Introduction
Percutaneous lung tumor ablation, which includes
methods such as radiofrequency ablation (RFA), microwave ablation
(MWA) and cryoablation, is considered as a safe and effective
treatment for several inoperable patients with non-small cell lung
cancer (NSCLC) or lung metastasis (1). Despite the advantage of reduced
trauma, percutaneous lung tumor ablation is still an invasive
procedure that can be accompanied by several complications. One of
the rarest, but yet most severe, complications is bronchopleural
fistula (BPF), with an incidence rate of 0.4-2.0% and a high
mortality rate of up to 20% (2-4).
To deepen our insights into this complication, the present study
reported the case of a patient with BPF following percutaneous
microwave ablation (MWA). A statement that the ethics committee
reviewed the application and decided that no ethics approval was
needed due to the nature of the study being that of a case study
would be appropriate. In the present study, the patient with
primary lung cancer choose to undergo microwave ablation due to
poor lung function and inability to tolerate surgery. Following
MWA, BPF, severe lung infection and empyema were reported.
Currently, there is no consensus or optimal treatment strategy for
BPF. Therefore, only a few studies on the treatment of BPF by
endobronchial unidirectional valve (EBV) implantation have been
conducted (5-7).
The current case study aimed to provide novel insights into the
treatment of BPF using EBV.
Case presentation
A 73-year-old man was admitted to the Shaoxing
Second Hospital (Shaoxing, China) due to a nodule in the lower lobe
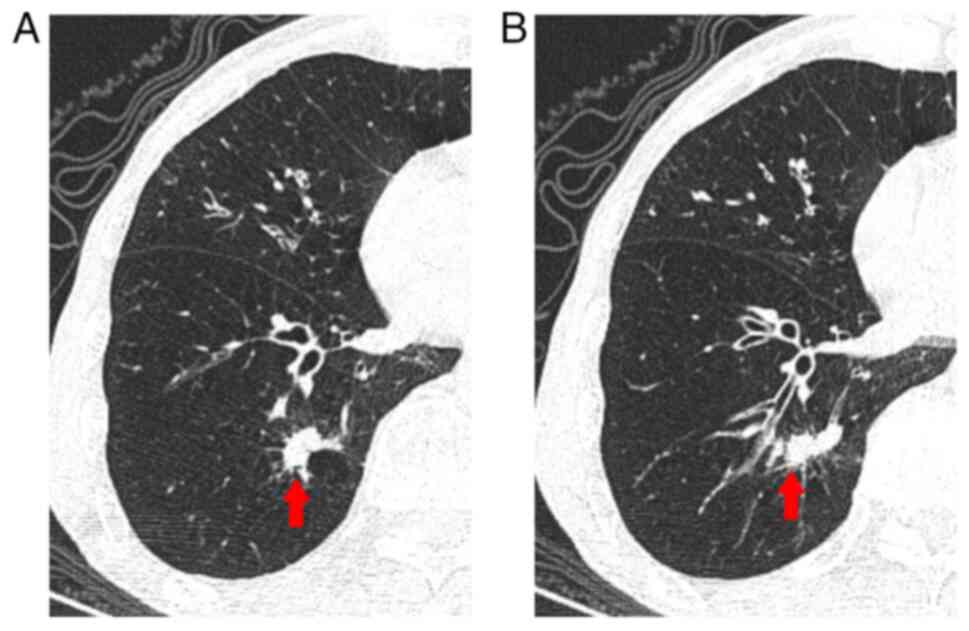
of the right lung. Chest computed tomography (CT) scan revealed a
22x10 mm2 irregular solid nodule in the same area
(Fig. 1A and B). The patient had a history of
complicated chronic bronchitis, emphysema and hypertension, with
well-controlled blood pressure. In addition to this emaciated
appearance, the physical examination results were non-specific,
with a body mass index of 16.4 kg/m2. Pulmonary function
analysis indicated that the mixed ventilation dysfunction was
mainly obstructive, while the pulmonary diffusion function was
severely decreased. A forced expiratory volume in one second
[FEV1(L)] of 1.01, accounting for 34.8% of the expected value. In
addition, the maximal voluntary ventilation (MVV) value was 29.6
l/min, accounting for 26.1% of the expected value. Additionally,
the laboratory tests revealed mild anemia (hemoglobin levels, 103
g/l; normal range, 130-175 g/l) and elevated serum squamous cell
carcinoma antigen levels (2.88 ng/ml; normal range, 0-1.5 ng/ml). A
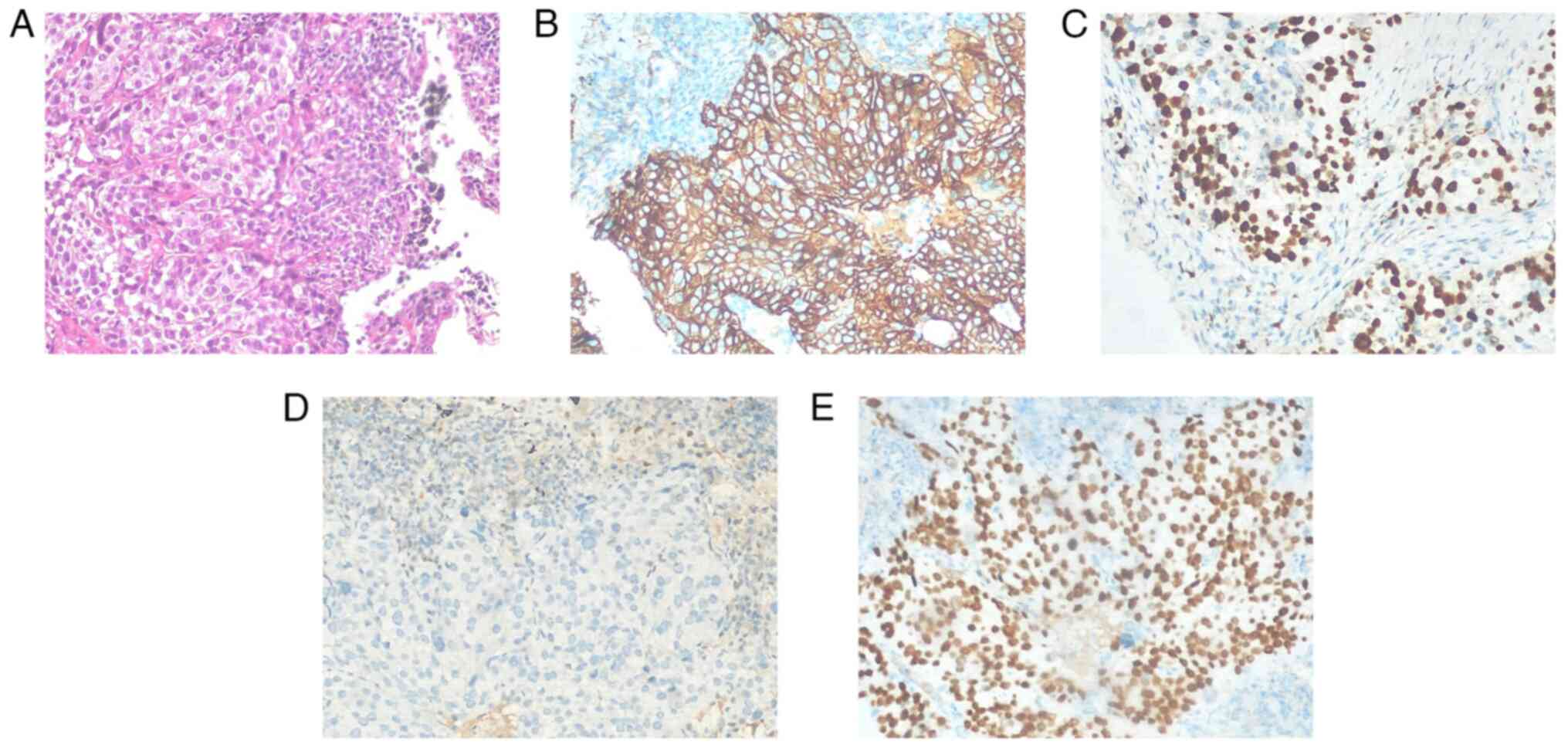
CT-guided puncture biopsy verified squamous cell carcinoma in the
lower lobe of the right lung (Fig.
2). Pathological and immunohistochemical analysis (Department
of Pathology, Shaoxing Second Hospital, Shaoxing, China). The
specimens were processed including fixation in 10% neutral
formalin, conventional paraffin embedding, 4-µm thick continuous
sections, light microscopy observation and hematoxylin-eosin
staining of pathological specimens. At the same time, the EnVision
two-step method of immunohistochemistry was used according to
standard protocols to label with antibodies to cytokeratin (cat.
no. BFM-0482), P53 (cat. no. BFM-0002), Ki-67 (cat. no. BFM-0398),
thyroid transcription factor-1 (cat. no. BFM-0379), napsin A (cat.
no. BFM-0499), P40 (cat. no. BFM-0062) and epidermal growth factor
receptor (EGFR; cat. no. BFM-0450; all pre-diluted; Hangzhou Baiyin
Biotechnology Co., Ltd.). The secondary antibody was from the
Universal SAB Detection Kit [cat. no. 760-500; Roche Diagnostics
(Shanghai) Co., Ltd.], which was a universal type. The reagents
were used according to the manufacturer's instructions. A
representative image of squamous cell carcinoma of the lower lobe
of the right lung was presented (Fig.
2A). The immunohistochemical results revealed positive staining
for EGFR (Fig. 2B), Ki-67 index of
40% (Fig. 2C), P53 mutation
(Fig. 2D) and positivity for P40
(Fig. 2E). Due to poor lung
function and inability to tolerate surgery, surgical treatment was
deemed unsuitable and the patient underwent MWA, which was approved
by the Medical Technology Management Committee of Shaoxing Second
Hospital (Shaoxing, China; approval no. G08 Tumor ablation
technology 2019-07-01).
Prior to MWA, the patient received 100 mg pethidine
intramuscularly. Subsequently, the patient was placed into the
prone position and 1% lidocaine was injected locally into the
pleural wall layer by layer. A total of two ECO-100al5 MWA needles
were used, which were connected to the ECO-100a1 MWA system [ECO
Medical Technology (Nanjing) Co., Ltd.]. The procedure involved a
double-needle ablation approach, according to the manufacturer's
guidelines (Fig. 3A and B). Needle 1 received 45 W radiation for 2
min, while needle 2 was treated with 45 W radiation for 2 min,
followed by 50 W radiation for 3 min. A water circulation cooling
system was used to maintain stable the surface temperature of the
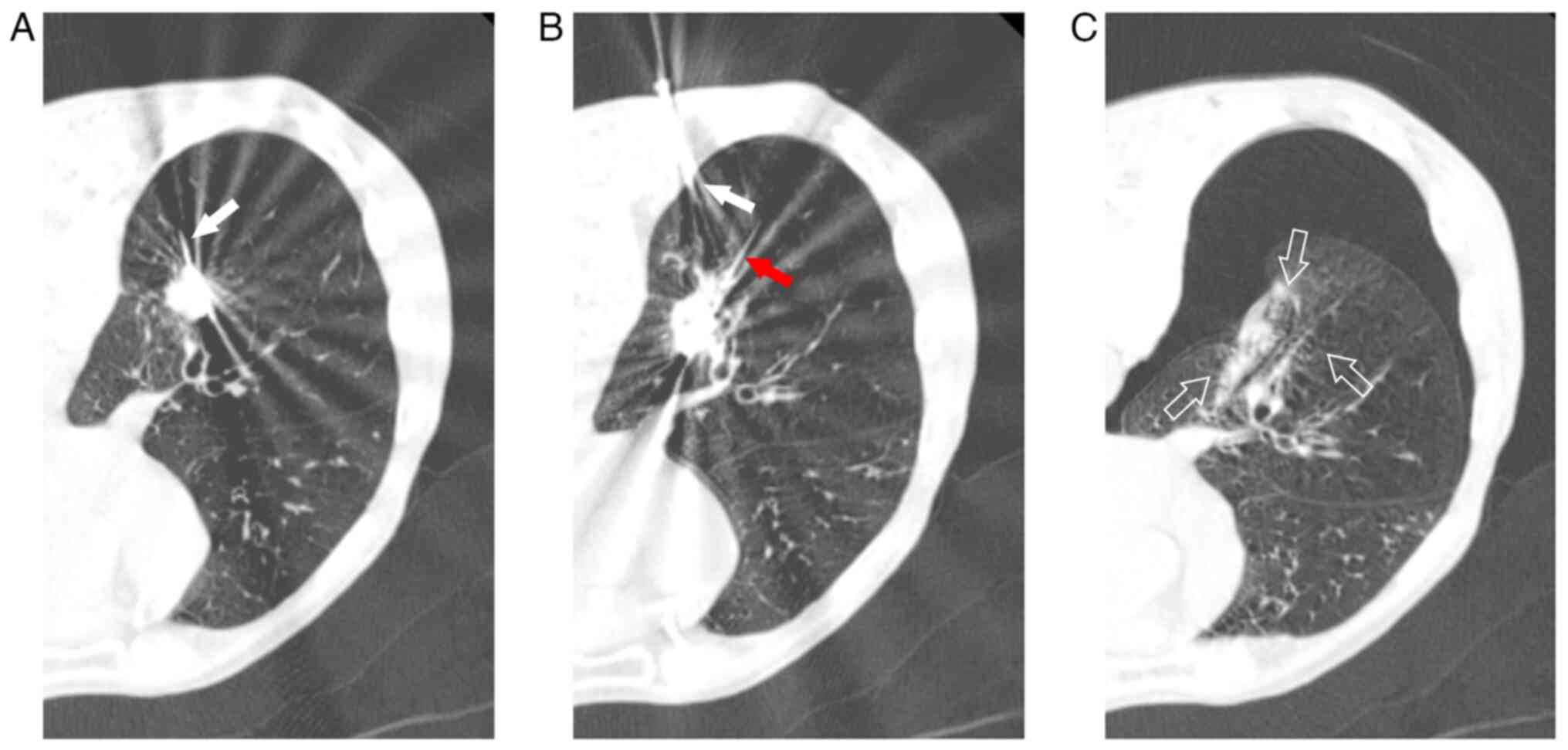
ablation needle. Prior to MWA, the revocation pathway was ablated,
while immediate chest examination revealed right pneumothorax
(Fig. 3C), necessitating closed
thoracic drainage due to air bubbles observed during coughing. At
six days following MWA, significant subcutaneous emphysema was
detected in the neck and chest, with chest CT scan showing enlarged
ablation and ground glass areas alongside a small pneumothorax with
pleural effusion (Fig. 4A). By day
14, the patient experienced fever, cough with yellow purulent
sputum, signs of pulmonary infection and encapsulated pleural
effusion on chest CT scan (Fig.
4B). Therefore, the patient was administered ceftazidime (2.0 g
twice daily) as an anti- microbial therapy. At 35 days after MWA,
chest CT scan revealed encapsulated effusion in the right lower
pleural cavity, gas-fluid flat and a 3-mm BPF in the posterior
basal A subsegment bronchus (B10a) of the right lower lobe
(Fig. 4C). Drainage was performed,
while air bubbles and purulent fluid were observed during coughing
and speaking. Pseudomonas aeruginosa was isolated from sputum and
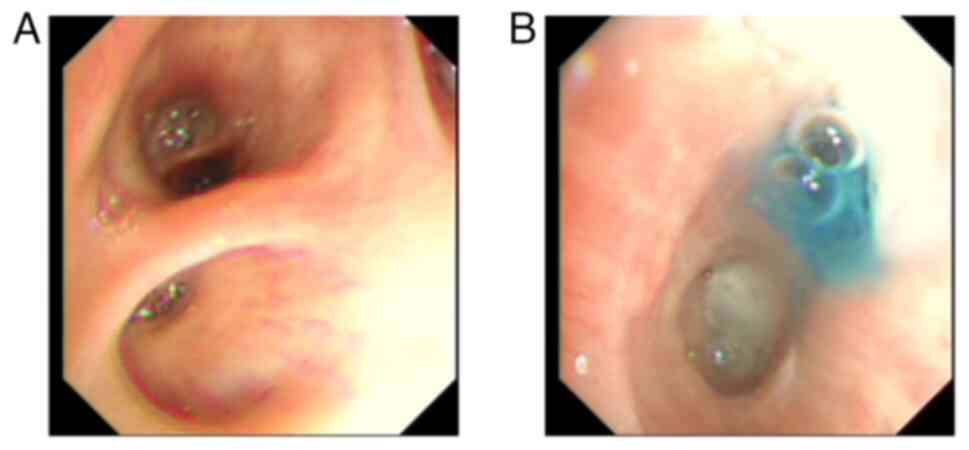
pleural effusion cultures. At 37 days following MWA, methylene blue
was injected via bronchoscopy into the thoracic drainage tube. The
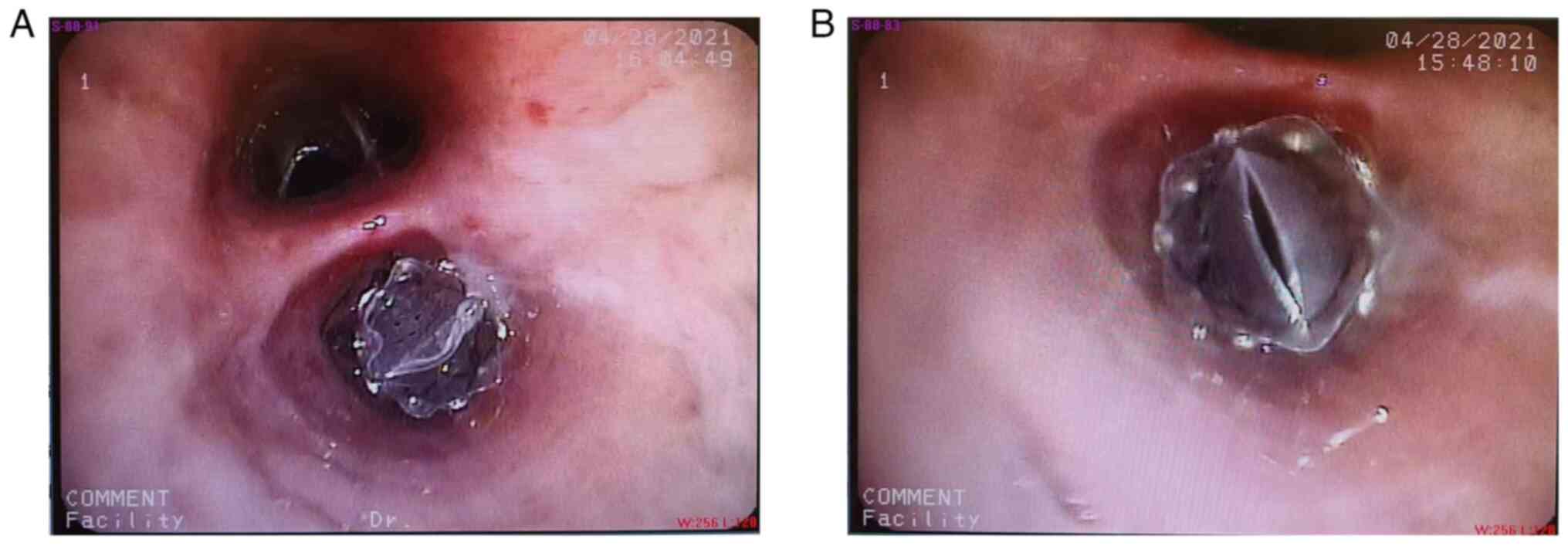
results verified the presence of BPF (Fig. 5A and B), thus leading to the implantation of an
EBV (EBV-TS-5.5; Pulmonx Corp.) in the right inferior lobe
posterior basal segment bronchus (B10) at 45 days after MWA
(Fig. 6A and B). EBV implantation markedly reduced air
leakage, which was entirely stopped five days after implantation,
thus allowing the removal of the thoracic drainage tube (Fig. 7A and B). Furthermore, at day 86 after EBV
implantation, chest CT scan revealed that the pulmonary infection
and narrowed pulmonary necrotic cavity had resolved (Fig. 7C and D; Table
I). No serious complications were recorded at 6-month
follow-up.
 | Table ILaboratory tests, computed tomography
scan and bronchoscopy after microwave ablation. |
Table I
Laboratory tests, computed tomography
scan and bronchoscopy after microwave ablation.
| Day after MWA | CT | Bronchoscopy | CRP, mg/l (0-5) | PCT, ng/m
(0-0.5) | WBC, 109/l
(3.5-9.5) | HGB, g/l
(130-175) |
|---|
| 6 | Subcutaneous
emphysema | - | 7.7 | 0.2 | 7.5 | 105 |
| 14 | Pulmonary infection
and encapsulated pleural effusion | - | 179.6 | 3.1 | 11.4 | 97 |
| 35 | BPF in B10 was
diagnosed | - | 36.9 | 0.15 | 14.0 | 69 |
| 37 | - | BPF was verified by
methylene blue injection | - | - | - | Infusion of two units
of suspended red blood cells |
| 43 | - | - | 104.6 | 1.2 | 7.3 | 98 |
| 45 | - | EBV implantation | - | - | - | - |
| 50 | Improvement of
pulmonary infection | - | 59.4 | 0.1 | 6.8 | 89 |
| 131 | Disappearance of
pulmonary infection and narrowed pulmonary necrotic cavity | - | 36.4 | 0.05 | 5.6 | 92 |
Discussion
Percutaneous lung tumor ablation is a minimally
invasive treatment option for lung tumors, offering both
effectiveness and safety. BPF is a rare but serious complication,
which can occur after lung tumor ablation. BPF is caused by the
abnormal communication between the bronchial tree and the pleural
cavity, and is characterized by persistent air leak (8). Due to the unrestricted effect of
tissue impedance on MWA, this method can be used to treat larger
tumors compared with RFA (9). In
addition, MWA can cause BPF more easily compared with RFA. However,
it can penetrate and effectively heat tissues with high impedance.
It has been reported that MWA can eliminate the obstacles caused by
RFA of inflatable lung. Therefore, microwave penetration of the
inflatable lung is more likely to cause distal structure heating
compared with RFA (10). In
addition, Brace et al (11)
indicate that RFA carries a 49% risk of tissue shrinkage compared
with 55% recorded for MWA. The two methods could elevate the risk
of BPF. Several factors, such as the presence of emphysema, play a
significant role in the development of BPF. Therefore, a previous
study demonstrated that emphysema, which is characterized by
reduced perfusion and ventilation of pulmonary parenchyma, could
enhance the susceptibility to thermal injury and the risk of
pulmonary abscesses (12). The
cavity after the excretion of necrotic tissue and pus can also
promote the development of BPF. Theoretically, ablation of tumors
near the pleura or bronchus is more likely to cause BPF, since the
ablation area more commonly involves the pleura or bronchus and
cause necrotic changes. It has been reported that there is a
significant association between the ablation area involving the
normal anterior pleura and the occurrence of pneumothorax (13). Subsequent experiments verified this
hypothesis and previous studies indicate that the changes around
the channel during ablation therapy were similar to those in the
ablation area, both leading to coagulation necrosis. This type of
injury can form fistulas along the needle channel between the
thermal ablation area and the pleural space (14,15).
Furthermore, infections can easily form an abscess after necrosis
in the ablation area. In turn, vascular occlusion in the necrotic
area can make wound healing more difficult, thus further affecting
the spontaneous closure of the ablation area. In addition,
excessive ablation can also lead to BPF, since it can promote
extensive necrosis of the lung tissue, increase the incidence of
infection and abscess and promote BPF formation after excretion of
the necrotic tissue.
In the present case study, the patient suffered from
lung squamous cell carcinoma, which was characterized by
spontaneous necrosis and originated from the bronchial wall.
Emerging evidence has suggested that the aforementioned
characteristics can contribute to the development of BPF (16). The occurrence of BPF in particular
cases can be associated with several factors, such as emphysema,
the close distance between the tumor and the bronchus/pleura (<1
cm) and excessive ablation using double-needle. In the present
study, the lung tumor exhibited irregular shape with a maximum
diameter of 22 mm. The present patient received double-needle MWA
for radical treatment to expand the ablation area, increase tissue
carbonization and promote necrosis. However, as the proximity of
the tumor to the trachea (B10) and pleura was <1 cm, it could
possibly result in sinus formation following bronchial wall and
pleural necrosis. In several patients BPF closure can occur
spontaneously after basic conservative therapy (17). However, if the air leak persists
for >3-5 days, surgical evaluation is recommended (18).
Although BPF commonly managed by surgery, the
majority of patients with NSCLC who opt for ablation are unsuitable
for surgical treatment. Therefore, endoscopic fistula closure has
become increasingly popular as a minimally invasive alternative.
Currently, several cases of successful bronchoscopy-mediated BPF
closure have been reported. Kodama et al (19) demonstrate that filling silica gel
into the leaking bronchus through a bronchoscope to obliterate the
fistula could treat BPF. Additionally, Powell et al
(20) showed that treatment with
n-butyl cyanoacrylate glue could immediately stop the air leakage
after surgery, while no recurrence was recorded during follow-up.
Arnaud et al (21) used
fibrin glue to treat the BPF after MWA. A CT scan was performed at
one month after hospitalization and revealed a persistent pulmonary
cavity with a bronchial fistula in the upper left lobe.
Nevertheless, it has been widely reported that EBV can successfully
treat BPF (5-7).
EBV can occlude fistula while allowing drainage of secretions and
trapped air (22). The function
contributes to reducing infections around the fistula and promoting
healing. EBV implantation may have a higher successful rate
compared with other endoscopic treatments for BPF.
In the current case study, the patient immediately
developed pneumothorax after ablation, thus indicating pleural
injury. After placing a chest drainage, the patient did not
experience sustained air leakage, possibly due to temporary wound
contraction and tissue necrosis- and carbonization-mediated
closure. Subsequently, due to tissue necrosis and detachment,
accompanied by pulmonary infection, the subsegment bronchus (B10a)
was connected to the pleural cavity. The tracheal fistula was ~3 mm
in diameter and it could not heal on its own following conservative
treatment. This condition could be associated with infection, since
both sputum and pleural fluid cultures were positive for
Pseudomonas aeruginosa, which is a highly invasive and
difficult to treat bacterium (23). To treat the aforementioned
infection, the patient received ceftazidime, as an antimicrobial
agent, and sufficient chest drainage therapy. When the infection
was controlled to a certain extent, EBV was selected to close the
fistula. EBV is an improved method to block the communication
between the thoracic cavity and the respiratory tract, thus
contributing to infection control. Andreetti et al (6) showed that following EBV implantation,
the patient still experienced chest leakage. Therefore, the
aforementioned patient received autologous platelet gel to close
the cavity and to definitively stop the air leak, while the
antibiotic pleural irrigation continued once daily. After the
fourth application of autologous platelet gel, the air leak
stopped. Following 58 days of EBV placement, the chest drain was
removed. In the present study, EBV implantation markedly reduced
air leakage, which was entirely stopped at five days
post-implantation, thus allowing the removal of the thoracic
drainage tube. The aforementioned observation could be due to the
fact that the fistula opening in the current case was located far
away in the lung subsegmental bronchus. Therefore, to reduce the
occurrence of BPF and the difficulty in treating BPF, the ablation
power and time for tumors near the bronchus should be reduced.
Additionally, it has been reported that the adverse events
occurring after EBV implantation include EBV translocation,
haemoptysis, pneumothorax and pneumonia. They are mostly mild and
can be treated by removing EBV (24). In the present case, no
complications of EBV have occurred.
Overall, the present case study indicated that
adequate drainage, infection control and improved nutritional
status are markedly involved in the success of EBV-mediated
treatment of BPF. EBV implantation after MWA is an effective method
for treating BPF.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XM, LH and JZ contributed to the conception and
design of the study. Data collection and analysis were performed by
XM and MQ. The manuscript was written by XM. XM and LH confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The requirement for ethics approval was waived by
the Ethics Committee of Shaoxing Second Hospital (Shaoxing, China)
due to the retrospective nature of the study.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of potentially identifying images or
data included in this article.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Palussiere J, Catena V and Buy X:
Percutaneous thermal ablation of lung tumors-radiofrequency,
microwave and cryotherapy: Where are we going? Diagn Interv
Imaging. 98:619–625. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kashima M, Yamakado K, Takaki H, Kodama H,
Yamada T, Uraki J and Nakatsuka A: Complications after 1000 lung
radiofrequency ablation sessions in 420 patients: A single center's
experiences. AJR Am J Roentgenol. 197:W576–W580. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kurilova I, Gonzalez-Aguirre A, Beets-Tan
RG, Erinjeri J, Petre EN, Gonen M, Bains M, Kemeny NE, Solomon SB
and Sofocleous CT: Microwave ablation in the management of
colorectal cancer pulmonary metastases. Cardiovasc Intervent
Radiol. 41:1530–1544. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zheng A, Yang X, Ye X, Huang G, Wei Z,
Wang J, Han X, Ni X and Meng M: Bronchopleural fistula after lung
ablation: Experience in two cases and literature review. Indian J
Cancer. 52:e41–e46. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Alexander ES, Terrance T, Martin DW and
Dupuy DE: Use of endobronchial valves for the treatment of
bronchopleural fistulas after thermal ablation of lung neoplasms. J
Vasc Interv Radiol. 23:1236–1240. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Andreetti C, Maurizi G, Cassiano F and
Rendina EA: Resolution of a lifethreatening complication after lung
radiofrequency ablation. Eur J Cardiothorac Surg. 46:e56–e58.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zo S, Song YJ, Kim BG, Jeong BH, Jeon K,
Cho JH and Kim H: Surgically intractable bronchopleural fistula
treated with endobronchial valve insertion by isolating the tract
with indigo carmine: A case report. Respir Med Case Rep.
27(29100972)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Abu-Hiljeh M and Blundin M: Emergency use
of an endobronchial one-way valve in the management of severe air
leak and massive subcutaneous emphysema. Lung. 188:253–257.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Páez-Carpio A, Gómez FM, Olivé GI, Paredes
P, Baetens T, Carrero E, Sánchez M and Vollmer I: Image-guided
percutaneous ablation for the treatment of lung malignancies:
Current state of the art. Insights Imaging. 12(57)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chan G, Kwan J, Samol J, Verma A and Pua
U: Remote right main pulmonary bronchus bronchopleural fistula
formation after microwave ablation of lung tumor. J Vasc Interv
Radiol. 30:1656–1658. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Brace CL, Diaz TA, Hinshaw JL and Lee FT
Jr: Tissue contraction caused by radiofrequency and microwave
ablation: A laboratory study in liver and lung. J Vasc Interv
Radiol. 21:1280–1286. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Alberti N, Buy X, Frulio N, Montaudon M,
Canella M, Gangi A, Crombe A and Palussière J: Rare complications
after lung percutaneous radiofrequency ablation: Incidence, risk
factors, prevention and management. Eur J Radiol. 85:1181–1191.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yoshimatsu R, Yamagami T, Terayama K,
Matsumoto T, Miura H and Nishimura TS: Delayed and recurrent
pneumothorax after radiofrequency ablation of lung tumors. Chest.
135:1002–1009. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Izaaryene J, Cohen F, Souteyrand P,
Rolland PH, Vidal V, Bartoli JM, Secq V and Gaubert JY:
Pathological effects of lung radiofrequency ablation that
contribute to pneumothorax, using a porcine model. Int J
Hyperthermia. 33:713–716. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lee KS, Takaki H, Yarmohammadi H,
Srimathveeravalli G, Luchins K, Monette S, Nair S, Kishore S and
Erinjeri JP: Pleural puncture that excludes the ablation zone
decreases the risk of pneumothorax after percutaneous microwave
ablation in porcine lung. J Vasc Interv Radiol. 26:1052–1058.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sakurai J, Hiraki T, Mukai T, Mimura H,
Yasui K, Gobara H, Hase S, Fujiwara H, Iguchi T, Tajiri N, et al:
Intractable pneumothorax due to bronchopleural fistula after
radiofrequency ablation of lung tumors. J Vasc Interv Radiol.
18:141–145. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cannella M, Cornelis F, Descat E, Ferron
S, Carteret T, Castagnède H and Palussière J: Bronchopleural
fistula after radiofrequency ablation of lung tumours. Cardiovasc
Intervent Radiol. 34 (Suppl 2):S171–S174. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Havelock T, Teoh R, Laws D and Gleeson F:
BTS Pleural Disease Guideline Group. Pleural procedures and
thoracic ultrasound: British thoracic society pleural disease
guideline 2010. Thorax. 65 (Suppl 2):ii61–ii76. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kodama H, Yamakado K, Murashima S, Takaki
H, Uraki J, Nakatsuka A, Shoumura S, Tarukawa T, Shimamoto A, Takao
M and Takeda K: Intractable bronchopleural fistula caused by
radiofrequency ablation: Endoscopic bronchial occlusion with
silicone embolic material. Br J Radiol. 82:e225–e227.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Powell DK and Baum S: Bronchopleural
fistula treated with N-butyl cyanoacrylate glue after ablation. J
Vasc Interv Radiol. 29:1692–1693. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Thivolet A, Menassel B, Chatté G, Tabutin
M, Bouhamama A, Pilleul F and Mastier C: Delayed bronchocutaneous
fistula without pneumothorax following a microwave ablation of a
recurrent pulmonary metastasis. Cardiovasc Intervent Radiol.
41:340–343. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Reed FM, Gilbert RC, Taylor DM and Toth
JW: Endobronchial valves for challenging air leaks. Ann Thorac
Surg. 100:1181–1186. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bassetti M, Vena A, Russo A, Croxatto A,
Calandra T and Guery B: Rational approach in the management of
Pseudomonas aeruginosa infections. Curr Opin Infect Dis.
31:578–586. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sciurba FC, Ernst A, Herth FJF, Strange C,
Criner GJ, Marquette CH, Kovitz KL, Chiacchierini RP, Goldin J and
McLennan G: VENT Study Research Group. A randomized study of
endobronchial valves for advanced emphysema. N Engl J Med.
363:1233–1244. 2010.PubMed/NCBI View Article : Google Scholar
|