Introduction
Chronic low back pain (CLBP) is a widespread public
and occupational health issue, affecting ~23% of the population,
with 11-12% experiencing disability (1,2).
CLBP lasts 12 weeks or more and is influenced by various factors,
including genetics, age, fitness level, weight and mental
well-being (3,4). A number of studies have shown that
the prevalence of CLBP steadily increases from the 3rd to the 6th
decade of life, with a higher prevalence observed among women
(3,4). In developed countries, 49-90% of
individuals will experience at least one episode of low back pain
in their lifetime, with the majority experiencing temporary relief
within 2 weeks. However, 2-7% of people will go on to develop CLBP
(3,4).
Duloxetine, a serotonin-noradrenaline reuptake
inhibitor, increases dopamine levels in the prefrontal cortex and
enhances the activity of noradrenergic and serotonergic neurons in
the descending spinal pathway; thus, alleviates neuropathic and
chronic pain (5,6). Clinical trials as well as systematic
reviews, have demonstrated its effectiveness in treating CLBP, with
significant improvements compared with placebo. A clinical trial
conducted by Skljarevski et al (7), involving 404 patients with
non-radicular CLBP over a 13-week period, demonstrated that the
duloxetine group exhibited improvement in functional outcomes
compared with the placebo group. Two previous systematic reviews by
Weng et al (8) and Hirase
et al (9) have been
conducted to investigate the effect of duloxetine on CLBP. However,
Weng et al (8) examined the
impact of duloxetine on CLBP and osteoarthritis, while Hirase et
al (9) performed a systematic
review without a meta-analysis due to heterogeneity.
Considering the lack of comprehensive investigations
into CLBP and the publication of recent clinical trials evaluating
the use of duloxetine, the present systematic review was conducted
to incorporate the most up-to-date evidence available as of the
publication date of the present study. The current review aimed to
not only assess the efficacy of duloxetine in managing CLBP but
also explore its impact on the quality of life of the patients and
the frequency of serious adverse effects.
Materials and methods
The present systematic review and meta-analysis
adhered to the guidelines outlined in the Preferred Reporting Items
for Systematic Reviews and Meta-Analyses (PRISMA) statement
(10), ensuring methodological
rigor and transparency throughout the research process.
Eligibility criteria
Randomized controlled trials (RCTs) that examined
duloxetine across various dosage regimens in individuals suffering
from chronic low back pain (CLBP) were included, compared with
placebo. Observational studies, those with duplicated or
overlapping populations, and those focusing solely on acute low
back pain were excluded. Furthermore, studies that used accompanied
treatment, such as exercise, electrotherapy or physiotherapy were
excluded from this meta-analysis to maintain the integrity and
relevance of the selected literature.
Search strategy
A comprehensive and systematic search strategy was
developed based on four prominent electronic bibliographic
databases: PubMed (https://pubmed.ncbi.nlm.nih.gov/), Web of Science
(https://0710oser8-1106-y-https-www-webofscience-com.mplbci.ekb.eg/wos/woscc/basic-search),
Cochrane Central (https://www.cochranelibrary.com/advanced-search) and
Scopus (https://07105seqa-1106-y-https-www-scopus-com.mplbci.ekb.eg/search/form.uri?display=basic#basic).
The present study aimed to maximize the retrieval of relevant
articles published from the inception of each database up to March
2024. The search strategy used was as follows: For PubMed,
(duloxetine hydrochloride OR duloxetine OR Cymbalta) AND (low back
pain OR back pain OR back pain OR backache); for Web of Science,
(duloxetine OR Cymbalta) AND (low back pain OR back pain OR back
pain OR backache); for Cochrane, (duloxetine OR Cymbalta) AND (low
back pain OR back pain OR back pain OR backache); and for Scopus,
(duloxetine OR Cymbalta) AND (low back pain OR back pain OR back
pain OR backache).
Study selection
A total of 2 independent reviewers performed the
study selection process. Initially, titles and abstracts were
screened to identify potentially eligible studies, followed by
full-text screening to confirm eligibility based on the predefined
inclusion and exclusion criteria.
Data extraction
Data extraction was conducted using a structured
electronic data extraction sheet. A total of 2 independent
reviewers extracted the following data from the included studies:
Study name, year of publication, study design, interventions,
duration of duloxetine treatment, sample size, mean age, male
percentage, weight, follow up duration and outcome measures.
Definition of outcomes parameters
The visual analogue scale (VAS) is a subjective,
self-reported, pain rating scale. It is used to track pain
regression. A handwritten mark placed at one point along the length
of a 10 cm line that represents a continuum between the two ends of
the scale; ‘no pain’ on the left end (0 cm) of the scale and ‘worst
pain’ on the right (10 cm). Measurements from the zero point (left
end) of the scale to the patients' marks are recorded in cm and are
interpreted as their pain (11).
The Brief Pain Inventory (BPI) is a self-reported
assessment. It provides information on the intensity of pain
(sensory dimension or BPI-severity) and the degree to which pain
interferes with function (reactive dimension or BPI-Interference).
BPI-I is rated on a scale of 0 (no interference) to 10 (interferes
completely). The average of 7 items or questions that the BPI-I
scale addresses (general activity, mood, walking ability, normal
work, relations with other people, sleep and enjoyment of life)
were reported (12). The
Roland-Morris Disability Questionnaire-24 (RMDQ-24) measures
physical disability due to CLBP on a scale ranging from 0 (no
disability) to 24 (severe disability) (13).
The SF-36 includes 36 questions. In total, 8
different sub-scores (physical and social functioning, physical and
emotional role limitations, mental health, energy, pain, and
general health perceptions), and a mental and physical summary
score can be obtained from these. A maximum score of 100 resembles
the best possible health state (14). Patients' global impression of
improvement (PGI-I) compares a participant's perception of
improvement at the time of assessment with treatment initiation
time. Score varies from 1 (very much better) to 7 (very much worse)
(15).
Of the included studies, only Raskin et al
(16) specifically addressed
depression, defining it according to DSM-IV criteria (17). While this definition was referenced
in the present study, none of the other 7 studies included a focus
on depression. Depression was defined according to the criteria of
DSM- IV (17). The included study
by Raskin et al (16) also
defined depression as in the DSM-IV. The diagnosis was confirmed by
the Mini International Neuropsychiatric Interview, a standardized
diagnostic interview based on DSM-IV criteria (17). Baseline disease severity was
defined by patients' scores on the Hamilton Depression Rating Scale
with 17 items (HAM-D) (18).
Patients were required to have a HAM-D total score of ≥18 at visits
1 and 2; a Mini-Mental State Examination (MMSE) score of ≥20 with
or without mild dementia; and at least one previous episode of
major depression. Patients with an MMSE score of 20 to 23 were
categorized as having mild dementia, while those with a score of
≥24 were categorized as having no dementia (18).
Quality assessment
The risk of bias in the included RCTs was assessed
using the well-established Cochrane collaboration tool (19). This evaluation included sequence
generation, allocation concealment, blinding, incomplete outcome
data, selective outcome reporting and other potential sources of
bias. Each study's bias risk was categorized as ‘Low’, ‘High’, or
‘Unclear’ ensuring a comprehensive understanding of the
methodological quality and robustness of the included studies.
Data analysis
For dichotomous data, risk ratios (RR) with 95%
confidence intervals (CIs) were used. For continuous data, the mean
differences (MD) with 95% CI were employed. Data synthesis was
performed using the R software (meta-package; v.6.5-0; https://www.r-project.org/). Visual inspection of the
forest plots and measurement of the Q and I2 statistics
were used to assess heterogeneity. Significant statistical
heterogeneity was indicated by the Q statistic P<0.1 or
I2>50%. Random effect model was used in the
meta-analysis.
Results
Search results
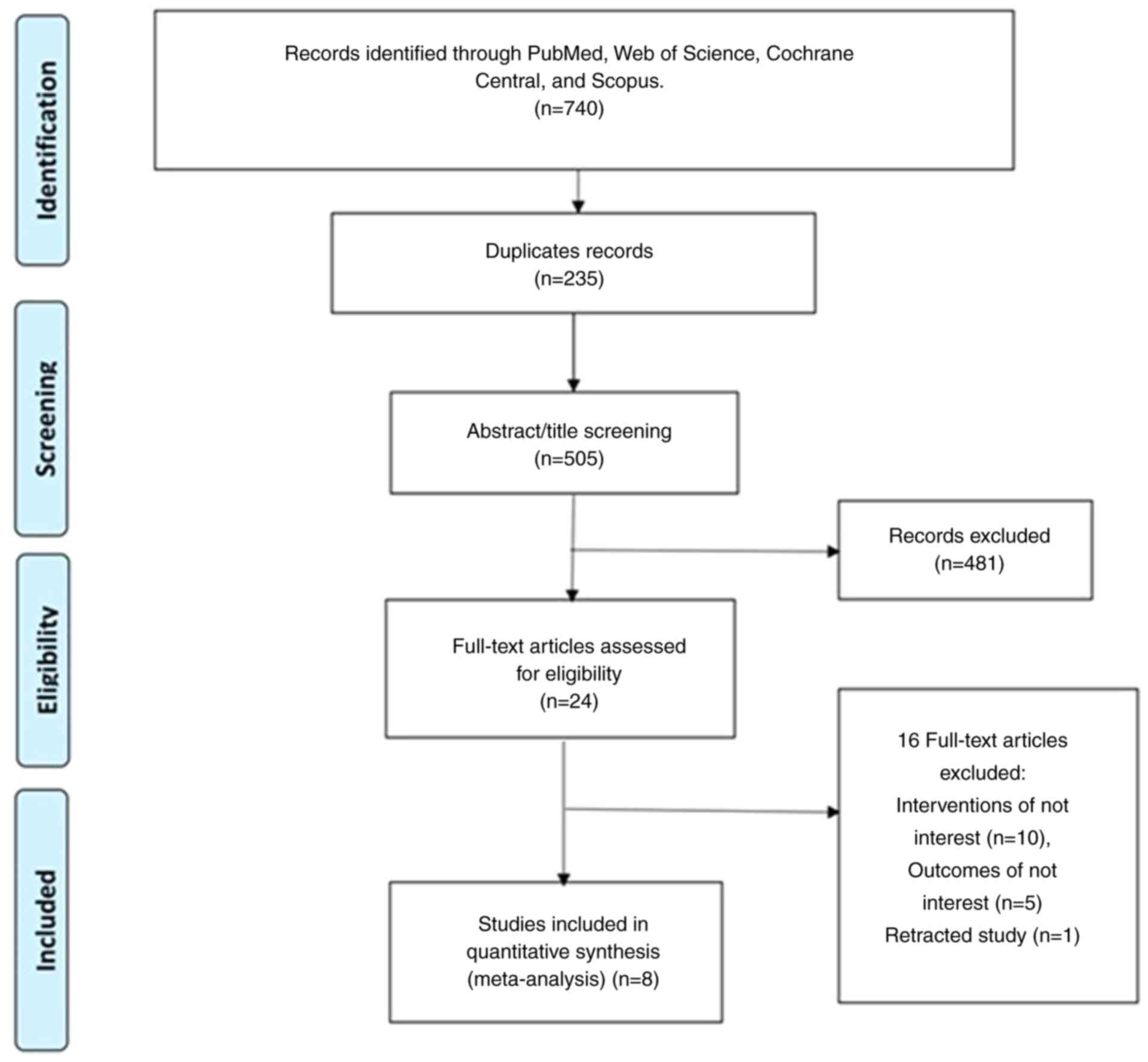
A total of 740 records were identified, 235
redundant entries were removed and 505 unique records were
reviewed. After assessing the full-text articles, the pool was
narrowed down to 24 relevant studies, and finally a total of 8
studies were included in the meta-analysis. Any discrepancies were
resolved through discussion and consensus to ensure the robustness
and integrity of the study selection process. The study selection
process is shown in Fig. 1.
Study characteristics
The included studies collectively enrolled a total
of 1,985 patients. These studies have exhibited diverse
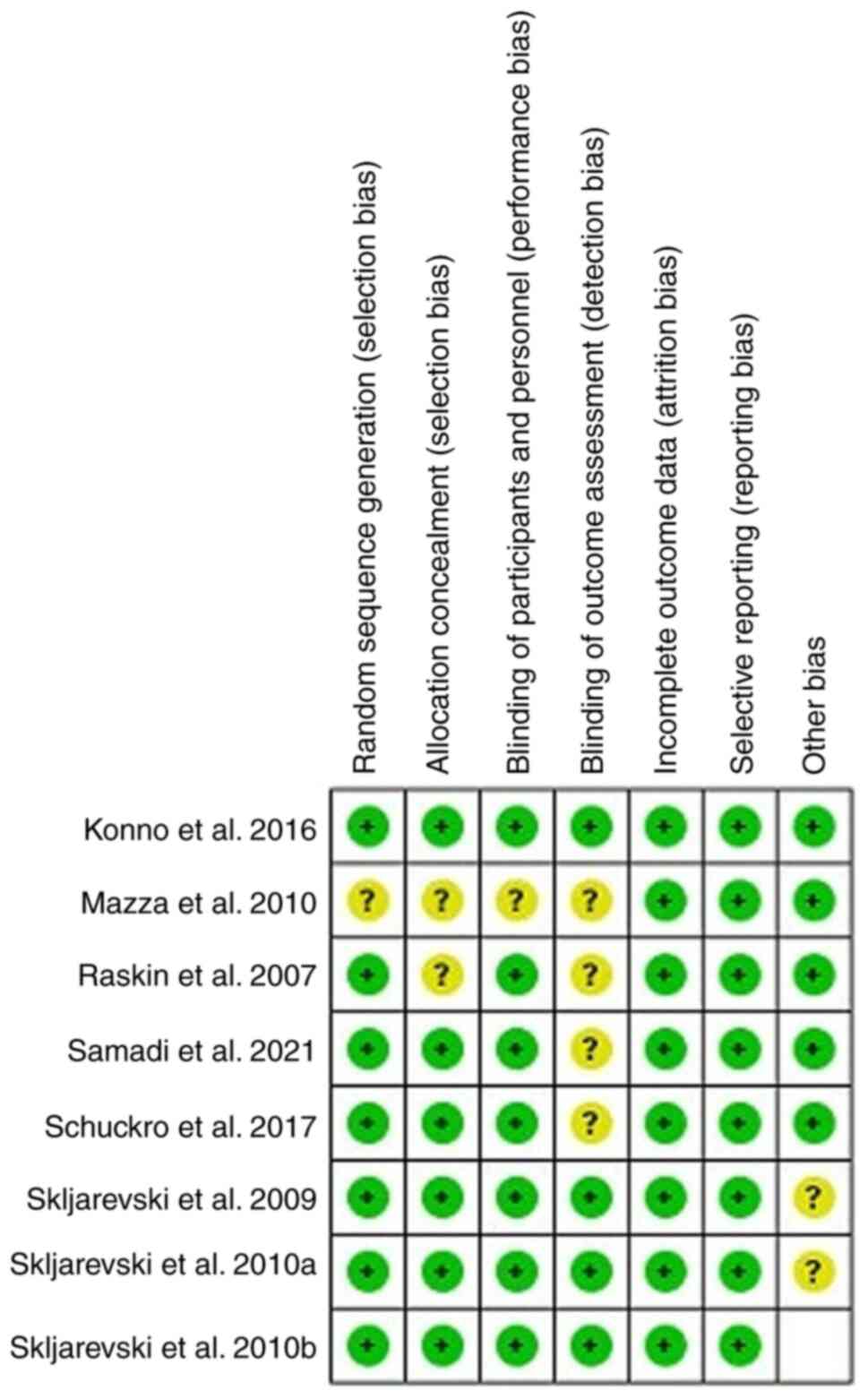
characteristics, as summarized in Table I. The methodological quality of
each study was evaluated using the Cochrane collaboration tool,
illuminating a spectrum ranging from moderate to high quality
(Fig. 2).
 | Table ISummary table of included studies;
RCT, randomized controlled trial; NA, not available. |
Table I
Summary table of included studies;
RCT, randomized controlled trial; NA, not available.
| First author,
year | Study design | Interventions | Duration of
duloxetine treatment in weeks | Sample size | Mean age (standard
deviation) (Intervention/control) | Male n (%)
(Intervention/control) | Weight (kg)
(Intervention/control) | Follow up in
weeks | Outcome
parameters | (Refs.) |
|---|
| Raskin et
al, 2007 | RCT | Duloxetine (60 mg)
vs. placebo | 8 | 311 | 72.6 (5.7)/73.3
(5.7) | 82 (39.6%)/44
(42.3%) | 80 (17.7)/79.7
(18.8) | 8 | VAS | (16) |
| Skljarevski et
al, 2009 | RCT | Duloxetine 60-120mg
vs. placebo | 13 | 404 | 52.9 (12.8)/54.0
(13.5) | 23 (39%)/53
(45.3%) | 84.9 (14.2)/81.9
(17.4) | 13 | Weekly mean 24-h
average pain, RMDQ-24), PGI-I, BPI | (24) |
| Mazza et al,
2010 | RCT | Duloxetine 60 mg
vs. Escitalopram 20 mg | 13 | 85 | 53.6 (13.5)/52
(12.4) | 18 (43.1)/17
(43.6) | 81.5 (18.6)/82.7
(16.9) | 13 | Weekly mean 24-h
average pain, CGI-S, SF-36 | (32) |
| Skljarevski et
al, 2010a | RCT | Duloxetine 60-120
mg vs. placebo | 13 | 236 | 51.8 (14.9)/51.2
(13.5) | 44 (38.3)/48
(39.7) | 76.2 (14.7)/75.9
(13.9) | 13 | BPI, RMDQ, PGI,
CGI-S, BPI-S,BPI-I, weekly means of the 24-hour average pain | (23) |
| Skljarevski et
al, 2010b | RCT | Duloxetine 60 mg
vs. placebo | 12 | 401 | 54.9 (13.7)/53.4
(14.2) | 80 (40.4)/75
(36.9) | 78.3 (15.8)/79.4
(14.7) | 12 | BPI, PGI-I,
RMDQ-24, BPI-S, BPI-I, SF-36 | (7) |
| Konno et al,
2016 | RCT | Duloxetine 60 mg
vs. placebo | 14 | 458 | 60.0 (13.2)/57.8
(13.7) | 115 (50.0)/104
(46.0) | 63.56 (12.75)/63.15
(13.42) | 14 | BPI, GGI-S,
RMDQ | (22) |
| Schukro et
al, 2016 | RCT | Duloxetine 120 mg
vs. placebo | 8 | 40 | 57.9 (13.4) | 20 (49%) | 80.5 (18.3) | 8 | VAS | (20) |
| Samadi et
al, 2021 | RCT | Duloxetine 30 mg
vs. placebo | 6 | 50 | 45.13 (15.41)/52
(11.53) | 7 (46.67%)/11
(68.75%) | NA | 6 | VAS, SF-36 | (21) |
Outcomes. Pain reduction and physical
improvement
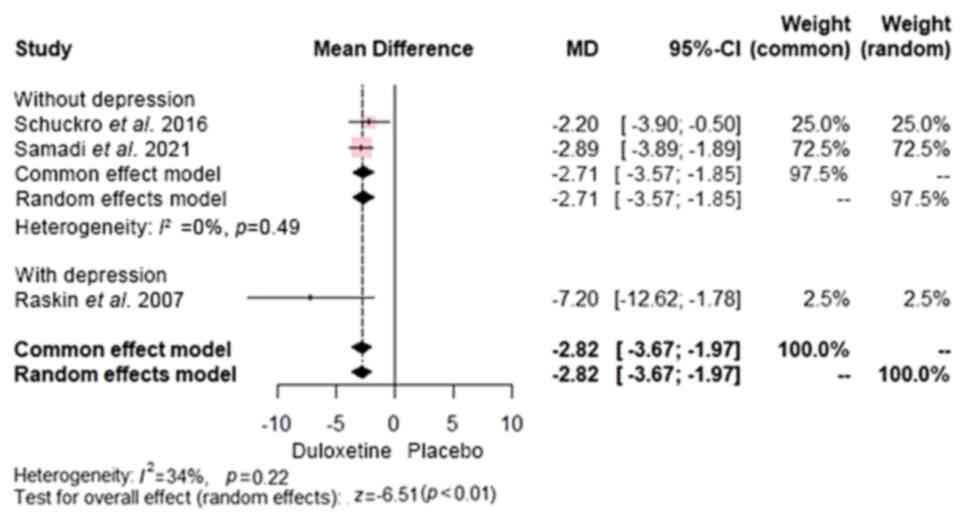
The results revealed that duloxetine was superior to
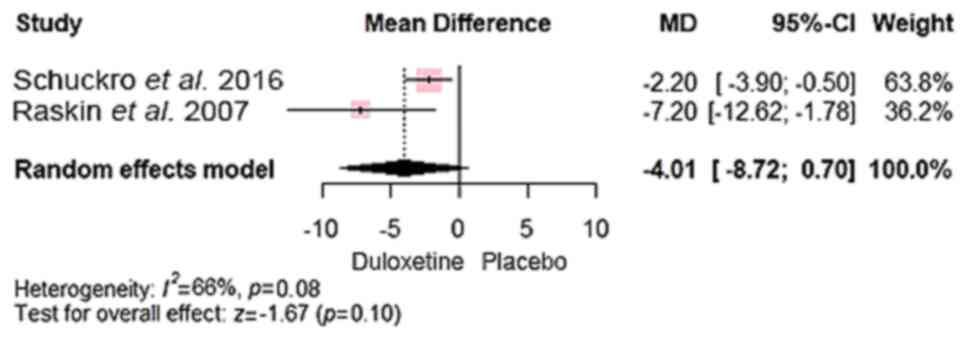
placebo in reducing pain intensity, as demonstrated by significant
improvements on both the VAS (20,21)
[MD, -2.82; 95% CI, (-3.67 to -1.97); P<0.0001]. A subgroup
analysis was conducted to differentiate patients with concomitant
depression from those with only CLBP. The results of both groups
(CLBP with and without depression) showed that duloxetine had
significant advantage over control [MD, -2.71; 95% CI, (-3.57 to
-1.85) and MD, -7.20; 95% CI, (-12.62 to -1.78), respectively]
(Fig. 3).
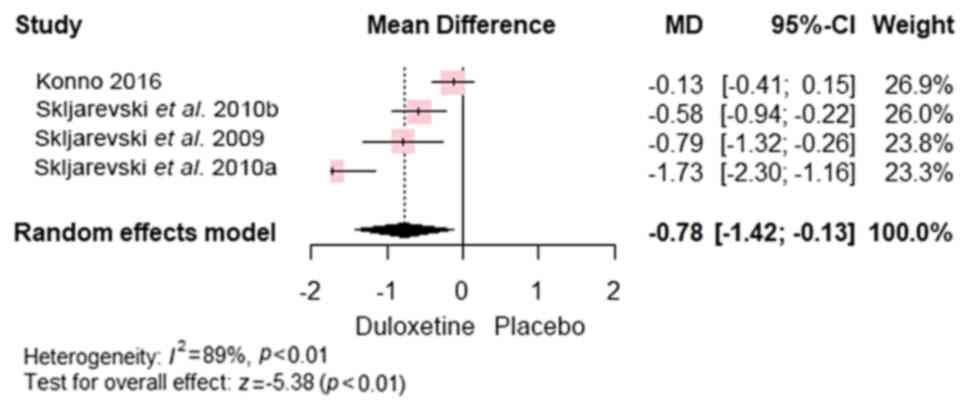
Furthermore, the overall mean difference favoured
duloxetine over control in terms of the BPI score (7,22-24)
[MD, -0.78; 95% CI, (-1.42 to -0.13); P<0.0001] (Fig. 4). In addition, the overall mean
difference between favoured duloxetine over control regarding the
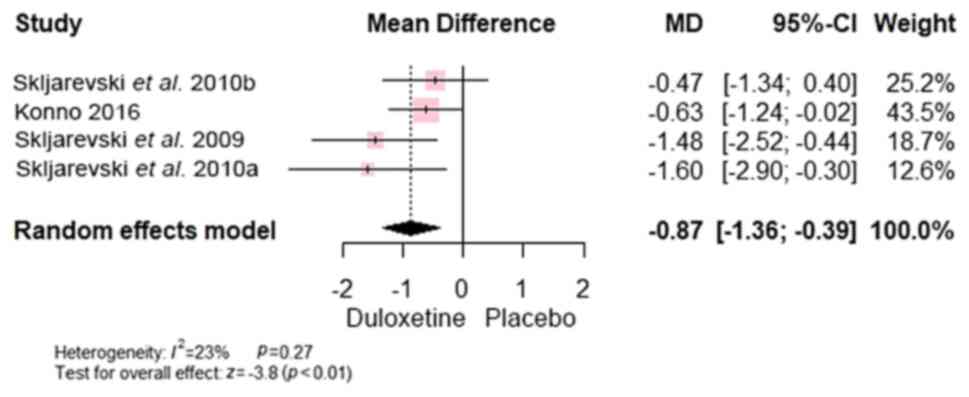
RMDQ-24 scale [MD, -0.87; 95% CI, (-1.36 to -0.39), P<0.0001]
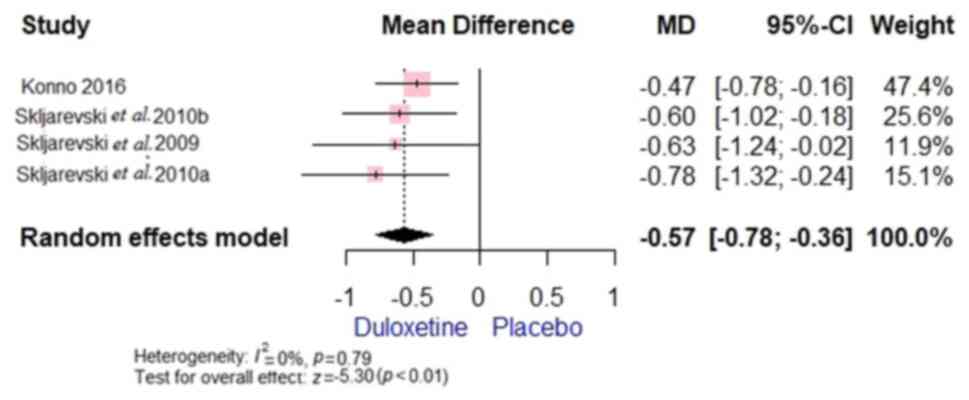
(Fig. 5), BPI-S average pain [MD,
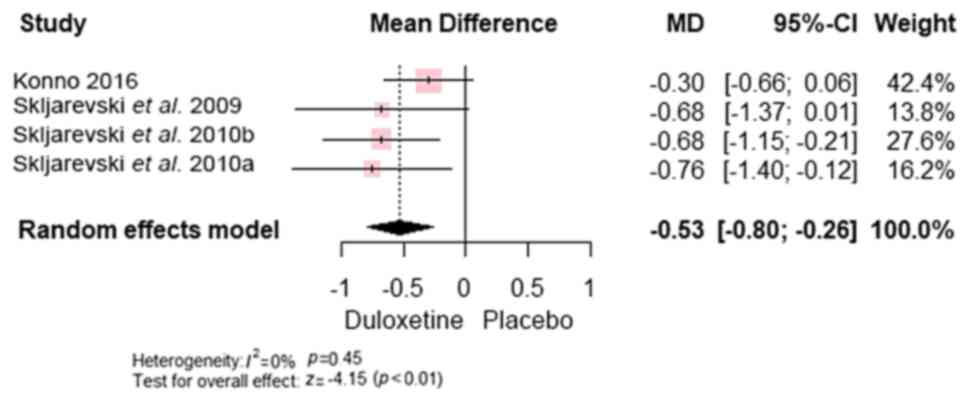
-0.57; 95% CI, (-0.78 to -0.36); P<0.00001] (Fig. 6) and worst pain [MD, -0.53; 95% CI,
(-0.80 to -0.26); P<0.0001] (Fig.
7).
Quality of life. One study (21) reported on impact of duloxetine on
overall SF-36 scores, and showed no significant difference between
the compared groups [MD, 4.30; 95% CI, (-3.59 to 12.19);
P>0.05].
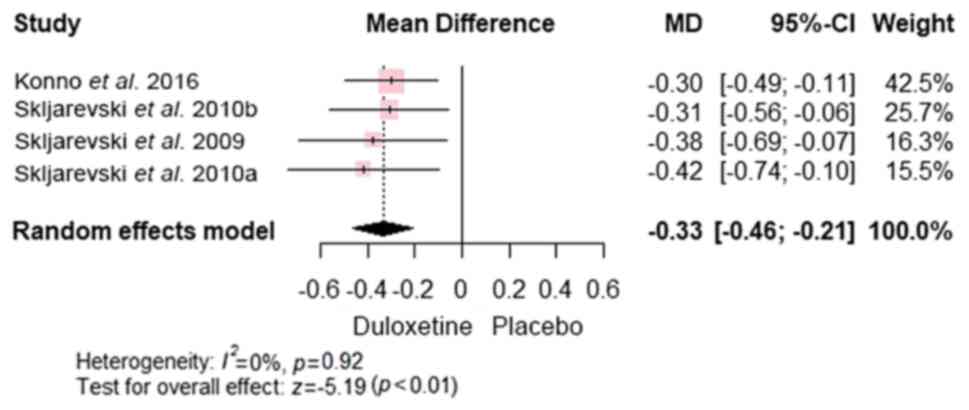
PGI-I. Assessment of PGI-I across the 4
studies (7,22-24)
revealed significant advantages of duloxetine over placebo [MD,
-0.33; 95% CI, (-0.46 to -0.21); P<0.0001)], with no significant
heterogeneity (P=0.92 and I2=0%) (Fig. 8).
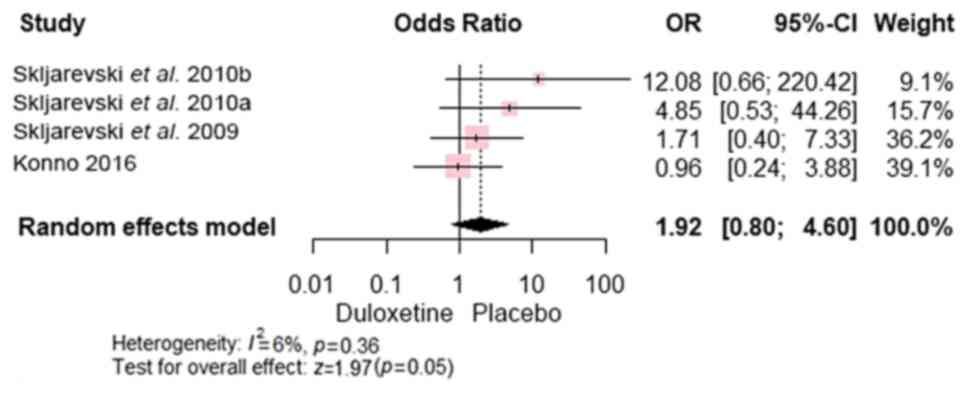
Safety and tolerability. In total, 7 studies
reported data on safety and tolerability while the study conducted
by Samadi et al (21) did
not report any data on side effects. The analysis revealed no
significant difference in the occurrence of serious adverse events
(chest pain, vertigo, dyspnoea, perioral numbness, perioral
numbness, myocardial infarction, toxic myopathy, asthma and alcohol
poisoning) between the duloxetine and control groups [RR, 1.92; 95%
CI, (0.80 to 4.60); P=0.054], with studies (7,22-24)
demonstrating homogeneity (P=0.36 and I2=6%) (Fig. 9).
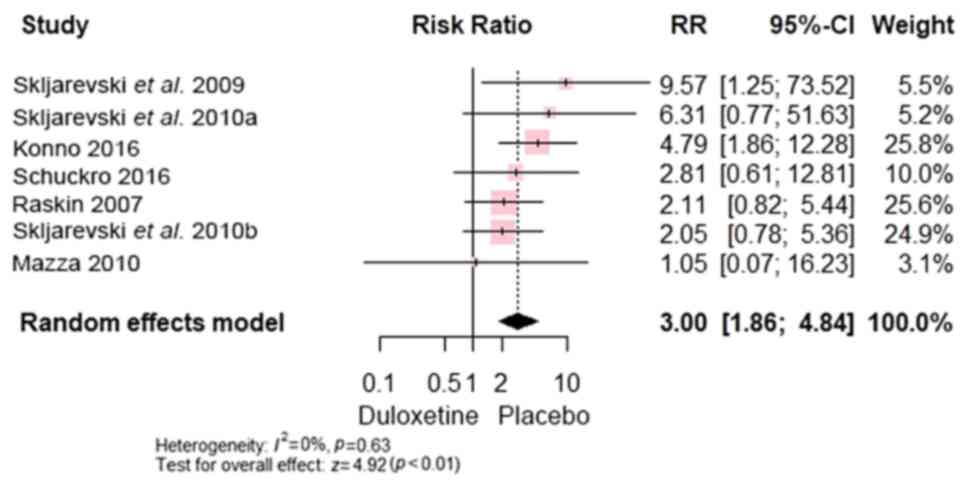
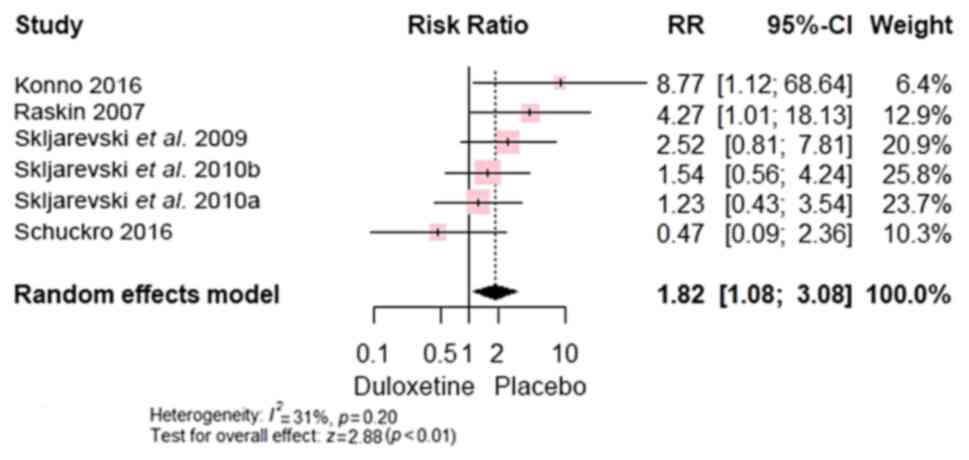
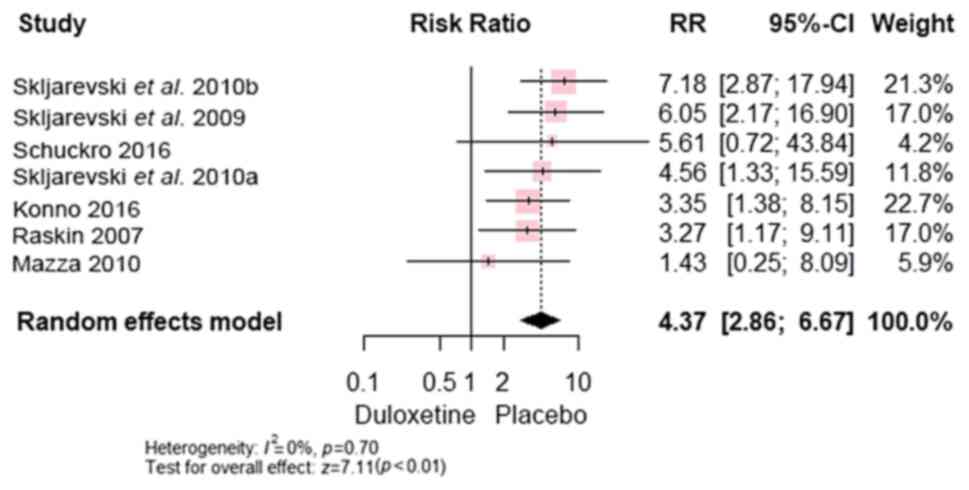
Compared with control, duloxetine exhibited more
constipation [RR, 3.00; 95% CI, (1.86 to 4.84); P<0.00001]
(Fig. 10), diarrhoea [RR, 1.82;
95% CI, (1.08 to 3.08); P<0.0001] (Fig. 11), nausea [RR, 4.37; 95% CI, (2.86
to 6.67); P<0.00001] (Fig. 12)
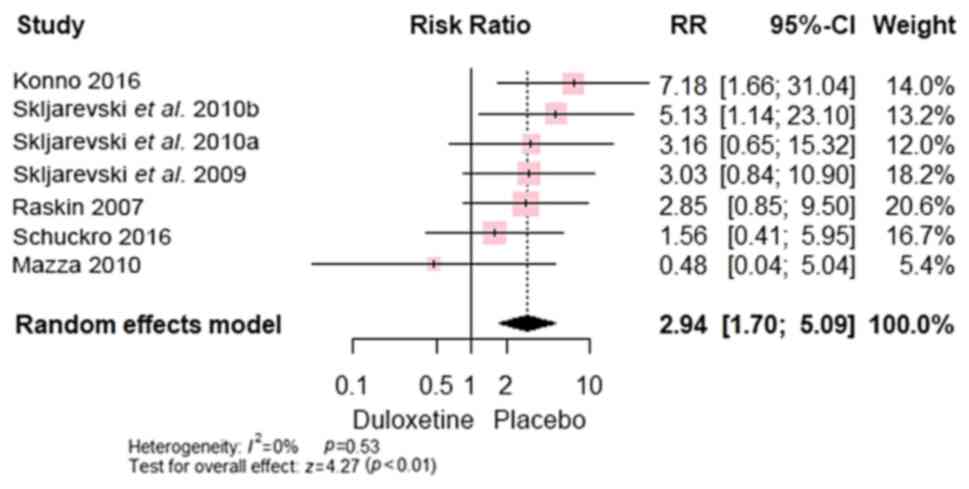
and dizziness [RR, 2.94; 95% CI, (1.70 to 5.09); P=0.0001]
(Fig. 13). In addition, no
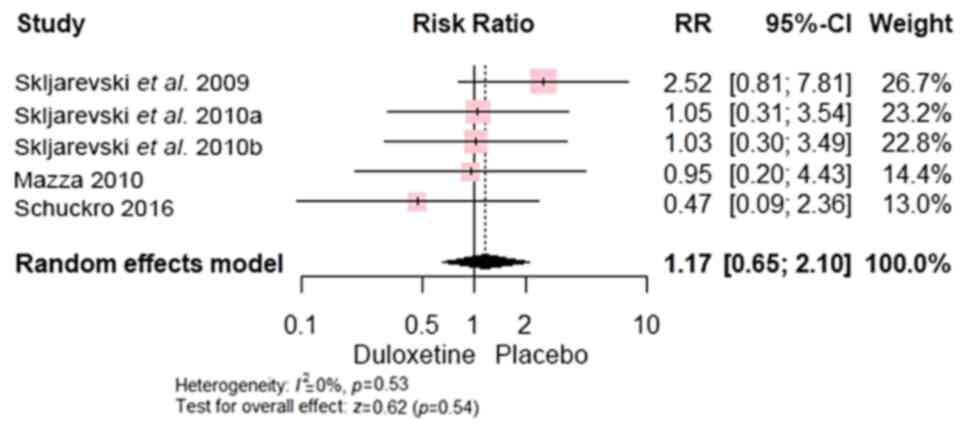
significant difference was found between both groups regarding
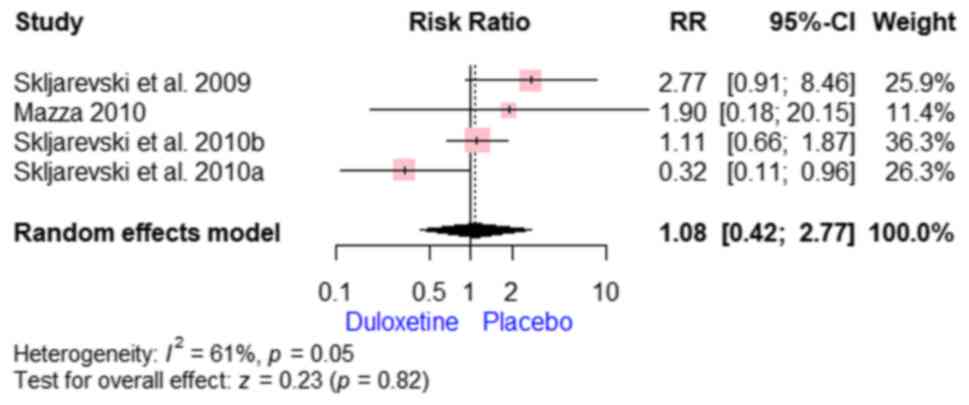
insomnia [RR, 1.17; 95% CI, (0.65 to 2.10); P=0.54] (Fig. 14) and headache [RR, 1.08; 95% CI,
(0.42 to 2.77); P=0.82] (Fig.
15).
Sensitivity analysis. A sensitivity analysis
was performed to evaluate the impact of excluding the study by
Samadi et al (21) that
included post-surgical patients. The aforementioned study only
reported on the VAS outcome. After removing it, the analysis did
not show significant difference between duloxetine and placebo in
terms of VAS outcome, as revealed in Fig. 16.
Discussion
The present study, including eight RCTs, provides
robust evidence, supporting the efficacy of duloxetine in reducing
pain and enhancing quality of life of patients with CLBP.
Duloxetine demonstrated superior outcomes over placebo in pain
reduction measured by the VAS, BPI, BPI-S, RMDQ-24 scales and worse
pain scales. In addition, duloxetine improved quality of life
assessed by the SF-36. Clinically, these results favor the
duloxetine group, indicating its efficacy in alleviating CLBP
symptoms and enhancing patients' overall well-being (25,26).
Although the overall trend supports the
effectiveness of duloxetine, individual study outcomes may vary.
Variations in the study design, patient populations and
methodological approaches contributed to the observed
heterogeneity. Discrepancies in the findings can be attributed to
factors such as differences in drug dosage, concomitant medication
usage, and patient characteristics such as age and comorbidities.
Methodological disparities, including study duration and outcome
measures, also contribute to variability among studies (27).
The superiority of the duloxetine group over the
placebo is scientifically grounded in its pharmacological mechanism
of action. As an antidepressant and serotonin-noradrenaline
reuptake inhibitor, duloxetine modulates neurotransmitter levels in
the central nervous system and attenuates pain-signaling pathways.
This neurobiological mechanism, coupled with the demonstrated
efficacy in clinical trials, elucidates why the duloxetine group
exhibited superior outcomes in terms of pain reduction and quality
of life improvement (28,29).
Previous studies have similarly highlighted the
efficacy of duloxetine in the management of CLBP, corroborating the
present findings (30,31). However, the present study advances
the existing literature by incorporating the most up-to-date
evidence through a comprehensive systematic review and
meta-analysis. These findings align with those of previous studies
that underscore the effectiveness of duloxetine in treating CLBP
(9). Nevertheless, the present
study provides additional insights and robust evidence, further
strengthening the existing body of literature on this topic.
The strengths of the present study include a
comprehensive methodology adhering to the PRISMA guidelines,
meticulous selection and analysis of relevant studies, and robust
assessment of study quality. However, limitations include the
observed heterogeneity across studies, potential biases inherent in
meta-analyses and limited number of included studies, which may
affect the generalizability of the findings.
Based on these results, future research should focus
on specific subpopulations, such as patients with depression and
CLBP, and explore the comparative efficacy of duloxetine in
conjunction with conservative therapies. Additionally, further
investigations into the efficacy of duloxetine across various types
of back pain and its long-term effects are warranted. Future
studies should also address the methodological inconsistencies and
biases observed in previous research to strengthen the evidence
base for the use of duloxetine in managing CLBP.
In conclusion, the present study has yielded
promising outcomes concerning pain alleviation and improvements in
the quality of life among individuals afflicted with CLBP. While
acknowledging limitations such as study heterogeneity and the
restricted number of included studies, the findings present
compelling evidence for the efficacy of duloxetine in mitigating
CLBP. These results highlight the potential of duloxetine as a
valuable therapeutic intervention in CLBP management, particularly
in individuals with comorbid depression. Further research is
imperative to address the prevailing limitations and
comprehensively validate these findings. Such efforts are crucial
for refining treatment approaches and advancing CLBP management on
a global scale.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MSAS, AZA and DB conceptualized and designed the
study, contributed to data analysis and interpretation, and played
a major role in writing the manuscript. HEM and ZB did the initial
screening while ZB and DB contributed to full text screening and
data interpretation. HEM and ZB curated and analyzed the data,
created visualizations, and contributed to the interpretation of
results. DB, HEM and ZB drafted the initial manuscript, synthesized
study findings, and incorporated feedback from co-authors. All
authors read and approved the final version of the manuscript. MSAS
and HEM confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alfalogy E, Mahfouz S, Elmedany S, Hariri
N and Fallatah S: Chronic low back pain: Prevalence, impact on
quality of life, and predictors of future disability. Cureus.
15(e45760)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kahere M, Hlongwa M and Ginindza TG: A
scoping review on the epidemiology of chronic low back pain among
adults in sub-saharan africa. Int J Environ Res Public Health.
19(2964)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nicol V, Verdaguer C, Daste C, Bisseriex
H, Lapeyre É, Lefèvre-Colau MM, Rannou F, Rören A, Facione J and
Nguyen C: Chronic low back pain: A narrative review of recent
international guidelines for diagnosis and conservative treatment.
J Clin Med. 12(1685)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hnatešen D, Pavić R, Radoš I, Dimitrijević
I, Budrovac D, Čebohin M and Gusar I: Quality of life and mental
distress in patients with chronic low back pain: A cross-sectional
study. Int J Environ Res Public Health. 19(10657)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Onuţu AH: Duloxetine, an antidepressant
with analgesic properties-A preliminary analysis. Rom J Anaesth
Intensive Care. 22:123–128. 2015.PubMed/NCBI
|
|
6
|
Dhaliwal JS, Spurling BC and Molla M:
Duloxetine. StatPearls. Treasure Island (FL): StatPearls Publishing
Copyright ©. 2024, StatPearls Publishing LLC.; 2024.
|
|
7
|
Skljarevski V, Zhang S, Desaiah D, Alaka
KJ, Palacios S, Miazgowski T and Patrick K: Duloxetine versus
placebo in patients with chronic low back pain: A 12-week,
fixed-dose, randomized, double-blind trial. J Pain. 11:1282–1290.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Weng C, Xu J, Wang Q, Lu W and Liu Z:
Efficacy and safety of duloxetine in osteoarthritis or chronic low
back pain: A systematic review and meta-analysis. Osteoarthritis
Cartilage. 28:721–734. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hirase T, Hirase J, Ling J, Kuo PH,
Hernandez GA, Giwa K and Marco R: Duloxetine for the treatment of
chronic low back pain: A systematic review of randomized
placebo-controlled trials. Cureus. 13(e15169)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Gift AG: Visual analogue scales:
Measurement of subjective phenomena. Nur Res. 38:286–288.
1989.PubMed/NCBI
|
|
12
|
Cleeland CS and Ryan KM: Pain assessment:
Global use of the brief pain inventory. Ann Acad Med Singap.
23:129–138. 1994.PubMed/NCBI
|
|
13
|
Stratford PW and Riddle DL: A roland
morris disability questionnaire target value to distinguish between
functional and dysfunctional states in people with low back pain.
Physiother Can. 68:29–35. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hays RD, Sherbourne CD and Mazel RM: The
rand 36-item health survey 1.0. Health Econ. 2:217–227.
1993.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Srikrishna S, Robinson D and Cardozo L:
Validation of the patient global impression of improvement (PGI-I)
for urogenital prolapse. Int Urogynecol J. 21:523–528.
2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Raskin J, Wiltse CG, Siegal A, Sheikh J,
Xu J, Dinkel JJ, Rotz BT and Mohs RC: Efficacy of duloxetine on
cognition, depression, and pain in elderly patients with major
depressive disorder: An 8-week, double-blind, placebo-controlled
trial. Am J Psychiatry. 164:900–909. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Stein DJ, Phillips KA, Bolton D, Fulford
KW, Sadler JZ and Kendler KS: What is a mental/psychiatric
disorder? From DSM-IV to DSM-V. Psychol Med. 40:1759–1765.
2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nixon N, Guo B, Garland A, Kaylor-Hughes
C, Nixon E and Morriss R: The bi-factor structure of the 17-item
hamilton depression rating scale in persistent major depression;
dimensional measurement of outcome. PLoS One.
15(e0241370)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Higgins JPT, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L and Sterne JAC:
Cochrane Bias Methods Group; Cochrane Statistical Methods Group.
The Cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Schukro RP, Oehmke MJ, Geroldinger A,
Heinze G, Kress HG and Pramhas S: Efficacy of duloxetine in chronic
low back pain with a neuropathic component: A randomized,
double-blind, placebo-controlled crossover trial. Anesthesiology.
124:150–158. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Samadi A, Salehian R, Kiani D and Jolfaei
AG: Effectiveness of duloxetine on severity of pain and quality of
life in chronic low back pain in patients who had posterior spinal
fixation. J Orth Trauma Rehabil. 6(144)2021.
|
|
22
|
Konno S, Oda N, Ochiai T and Alev L:
Randomized, double-blind, placebo-controlled phase III trial of
duloxetine monotherapy in Japanese patients with chronic low back
pain. Spine (Phila Pa 1976). 41:1709–1717. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Skljarevski V, Desaiah D, Liu-Seifert H,
Zhang Q, Chappell AS, Detke MJ, Iyengar S, Atkinson JH and Backonja
M: Efficacy and safety of duloxetine in patients with chronic low
back pain. Spine (Phila Pa 1976). 35:E578–E585. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Skljarevski V, Ossanna M, Liu-Seifert H,
Zhang Q, Chappell A, Iyengar S, Detke M and Backonja M: A
double-blind, randomized trial of duloxetine versus placebo in the
management of chronic low back pain. Eur J Neurol. 16:1041–1048.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Brown JP and Boulay LJ: Clinical
experience with duloxetine in the management of chronic
musculoskeletal pain. A focus on osteoarthritis of the knee. Ther
Adv Musculoskelet Dis. 5:291–304. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rodrigues-Amorim D, Olivares JM, Spuch C
and Rivera-Baltanás T: A systematic review of efficacy, safety, and
tolerability of duloxetine. Front Psychiatry.
11(554899)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jia Z, Yu J, Zhao C, Ren H and Luo F:
Outcomes and predictors of response of duloxetine for the treatment
of persistent idiopathic dentoalveolar pain: A retrospective
multicenter observational study. J Pain Res. 15:3031–3041.
2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yasuda H, Hotta N, Nakao K, Kasuga M,
Kashiwagi A and Kawamori R: Superiority of duloxetine to placebo in
improving diabetic neuropathic pain: Results of a randomized
controlled trial in Japan. J Diabetes Investig. 2:132–139.
2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Obata H: Analgesic mechanisms of
antidepressants for neuropathic pain. Int J Mol Sci.
18(2483)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Onda A and Kimura M: Reduction in anxiety
during treatment with exercise and duloxetine is related to
improvement of low back pain-related disability in patients with
non-specific chronic low back pain. Fukushima J Med Sci.
66:148–155. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ferreira GE, McLachlan AJ, Lin C-WC, Zadro
JR, Abdel-Shaheed C, O'Keeffe M and Maher CG: Efficacy and safety
of antidepressants for the treatment of back pain and
osteoarthritis: Systematic review and meta-analysis. BMJ.
372(m4825)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mazza M, Mazza O, Pazzaglia C, Padua L and
Mazza S: Escitalopram 20 mg versus duloxetine 60 mg for the
treatment of chronic low back pain. Expert Opin Pharmacother.
11:1049–1052. 2010.PubMed/NCBI View Article : Google Scholar
|