van der Hoek W: Laboratory methods for case finding in human
psittacosis outbreaks: A systematic review. BMC Infect Dis.
18(442)2018.PubMed/NCBI View Article : Google Scholar
|
|
84
|
She RC, Thurber A, Hymas WC, Stevenson J,
Langer J, Litwin CM and Petti CA: Limited utility of culture for
Mycoplasma pneumoniae and Chlamydophila pneumoniae for diagnosis of
respiratory tract infections. J Clin Microbiol. 48:3380–3382.
2010.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Hyman CL, Roblin PM, Gaydos CA, Quinn TC,
Schachter J and Hammerschlag MR: Prevalence of asymptomatic
nasopharyngeal carriage of Chlamydia pneumoniae in subjectively
healthy adults: Assessment by polymerase chain reaction-enzyme
immunoassay and culture. Clin Infect Dis. 20:1174–1178.
1995.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Kohlhoff SA and Hammerschlag MR: Treatment
of Chlamydial infections: 2014 update. Expert Opin Pharmacother.
16:205–212. 2015.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Roblin PM, Kohlhoff SA, Parker C and
Hammerschlag MR: In vitro activity of CEM-101, a new fluoroketolide
antibiotic, against Chlamydia trachomatis and Chlamydia
(Chlamydophila) pneumoniae. Antimicrob Agents Chemother.
54:1358–1359. 2010.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Chotikanatis K, Kohlhoff SA and
Hammerschlag MR: In vitro activity of nemonoxacin, a novel
nonfluorinated quinolone antibiotic, against Chlamydia trachomatis
and Chlamydia pneumoniae. Antimicrob Agents Chemother.
58:1800–1801. 2014.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Biedenbach DJ, Huband MD, Hackel M, de
Jonge BL, Sahm DF and Bradford PA: In vitro activity of AZD0914, a
novel bacterial DNA gyrase/topoisomerase IV inhibitor, against
clinically relevant gram-positive and fastidious gram-negative
pathogens. Antimicrob Agents Chemother. 59:6053–6063.
2015.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Van Rensburg DJ, Perng RP, Mitha IH,
Bester AJ, Kasumba J, Wu RG, Ho ML, Chang LW, Chung DT, Chang YT,
et al: Efficacy and safety of nemonoxacin versus levofloxacin for
community-acquired pneumonia. Antimicrob Agents Chemother.
54:4098–4106. 2010.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Liu Y, Zhang Y, Wu J, Zhu D, Sun S, Zhao
L, Wang X, Liu H, Ren Z, Wang C, et al: A randomized, double-blind,
multicenter Phase II study comparing the efficacy and safety of
oral nemonoxacin with oral levofloxacin in the treatment of
community-acquired pneumonia. J Microbiol Immunol Infect.
50:811–820. 2017.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Barrera CM, Mykietiuk A, Metev H, Nitu MF,
Karimjee N, Doreski PA, Mitha I, Tanaseanu CM, Molina JM,
Antonovsky Y, et al: Efficacy and safety of oral solithromycin
versus oral moxifloxacin for treatment of community-acquired
bacterial pneumonia: A global, double-blind, multicentre,
randomised, active-controlled, non-inferiority trial
(SOLITAIRE-ORAL). Lancet Infect Dis. 16:421–430. 2016.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Mandell LA, Wunderink RG, Anzueto A,
Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher
DM, Niederman MS, et al: Infectious Diseases Society of
America/American Thoracic Society consensus guidelines on the
management of community-acquired pneumonia in adults. Clin Infect
Dis. 44 (Suppl 2):S27–S72. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
94
|
Kashyap S and Sarkar M: Mycoplasma
pneumonia: Clinical features and management. Lung India. 27:75–85.
2010.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Cao B, Zhao CJ, Yin YD, Zhao F, Song SF,
Bai L, Zhang JZ, Liu YM, Zhang YY, Wang H and Wang C: High
prevalence of macrolide resistance in Mycoplasma pneumoniae
isolates from adult and adolescent patients with respiratory tract
infection in China. Clin Infect Dis. 51:189–194. 2010.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Shimizu T: Inflammation-inducing Factors
of Mycoplasma pneumoniae. Front Microbiol. 7(414)2016.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Principi N and Esposito S: Emerging role
of Mycoplasma pneumoniae and Chlamydia pneumoniae in paediatric
respiratory-tract infections. Lancet Infect Dis. 1:334–344.
2001.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Sánchez-Vargas FM and Gómez-Duarte OG:
Mycoplasma pneumoniae-an emerging extra-pulmonary pathogen. Clin
Microbiol Infect. 14:105–117. 2008.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Gacouin A, Le Tulzo Y, Lavoue S, Camus C,
Hoff J, Bassen R, Arvieux C, Heurtin C and Thomas R: Severe
pneumonia due to Legionella pneumophila: prognostic factors, impact
of delayed appropriate antimicrobial therapy. Intensive Care Med.
28:686–691. 2002.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Heath CH, Grove DI and Looke DF: Delay in
appropriate therapy of Legionella pneumonia associated with
increased mortality. Eur J Clin Microbiol Infect Dis. 15:286–290.
1996.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Edelstein PH: Legionnaires' disease. Clin
Infect Dis. 16:741–747. 1993.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Swanson DJ, Sung RJ, Fine MJ, Orloff JJ,
Chu SY and Yu VL: Erythromycin ototoxicity: Prospective assessment
with serum concentrations and audiograms in a study of patients
with pneumonia. Am J Med. 92:61–68. 1992.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Howden BP, Stuart RL, Tallis G, Bailey M
and Johnson PD: Treatment and outcome of 104 hospitalized patients
with legionnaires' disease. Intern Med J. 33:484–488.
2003.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Plouffe JF, Breiman RF, Fields BS, Herbert
M, Inverso J, Knirsch C, Kolokathis A, Marrie TJ, Nicolle L and
Schwartz DB: Azithromycin in the treatment of Legionella pneumonia
requiring hospitalization. Clin Infect Dis. 37:1475–1480.
2003.PubMed/NCBI View
Article : Google Scholar
|
|
105
|
Dorrell L, Fulton B and Ong EL:
Intravenous azithromycin as salvage therapy in a patient with
Legionnaire's disease. Thorax. 49:620–621. 1994.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Sabrià M, Pedro-Botet ML, Gómez J, Roig J,
Vilaseca B, Sopena N and Baños V: Legionnaires Disease Therapy
Group. Fluoroquinolones vs macrolides in the treatment of
Legionnaires disease. Chest. 128:1401–1405. 2005.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Dunbar LM, Khashab MM, Kahn JB, Zadeikis
N, Xiang JX and Tennenberg AM: Efficacy of 750-mg, 5-day
levofloxacin in the treatment of community-acquired pneumonia
caused by atypical pathogens. Curr Med Res Opin. 20:555–563.
2004.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Burdet C, Lepeule R, Duval X, Caseris M,
Rioux C, Lucet JC and Yazdanpanah Y: Quinolones versus macrolides
in the treatment of legionellosis: A systematic review and
meta-analysis. J Antimicrob Chemother. 69:2354–2360.
2014.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Bruin JP, Koshkolda T, IJzerman EP, Lück
C, Diederen BM, Den Boer JW and Mouton JW: Isolation of
ciprofloxacin-resistant Legionella pneumophila in a patient with
severe pneumonia. J Antimicrob Chemother. 69:2869–2871.
2014.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Shadoud L, Almahmoud I, Jarraud S, Etienne
J, Larrat S, Schwebel C, Timsit JF, Schneider D and Maurin M:
Hidden Selection of Bacterial Resistance to Fluoroquinolones In
Vivo: The Case of Legionella pneumophila and Humans. EBioMedicine.
2:1179–1185. 2015.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Arnold FW, Summersgill JT and Ramirez JA:
Role of atypical pathogens in the etiology of community-acquired
pneumonia. Semin Respir Crit Care Med. 37:819–828. 2016.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Georgakopoulou VE, Lempesis IG, Sklapani
P, Trakas N and Spandidos DA: Exploring the pathogenetic mechanisms
of Mycoplasma pneumonia (Review). Exp Ther Med.
28(271)2024.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Waites KB, Xiao L, Liu Y, Balish MF and
Atkinson TP: Mycoplasma pneumoniae from the respiratory tract and
beyond. Clin Microbiol Rev. 30:747–809. 2017.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Liu KT, Yang KY, Lee YC and Perng RP: Risk
factor analysis of acute respiratory distress syndrome amoςινφng
hospitalized patients with Chlamydophila pneumoniae pneumonia. J
Chin Med Assoc. 70:318–323. 2007.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Wingfield T, Rowell S, Peel A, Puli D,
Guleri A and Sharma R: Legionella pneumonia cases over a five-year
period: A descriptive, retrospective study of outcomes in a UK
district hospital. Clin Med (Lond). 13:152–159. 2013.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Housset B: Rising to the challenge of
resistance: A case study-based discussion. Int J Antimicrob Agents.
29 (Suppl 1):S11–S16. 2007.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Cunningham AF, Johnston SL, Julious SA,
Lampe FC and Ward ME: Chronic Chlamydia pneumoniae infection and
asthma exacerbations in children. Eur Respir J. 11:345–349.
1998.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Von Hertzen L, Töyrylä M, Gimishanov A,
Bloigu A, Leinonen M, Saikku P and Haahtela T: Asthma, atopy and
Chlamydia pneumoniae antibodies in adults. Clin Exp Allergy.
29:522–528. 1999.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Koyi H, Brandén E, Gnarpe J, Gnarpe H and
Steen B: An association between chronic infection with Chlamydia
pneumoniae and lung cancer. A prospective 2-year study. APMIS.
109:572–580. 2001.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Kocazeybek B: Chronic Chlamydophila
pneumoniae infection in lung cancer, a risk factor: A case-control
study. J Med Microbiol. 52:721–726. 2003.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Littman AJ, White E, Jackson LA,
Thornquist MD, Gaydos CA, Goodman GE and Vaughan TL: Chlamydia
pneumoniae infection and risk of lung cancer. Cancer Epidemiol
Biomarkers Prev. 13:1624–1630. 2004.PubMed/NCBI
|
|
122
|
Koh WP, Chow VT, Phoon MC, Ramachandran N
and Seow A: Lack of association between chronic Chlamydophila
pneumoniae infection and lung cancer among nonsmoking Chinese women
in Singapore. Int J Cancer. 114:502–504. 2005.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Xue L, Liang YH, Gao YY and Wang XJ:
Clinical study of chlamydia pneumoniae infection in patients with
coronary heart disease. BMC Cardiovasc Disord.
19(110)2019.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Dogra J: Oral azithromycin in extended
dosage schedule for chronic, subclinical Chlamydia pneumoniae
infection causing coronary artery disease: A probable cure in
sight? Results of a controlled preliminary trial. Int J Gen Med.
5:505–509. 2012.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Su X and Chen HL: Chlamydia pneumoniae
infection and cerebral infarction risk: A meta-analysis. Int J
Stroke. 9:356–364. 2014.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Huong Ple T, Hien PT, Lan NT, Binh TQ,
Tuan DM and Anh DD: First report on prevalence and risk factors of
severe atypical pneumonia in Vietnamese children aged 1-15 years.
BMC Public Health. 14(1304)2014.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Morozumi M, Iwata S, Hasegawa K, Chiba N,
Takayanagi R, Matsubara K, Nakayama E, Sunakawa K and Ubukata K:
Acute Respiratory Diseases Study Group. Increased macrolide
resistance of Mycoplasma pneumoniae in pediatric patients with
community-acquired pneumonia. Antimicrob Agents Chemother.
52:348–350. 2008.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Dumke R, von Baum H, Lück PC and Jacobs E:
Occurrence of macrolide-resistant Mycoplasma pneumoniae strains in
Germany. Clin Microbiol Infect. 16:613–616. 2010.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Peuchant O, Ménard A, Renaudin H, Morozumi
M, Ubukata K, Bébéar CM and Pereyre S: Increased macrolide
resistance of Mycoplasma pneumoniae in France directly detected in
clinical specimens by real-time PCR and melting curve analysis. J
Antimicrob Chemother. 64:52–58. 2009.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Principi N and Esposito S:
Macrolide-resistant Mycoplasma pneumoniae: Its role in respiratory
infection. J Antimicrob Chemother. 68:506–511. 2013.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Zhou Y, Zhang Y, Sheng Y, Zhang L, Shen Z
and Chen Z: More complications occur in macrolide-resistant than in
macrolide-sensitive Mycoplasma pneumoniae pneumonia. Antimicrob
Agents Chemother. 58:1034–1038. 2014.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Gautam J and Krawiec C: Chlamydia
Pneumonia. StatPearls Publishing, Treasure Island, FL, 2024.
Available from: https://www.ncbi.nlm.nih.gov/books/NBK560874/.
|
|
133
|
Marchese S, Marchese G, Paviglianiti G,
Lapi M, Ottoveggio G, Pipitone G and Corsello G: A pediatric case
of Chlamydia psittaci caused severe Acute Respiratory Distress
Syndrome (ARDS) in Italy. Ital J Pediatr. 49(107)2023.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Hashimoto K, Nishimura S and Akagi M: Lung
adenocarcinoma presenting as a soft tissue metastasis to the
shoulder: A case report. Medicina (Kaunas). 57(181)2021.PubMed/NCBI View Article : Google Scholar
|