1. Introduction
Asthma is a common chronic respiratory condition
marked by inflammation of the airways, the reversible obstruction
of airflow and an increased sensitivity of the bronchi (1). This disease affects millions of
individuals globally, markedly affecting their quality of life
(QoL) and placing a substantial burden on healthcare systems.
According to the Centers for Disease Control and Prevention (CDC),
asthma affects ~25 million Americans, which translates to ~1 in 13
individuals (2). Globally, the
prevalence of asthma varies; however, it remains a critical public
health challenge (3).
In pregnant women, the prevalence of asthma is
noteworthy, affecting 8-13% of pregnancies (4). Pregnancy can alter the course of
asthma, with approximately one-third of pregnant women experiencing
more severe symptoms, one-third remaining stable and one-third
observing an improvement in their asthma condition (5). This variability introduces
significant challenges in the management of asthma during
pregnancy, necessitating a nuanced approach to treatment that
considers both maternal and fetal health. Multiple factors compound
the management of asthma in pregnancy, increasing both maternal and
fetal risks (6,7). These risks underscore the importance
of optimal asthma management during pregnancy.
The physiological changes that occur during
pregnancy, such as the increased tidal volume and decreased
functional residual capacity, can complicate the standard asthma
treatment protocols (8).
Furthermore, the safety of various asthma medications during
pregnancy continues to be a pivotal concern, with ongoing research
focusing on understanding the teratogenic effects of traditional
and newer asthma medications (6).
The present review discusses the novel challenges in
the management of asthma during pregnancy, particularly focusing on
the physiological, pharmacological and therapeutic complexities
introduced by pregnancy. The present review also discusses recent
advancements in treatment strategies that have emerged in response
to these challenges. These achievements include the development and
increased use of biologics in pregnancy, innovations in
personalized medicine approaches, and the integration of digital
health tools into patient monitoring and management strategies.
2. Physiological changes that occur during
pregnancy
Physiological changes occurring in
pregnancy
Pregnancy induces notable physiological changes that
can affect almost every organ system, including the respiratory
system. These changes can exacerbate asthma or influence the
effectiveness and pharmacokinetics of medications for asthma
management.
Respiratory and cardiovascular
changes
During pregnancy, several physiological changes
occur that can complicate asthma management. Increased progesterone
levels stimulate the respiratory center in the medulla, resulting
in a 40% increase in tidal volume and a 50% increase in minute
ventilation, while the respiratory rate remains stable (9). These changes are essential to meet
the heightened oxygen demands of both the mother and fetus.
However, they also reduce arterial partial pressure of carbon
dioxide and elevate respiratory alkalosis, potentially exacerbating
airway hyperresponsiveness and altering responses to asthma
medications (10).
The cardiovascular system also undergoes significant
adaptations during pregnancy, with the blood volume increasing by
30-50% to ensure adequate placental perfusion and meet the
metabolic needs of the mother and fetus. This hemodilution reduces
drug concentrations in the bloodstream, and combined with the
enhanced renal blood flow, it can lead to the more rapid clearance
of asthma medications, such as bronchodilators and corticosteroids.
Consequently, standard doses may not achieve therapeutic levels,
necessitating careful and potentially frequent adjustments to
dosing regimens (11).
Immunologic and mechanical
changes
Immunologically, pregnancy induces a shift from a
T-helper 1 (ThH1) to a T-helper-2 (ThH2) dominance to prevent fetal
rejection. However, this ThH2-dominant response also increases
antibody production and the likelihood of developing allergic
reactions, common triggers for asthma exacerbations. For pregnant
women with asthma, this shift can increase the frequency and
severity of symptoms, particularly if their asthma is
allergy-driven. Therefore, healthcare providers need to reassess
treatment plans to accommodate these immunological changes,
potentially requiring more aggressive or different therapeutic
approaches (12).
Mechanically, the growing uterus elevates the
diaphragm, reducing the space within the thoracic cavity and
decreasing total lung capacity. This elevation primarily affects
the residual volume, the amount of air remaining in the lungs
following a forced exhalation. Although vital capacity remains
stable, the reduced lung space can cause a sensation of labored
breathing. Asthma sufferers may experience increased shortness of
breath, necessitating adjustments in management strategies, such as
the more frequent use of inhaled bronchodilators or changes in the
timing of medication administration (13).
3. Medication safety and treatment
strategies
Medication safety
Ensuring the safety of asthma medications during
pregnancy is critical because of the potential risks to both the
mother and the fetus. Recent research has provided considerable
insight into the safety profiles of various asthma medications when
used during pregnancy.
Inhaled corticosteroids (ICS) and oral
corticosteroids
ICS are the cornerstone of asthma management and
have been extensively studied for their safety during pregnancy.
Oral corticosteroids are used to treat severe asthma attacks
(14).
The study by Schatz et al (15) assessed the safety of various asthma
and allergy medications during pregnancy among 824 women with
asthma and 678 controls without asthma. Medications analyzed
included β-agonists and corticosteroids. The primary outcomes
investigated were major congenital malformations, preeclampsia,
preterm birth, a low birth weight and infants who were small for
their gestational age. Their study found no significant
associations between the use of these medications and an increased
risk of major congenital malformations or other adverse perinatal
outcomes, except for oral corticosteroids, which were associated
with an increased risk of preeclampsia [odds ratio (OR), 2.0]
(15). Furthermore, a
dose-response association was noted with corticosteroid exposure
and adverse outcomes, such as preeclampsia, preterm birth and low
birth weight. However, their study highlighted that the risks
associated with severe asthma potentially outweigh the risks posed
by medications, supporting the continued use of oral
corticosteroids when necessary, during pregnancy (15).
The study by Martel et al (16) examined the potential risks
associated with the use of ICS during pregnancy, specifically as
regards pregnancy-induced hypertension (PIH) and pre-eclampsia
among asthmatic women. Utilizing data from three Quebec health
databases, their study analyzed 3,505 women with asthma across
4,593 pregnancies (16). The
findings of their study indicated no significant association
between the use of ICS and an increased risk of developing PIH or
pre-eclampsia. However, the use of oral corticosteroids was
associated with a significant increase in the risk of developing
PIH, with a trend toward an increased risk of pre-eclampsia
(16).
Another study by Bracken et al (17) investigated the impact of asthma and
its treatment on pregnancy outcomes, specifically focusing on
preterm delivery and intrauterine growth restriction among 2,205
pregnant women, including 873 women with a history of asthma. Their
study found that while asthma severity and symptoms did not
significantly affect the risk of preterm delivery, the use of
certain medications, particularly oral corticosteroids, was
associated with an increased risk of preterm delivery (17). Women using oral corticosteroids
experienced reductions in gestational length by ~2.22 weeks
(17).
In addition, the study by Blais et al
(18) investigated the risk of
congenital malformations associated with the use of ICS during the
first trimester of pregnancy among women with asthma. The cohort of
their study comprised 4,561 pregnancies from women who delivered
between 1990 and 2000. Their study specifically evaluated the
association between varying doses of ICS and the incidence of
congenital malformations (18).
The key findings were that 9.2% of the pregnancies resulted in
congenital malformations, with 6.1% being classified as major
malformations. Of note, ~40% of the women used ICS during the first
trimester, with only 5.3% using doses >500 mg/day. The adjusted
OR for all congenital malformations with ICS use was 0.77 for doses
between 1-500 mg/day, 0.41 for doses between 501-1,000 mg/day, and
1.00 for doses >1,000 mg/day. For major malformations, the
adjusted ORs were 0.90, 0.56 and 1.67 for the respective dose
categories, respectively (18).
Their study concluded that the use of ICS during the first
trimester was not associated with an increased risk of congenital
malformations, even at higher doses. These results support the
safety of ICS treatment during pregnancy, aligning with
recommendations to maintain asthma control to prevent adverse
maternal and fetal outcomes (18).
The study by Bakhireva et al (19) evaluated the effects of asthma
medications, specifically ICS and oral corticosteroids on fetal
growth during pregnancy. The study included 654 infants born to
mothers with asthma and 303 infants born to mothers without asthma
as controls. The key findings indicated that the use of systemic
corticosteroids was associated with a decrease in mean birth
weight, with infants born to these mothers weighing ~200 g less
compared to those exposed only to β-agonists or no asthma
medications. However, no significant differences were found in the
incidence of small for gestational age infants among the different
medication groups, including ICS users (19). That study also noted that mean
birth length and head circumference did not significantly differ
among the groups. That study concluded that while systemic
corticosteroids had a minimal effect on birth weight, the use of
ICS did not impair fetal growth (19).
Furthermore, the study by Otsuka et al
(20) investigated the safety and
efficacy of ICS for asthma management in pregnant women. That
retrospective study reviewed the records of 592 asthmatic pregnant
women who delivered at a Japanese hospital between 1987 and 2003.
The key findings of that study indicated that the use of ICS
increased significantly over the study period, from 0% in 1987-1989
to 83.3% in 2000-2003. The incidence of intrapartum asthma attacks
markedly decreased in women treated with ICS, with no attacks
reported from 2000 to 2003, contrasting with a 1.38% incidence from
1995 to 1999 among those treated with inhaled β-agonists alone
(20). Perinatal abnormalities
were more common among untreated women and those with severe
asthma. The incidence of these abnormalities decreased
significantly from 59.6% before 1995 to 26.2% after 1995 in the
treated group, particularly among those using ICS. Comparisons
revealed no significant differences in perinatal outcomes between
women treated with ICSs and those treated with other asthma
medications, apart from a slightly higher incidence of premature
rupture of membranes in the ICS group, which still fell within the
expected range. Their study concluded that ICS are safe and
effective for preventing asthma attacks during pregnancy and
reducing perinatal abnormalities (20).
Rahimi et al (21) performed a large meta-analysis which
demonstrated that ICS do not elevate the risk of significant
malformations, preterm delivery, low birth weight, or
pregnancy-induced hypertension. Notably, they enhance symptom
management and are beneficial in treating asthma, rendering them
safe for use during pregnancy (21). These findings are critical as they
support the continued use of ICS in pregnant women with asthma to
maintain asthma control and reduce exacerbations.
β-agonists
As regards the use of β-agonists during pregnancy,
studies have extensively evaluated the safety of short-acting
β-agonists (SABAs) and long-acting β-agonists (LABAs) to ensure
they do not pose significant risks to the developing fetus
(22,23). Research has consistently shown that
these medications, crucial for asthma management, are generally
safe for use during pregnancy and are not associated with major
teratogenic risks (22,23). This means that the use of SABAs and
LABAs does not increase the likelihood of congenital anomalies or
developmental issues in the fetus. Therefore, they serve as an
effective and safe component of an asthma treatment regimen during
pregnancy, guaranteeing optimal asthma control for the mother,
without jeopardizing the health of the fetus. This safety profile
supports the continued use of these medications by pregnant women
who require asthma management, aligning with current medical
guidelines and practices (22,23).
The study by Lao and Huengsburg (24) retrospectively analyzed the outcomes
of pregnancy and labor in 87 asthmatic mothers compared to a
matched group of non-asthmatic controls. The primary focus was on
evaluating the safety and impact of bronchodilator therapy,
including the use of β-agonists, during pregnancy. The results of
their study indicated that bronchodilator therapy in asthmatic
mothers was not associated with an increased incidence of adverse
outcomes, such as preterm delivery, post-term delivery, low birth
weight, instrumental deliveries, postpartum hemorrhage, or
perinatal complications. However, there was a higher incidence of
caesarean sections in asthmatic mothers, particularly among those
receiving bronchodilator treatment. This was possibly linked to a
slightly higher rate of labor induction in these patients (24). Additionally, that study found that
well-controlled asthma during pregnancy, even with the use of
bronchodilator therapy, did not significantly differ from normal
controls in terms of overall pregnancy outcomes. The presence or
history of asthma, when managed effectively, did not adversely
influence the outcome of labor, with factors such as labor
induction and epidural analgesia playing a more significant role.
The findings suggest that with appropriate management, including
the use of bronchodilators, the risks associated with asthma in
pregnancy can be minimized, supporting the safety of β-agonists
during this period (24).
The study by Lin et al (25) explored the association between the
maternal use of asthma medications during the periconceptional
period and the risk of gastroschisis, a congenital abdominal wall
defect. That case-control study used data from the National Birth
Defects Prevention Study, covering births from 1997 to 2002,
including 381 cases of gastroschisis and 4,121 controls without
malformations (25). That study
found that the maternal use of bronchodilators during the
periconceptional period was associated with an increased risk of
gastroschisis [adjusted OR, 2.06; 95% confidence interval (CI),
1.19-3.59]. The risk was particularly elevated for women who used
multiple bronchodilators concurrently during this period,
suggesting a potential dose-response association (25). The findings of that study indicate
a potential association between bronchodilator use and an increased
risk of gastroschisis, although that study could not definitively
separate the effects of medication from the effects of asthma
severity. The authors of that study highlighted the need for
further research to determine whether the increased risk is due to
the medication itself or the underlying uncontrolled asthma
(25). That study underscored the
importance of carefully managing asthma during pregnancy, while
considering the potential risks and benefits of asthma medications
(25).
Another study by Clifton et al (26) examined the effects of inhaled
glucocorticoids and combination therapy with LABAs on placental
function and neonatal birthweight in pregnancies complicated by
asthma. Their study included 41 pregnant women with asthma and a
control group of 20 non-asthmatic women. The asthmatic group was
further divided based on their medication: Budesonide alone,
fluticasone propionate alone, and a combination of fluticasone
propionate with the LABA salmeterol (26). The key findings were that the use
of inhaled budesonide was associated with an increased placental
11β-HSD-2 activity, an enzyme critical for protecting the fetus
from excess maternal glucocorticoids. This group also exhibited
normal birthweight outcomes, suggesting that inhaled budesonide
does not adversely affect fetal growth. By contrast, the
combination therapy group (fluticasone/salmeterol) exhibited
reduced birthweight centiles, although without significant changes
in placental 11β-HSD-2 activity (26). This suggests a potential impact of
LABAs on fetal growth, although the small sample size necessitates
further investigation. The study by Clifton et al (26) concluded that while ICS alone
appeared safe and beneficial in managing asthma during pregnancy,
the combination with LABAs may require closer monitoring.
Leukotriene receptor antagonists
Numerous studies have examined the safety and
effects of leukotriene-receptor antagonists (LTRAs), such as
montelukast and pranlukast, on both the mother and the baby during
pregnancy.
For example, in 2007, Bakhireva et al
(27) assessed the safety of LTRAs
by comparing perinatal outcomes among women who used LTRAs, those
who used short-acting β-agonists and women without asthma. Their
findings demonstrated that LTRAs were not associated with increased
risks of adverse outcomes, such as pregnancy loss, gestational
diabetes, preeclampsia, low maternal weight gain, preterm delivery,
low Apgar scores, or reduced birth length and head circumference.
They noted a slight decrease in birth weight among infants born to
LTRA users, likely due to the severity of the asthma of the mother
(27).
Sarkar et al (28) in 2009 also examined the use of
montelukast during pregnancy in a multicenter, prospective,
comparative study. Their research involved 180 montelukast-exposed
pregnancies, resulting in 160 live births. Their study noted lower
birth weights and shorter gestational ages for montelukast-exposed
infants but found no significant increase in major malformations
(28).
Subsequently, Koren et al (29) in 2010 observed statistically
smaller babies and shorter gestational ages in 180 cases of
pregnant women exposed to montelukast compared to groups not
exposed to teratogens. However, these differences were not
significant when compared to a disease-matched group. It was found
that ~25% of the newborns experienced fetal distress, with only one
reported case of major malformation among the 143 infants exposed
during organogenesis (29). The
conclusion of that study was that montelukast does not
significantly increase the risk of major malformations in the
general population beyond the baseline risk (29).
In 2017 Cavero-Carbonell et al (30) conducted a Danish study analyzing
registry data to evaluate pregnancy outcomes related to montelukast
exposure. In montelukast-exposed pregnancies, that study included
754,300 singleton pregnancies and found increased risks of preterm
birth and maternal complications, such as preeclampsia and
gestational diabetes. However, that study found no significant
increase in major congenital anomalies, indicating that the
severity of the underlying maternal asthma may have a greater
influence on montelukast-related risks than the medication itself
(30).
Subsequently, in 2022, Hatakeyama et al
(31) focused on montelukast and
pranlukast, analyzing outcomes from 231 pregnant women exposed to
these medications during the first trimester compared to control
groups. Their study reported a 1.9% incidence of major congenital
anomalies, with no significant increase in risk indicated by
multivariable logistic regression analysis. The results of that
study suggested that montelukast and pranlukast do not elevate the
risk of major congenital anomalies, reinforcing their safety for
asthma management during pregnancy (31).
Iin 2023, Tsai et al (32) utilized Taiwan's National Health
Insurance Research Database to study the association between LTRA
use during pregnancy and the occurrence of neuropsychiatric events
(NEs) in offspring. That study, which covered 576,157
mother-offspring pairs, including 1,995 children exposed to LTRAs,
found no significant associations between prenatal LTRA exposure
and the development of attention deficit hyperactivity disorder
(ADHD), autism spectrum disorder (ASD), or Tourette syndrome in
children (32). The findingsof
that study indicate that LTRAs do not increase the risk of NEs in
offspring, providing reassurance for their use in pregnant women
with asthma or allergic rhinitis (32).
Overall, these studies suggest that while LTRAs,
such as montelukast and pranlukast may be associated with certain
risks such as a lower birth weight and a shorter gestational age,
they do not significantly increase the risk of major birth defects
and are generally safe for managing asthma during pregnancy.
However, the underlying maternal asthma may contribute to the
observed risks, and further studies are recommended to clarify
these associations. They can be a valuable part of a comprehensive
asthma management plan, particularly for women who do not respond
well to other types of medications. Further research is required on
these associations to guarantee the safety and effectiveness of
LTRAs in pregnant populations.
Biologics
Researchers have evaluated newer biological
therapies for pregnant populations, such as omalizumab, which
targets IgE antibodies. Limited studies suggest that omalizumab is
safe during pregnancy (as mentioned below). The ‘Xolair Pregnancy
Registry (EXPECT)’ report is a summary of a study that investigated
the safety of omalizumab use during pregnancy (33). That study focused on the outcomes
for the mother, the pregnancy and the baby, including the number of
birth defects. That study included 191 pregnant women exposed to
omalizumab, with data collection occurring at various stages from
enrollment to 18 months post-delivery. The outcomes for 169
pregnancies indicated 156 live births, 11 spontaneous abortions,
one stillbirth and one elective termination. Among the live births,
14.5% of infants were born prematurely, 10.9% were small for their
gestational age, and 3.2% had low birth weights. There were 20
infants with confirmed congenital anomalies, with 7 infants having
major defects and no unusual pattern of anomalies (33). Overall, the results of that study
suggest that omalizumab exposure during pregnancy does not
significantly deviate from expected congenital anomaly rates in the
general population or among asthma sufferers, indicating no
additional risk from the drug during pregnancy (33).
The study by Namazy et al (34) in 2020 compared the pregnancy
outcomes of women treated with omalizumab and a disease-matched
cohort not receiving omalizumab. Their study monitored 250 pregnant
women with asthma who received omalizumab treatment and compared
their outcomes with those of 1,153 pregnant women with
moderate-to-severe asthma who did not receive the treatment. The
results of their study revealed that the prevalence of major
congenital anomalies was 8.1% in the omalizumab-exposed group and
8.9% in the comparator group, indicating no significant difference
in the risk of congenital anomalies between the two groups.
Furthermore, the rates of live births and premature births were
similar between the two groups, suggesting that the use of
omalizumab during pregnancy does not increase the risk of these
outcomes compared to a similar population not treated with the drug
(34).
Gemicioğlu et al (35) in 2021 examined the safety of
omalizumab treatment in pregnant patients with asthma and found it
to be safe for both mothers and their infants. The researchers
observed improvements in asthma control measures during and after
pregnancy compared to before treatment initiation, after analyzing
data from 20 pregnant women treated with omalizumab. While 36.4% of
the women experienced asthma exacerbations during pregnancy, there
were no congenital anomalies among the 23 infants born, although
there were instances of low birth weight and premature births. That
study concluded that omalizumab does not significantly increase
risks for pregnant patients or their offspring (35).
In 2022, Shakuntulla and Chiarella (36) provided an extensive analysis of the
safety of using biologics in pregnant women with atopic diseases.
The focus of their study was on seven FDA-approved biologics:
Omalizumab, mepolizumab, reslizumab, benralizumab, dupilumab,
tezepelumab and tralokinumab. The data compiled from various
sources, primarily case reports and observational studies, involved
a total of 313 pregnancies (36).
Their study found no significant evidence that these biologics
negatively affect pregnancy outcomes, including preterm delivery,
low birth weight, or congenital malformations. This suggests that
the underlying atopic condition worsening is more likely to affect
pregnancy viability than the biologics themselves. However, the
authors of that study call for more extensive prospective studies
and registries to better assess the long-term safety and effects of
biologics on maternal and fetal health, acknowledging the
limitations of the current data due to the small sample sizes and
lack of controlled studies (36).
Collectively, the data remain relatively limited,
and biologics are typically reserved for patients with severe
asthma who do not respond to other treatments.
Innovative therapeutic approaches
In addition to the ongoing evaluation of medication
safety, there have been significant advancements in personalized
care plans, biologic treatments for severe asthma, the use of
inhaled corticosteroids and enhanced monitoring techniques during
pregnancy (37).
Integrated care approaches
Healthcare professionals, including obstetricians,
pulmonologists, allergists, and primary care providers, have
recognized the efficacy of multidisciplinary care models in
managing asthma during pregnancy. Research has shown that these
integrated care approaches significantly improve the outcomes of
pregnant women with asthma by providing comprehensive and cohesive
care. The key components of such models typically include
customized asthma action plans tailored to meet the unique needs of
each patient. These plans outline detailed strategies for managing
symptoms and provide clear instructions on how to adjust
medications safely during pregnancy. These approaches require
regular monitoring to promptly identify and address any changes in
the condition of the patient or response to treatment. This
proactive surveillance helps mitigate the risks associated with
asthma exacerbations during pregnancy, which can pose significant
health risks to both the mother and fetus. Adjustments to therapy
are based on a thorough assessment of the current condition of the
patient, considering factors, such as gestational age and any other
complications or comorbidities (38).
Utilizing telemedicine and digital
health tools
The advent of telemedicine and digital health tools
has transformed the management of chronic diseases, including
asthma during pregnancy. These technologies enable the continuous
monitoring and real-time data collection, making it easier for
healthcare providers to make informed decisions and adjust
treatment plans as needed without the necessity of frequent
face-to-face visits. This is particularly advantageous for pregnant
women, who may find frequent trips to healthcare facilities
challenging.
Digital tools, such as mobile apps for tracking
symptoms and medication use, as well as wearable devices that
monitor respiratory function, can improve patient engagement and
self-management. These technologies allow patients to record and
report their symptoms in real-time, facilitating timely
interventions by healthcare providers. Furthermore, telemedicine
provides a platform for virtual consultations, which are crucial
during times when access to direct medical care is limited
(39,40).
Updates on medication safety and
management strategies
Recent advances in research regarding the safety and
efficacy of asthma medications during pregnancy have led to
significant updates in standard care protocols. These guidelines
are critical for ensuring the health and safety of both the mother
and the unborn child. These updated protocols focus on maintaining
optimal asthma control by administering the safest possible
medications at the lowest effective doses.
The guidelines advocate for a careful assessment of
the risks and benefits of each medication, considering the latest
evidence on their safety profiles during pregnancy. This approach
not only helps in preventing asthma exacerbations, but also
minimizes the potential risks associated with medication exposure
during pregnancy. The goal is to strike a balance between effective
asthma control and the imperative to protect fetal development,
thereby reducing the likelihood of complications, such as pre-term
birth and a low birth weight associated with poorly controlled
asthma. These updated protocols serve as a crucial resource for
healthcare providers, ensuring that pregnant women with asthma
receive the most effective and safest possible care (41).
Personalized medicine
Personalized medicine represents a transformative
approach to healthcare, particularly in the management of asthma
during pregnancy, by tailoring treatment to the individual
characteristics of each patient. By specifically aligning care with
the physiological and genetic profile of pregnant women, this
personalized approach enhances the efficacy and safety of asthma
management (42).
Pharmacogenomics
The field of pharmacogenomics has emerged as a
pivotal component of personalized medicine, providing insight into
the genetic factors that influence the onset and progression of
asthma, as well as responses to specific therapies. By
understanding the genetic makeup of an individual, clinicians can
predict how well a patient may respond to certain asthma
medications, potentially avoiding ineffective treatments and
reducing the likelihood of adverse reactions. For instance, certain
genetic markers can indicate a higher likelihood of success with
specific bronchodilators or corticosteroids, enabling a more
targeted and effective management strategy. This precision in
selecting medications is particularly critical during pregnancy,
where the safety of drugs becomes even more paramount to avoid any
harm to both the mother and the developing fetus (43-45).
Environmental and lifestyle
modifications
Environmental and lifestyle factors play a critical
role in asthma management. Personalized medicine also includes
tailored advice on managing these factors during pregnancy, which
can have a profound impact on asthma control (46).
For example, healthcare providers may recommend
specific strategies to mitigate exposure to allergens that are
known to trigger the asthma symptoms of an individual, such as pet
dander, dust mites or pollen. Guidance on avoiding allergens and
irritants is essential for managing asthma during pregnancy. This
includes practical advice on maintaining a clean, allergen-free
home environment, such as strategies to reduce exposure to pet
dander, dust mites, and mold. In occupational settings, pregnant
women with asthma should receive recommendations on protective
measures to minimize exposure to workplace irritants. These
proactive measures contribute to controlling symptoms, reducing the
risk of exacerbations, and creating a supportive respiratory
environment during pregnancy (47,48).
Smoking cessation is critical for pregnant women
with asthma as it exacerbates asthma symptoms and poses significant
risks to both maternal and fetal health (49). Tailored smoking cessation programs
should be readily available, offering support, counseling and
resources to help pregnant women quit smoking and adopt a
tobacco-free lifestyle. These programs are essential for mitigating
the harmful effects of smoking, promoting healthier pregnancies,
and lessening the respiratory burden on both the mother and the
developing fetus. Healthcare professionals should make it a
priority to provide assistance and access to resources for pregnant
women to quit smoking. Midwives play a crucial role in this effort
by offering guidance, counseling, and resources to support pregnant
women in quitting smoking. They can provide personalized care
plans, education about the risks of smoking during pregnancy, and
ongoing support throughout the quitting process. Additionally,
midwives can collaborate with other healthcare providers to ensure
that pregnant women have access to comprehensive smoking cessation
programs and resources tailored to their specific needs (50-53).
Healthcare professionals play a crucial role in
supporting the well-being of pregnant women, particularly those
suffering from asthma. Encouraging pregnant women with asthma to
receive flu vaccinations is essential for safeguarding their health
and the health of their unborn children (54).
Pregnant women, particularly those with asthma, need
to be vaccinated against the flu for several reasons. Pregnant
women, including those with asthma, are at a higher risk of
experiencing severe flu complications due to changes in their
immune system and respiratory function during pregnancy. Asthma can
further exacerbate these risks, rendering pregnant women more
vulnerable to complications, such as pneumonia and respiratory
distress. Flu vaccination can help protect pregnant women from
contracting the flu, reducing the likelihood of experiencing severe
illness and its associated complications. By avoiding the flu,
pregnant women can maintain their overall health and well-being
during pregnancy. Flu vaccination during pregnancy not only
benefits the mother, but also provides protection to the baby.
Studies have shown that maternal flu vaccination lowers the risk of
flu-related complications in newborns, such as premature birth and
low birth weight. Additionally, the response of the mother to the
flu vaccine can pass on antibodies to the baby, providing some
immunity during the early months of life when the baby is too young
for vaccination. Moreover, vaccinating against the flu can help
pregnant women with asthma prevent flu-related asthma
exacerbations. Respiratory infections, including the flu, can
trigger asthma symptoms and lead to the worsening of asthma
control. By reducing the risk of flu infection, vaccination can
help maintain asthma control and minimize the need for
asthma-related medical interventions during pregnancy (55,56).
Healthcare providers can also personalize dietary
recommendations to improve respiratory health, including
anti-inflammatory foods that aid in managing asthma symptoms.
Advice on physical activity, tailored to the condition of the
patient and stage of pregnancy, can further support respiratory
function and overall well-being (57,58).
Immunological profiling
Immunological profiling is an advanced technique
that involves the detailed analysis of immune markers, such as
cytokine levels and other inflammatory indicators, which can vary
significantly among individuals. This approach allows clinicians to
identify specific inflammatory pathways active in a patient and
tailor treatments accordingly. For pregnant women, particularly
those with severe or difficult-to-control asthma, this can mean a
more precise treatment plan that directly targets the underlying
mechanisms of their asthma. By tailoring therapy to the unique
immunological profile of each individual, it is possible to achieve
better asthma control, reduce the frequency of exacerbations, and
minimize the need for systemic medications that may pose risks
during pregnancy. This targeted approach ensures that management
strategies are both effective and safe, adhering to the principle
of ‘precision medicine’ in a clinical context where both maternal
and fetal health are the priority (59).
4. Implications for maternal and fetal
health
Maternal outcomes
Effective asthma management during pregnancy is
crucial for optimizing maternal health outcomes both during and
after pregnancy. Uncontrolled asthma poses significant risks,
including increased rates of hypertension and preeclampsia,
complications that can lead to further maternal morbidity (60).
Hypertension and preeclampsia
Poorly controlled asthma during pregnancy poses a
significant risk for the development of hypertension and
preeclampsia in affected women. The inflammatory processes inherent
in uncontrolled asthma can potentially lead to vascular
dysfunction, thereby exacerbating the risk of hypertensive
disorders during pregnancy. Chronic inflammation associated with
asthma may disrupt normal vascular homeostasis, leading to
endothelial dysfunction and increased vascular resistance, both of
which are hallmark features of hypertension and preeclampsia.
Furthermore, the systemic inflammatory response characteristic of
uncontrolled asthma can promote oxidative stress and endothelial
injury, further predisposing pregnant women to developing these
complications. In addition, when asthma gets worse,
pro-inflammatory cytokines and mediators are released. These can
cause endothelial activation and dysfunction, which raises the risk
of high blood pressure and preeclampsia (61-63).
A previous meta-analysis revealed that maternal
asthma is associated with a 45% higher risk of developing
pregnancy-induced hypertension (PIH) [relative risk (RR), 1.45; 95%
CI, 1.29-1.63], and the risk of transient hypertension of pregnancy
is doubled (RR, 2.00; 95% CI, 1.52-2.63) (58). Additionally, women with asthma have
a 28% increased risk of developing preeclampsia or eclampsia (RR,
1.28; 95% CI, 1.25-1.32). Specifically, the risk of developing
preeclampsia alone is 43% higher (RR, 1.43; 95% CI, 1.31-1.57) and
for eclampsia, it is also 56% higher (RR, 1.56; 95% CI, 1.13-2.15)
(62).
Cesarean delivery and complicated
labor
Exacerbations of asthma during pregnancy have been
associated with increased risks of cesarean delivery and
complicated labor. The underlying inflammation and physiological
changes caused by asthma can have an impact on labor progression
and outcomes (64). A previous
study found that 27.1% of pregnant women with asthma who
experienced exacerbations underwent cesarean sections, which is
significantly higher than the 18.9% rate observed in asthmatic
women without exacerbations (65).
After excluding cases with direct indications for cesarean
sections, such as fetopelvic disproportion or failed induction, the
rate still remained higher (7.3 vs. 5.3%) in women with asthma
exacerbations (65).
Post-pregnancy recovery
Effective asthma control during pregnancy also
affects post-pregnancy recovery, influencing recovery time and the
risk of developing postpartum complications. Effective asthma
control reduces the risk of developing postpartum hemorrhage and
infections, which are more prevalent among women with systemic
inflammation due to uncontrolled asthma (66,67).
Wang et al (62) observed
that pregnant women with asthma faced a heightened risk of
experiencing deep vein thrombosis (DVT) and pulmonary embolism (PE)
compared to those without asthma. More specifically, according to
their study, the risk of developing venous thromboembolism was
2.60-fold higher (adjusted OR, 2.60; 95% CI, 2.41-2.80), the risk
of developing PE was 3.80-fold higher (adjusted OR, 3.80; 95% CI,
3.41-4.24) and the risk of developing DVT was 2.04-fold higher
(adjusted OR, 2.04; 95% CI, 1.84-2.25) in asthmatic women (66). This observation is corroborated by
findings of the study by Mendola et al (67), which found that women with asthma
had a significantly higher risk of developing PE during pregnancy.
Specifically, the risk of PE was 1.71-fold higher in women with
asthma compared to those without asthma (adjusted OR 1.71, 95% CI,
1.05-2.79) (67).
It is widely recognized that pregnancy inherently
entails an increased risk of hypercoagulability and venous stasis,
both of which contribute to the development of thromboembolic
events (67). Furthermore, asthma
itself has been identified as a prothrombotic condition (68).
Impact of pregnancy on asthma
The study conducted by Schatz et al (69) involving 330 pregnant women revealed
that asthma worsened in 35% of the participants, remained stable in
33% participants and improved in 28% participants (with uncertainty
in 4%) during pregnancy.
Stenius-Aarniala et al (70), in a prospective study of 504
pregnant women with asthma, noted that 47 women experienced acute
attacks, predominantly occurring between the 17th and 24th week of
pregnancy. This was attributed to a reduction or cessation of
medication early in pregnancy, leading to symptom exacerbation a
few weeks later.
In another study, Kim et al (65) compared 3,357 pregnant asthmatic
patients with 50,355 non-pregnant asthmatic patients, finding a
higher rate of asthma-related hospitalizations among pregnant
patients, with increasing proportions throughout pregnancy
trimesters. They observed a prevalence of 5.3% for asthma
exacerbations during pregnancy, with those experiencing acute
exacerbations requiring more intensive asthma-related healthcare
(65).
Furthermore, another prospective cohort study of 146
asthmatic pregnant women noted severe exacerbations in 36% of
cases, with viral respiratory infections and discontinuation of
inhaled corticosteroids being major contributing factors (71). Additionally, psychological changes
occurring during pregnancy, such as heightened emotional
vulnerability and stress, have been identified as potential
triggers for asthma exacerbations (72).
Impact on maternal QoL
The study by Fazel et al (73) included 1,603 pregnant women, of
whom 34 (2.1%) were diagnosed with asthma. Among these, 38% had
well-controlled asthma, while 62% had partly or poorly controlled
asthma. Quality of life (QoL) scores varied significantly depending
on asthma severity and control. Women with moderate to severe
persistent asthma had lower median QoL scores across various
domains, including symptoms (39.3 vs. 65.5), activities (58.4 vs.
70.1) and emotional well-being (45.7 vs. 62.9), compared to those
with intermittent or mild persistent asthma. The overall QoL was
higher in women with well-controlled asthma (median score of 69.6)
compared to those with partly or poorly controlled asthma (median
score of 55.8) (73). The study by
Powell et al (74) focused
on pregnant women with asthma, examining their QoL and related
psychosocial factors. It involved 125 participants with an average
age of 28.3 years and a mean gestational age of 16.4 weeks. The
Asthma Control Questionnaire (ACQ) revealed that 30.4% of women had
controlled asthma, 42.4% had partly controlled asthma and 27.2% had
uncontrolled asthma. Their study found that women generally
reported a good QoL, with a median Asthma Quality of Life
Questionnaire-Marks (AQLQ-M) total score of 0.88 out of 10,
indicating relatively low impairment. Anxiety was measured with the
Six-Item Short-Form State Trait Anxiety Inventory (STAI-6), where
participants had a median score of 26.7, suggesting low anxiety
levels. However, a poorer QoL was significantly associated with
greater levels of anxiety (P<0.0001), more negative illness
perceptions, and the need for maintenance with ICS (P=0.023)
(74).
Fetal outcomes
The impact of asthma on fetal development is
profound, with several studies (as presented below) highlighting
increased risks of adverse outcomes, such as a low birth weight,
preterm birth and perinatal morbidity.
Low birth weight and small for gestational
age. Asthma, particularly when poorly controlled, is associated
with a higher risk of delivering low birth weight and small for
gestational age infants. Active asthma flare-ups during pregnancy
raise the risk of these outcomes by affecting fetal growth. This is
most likely because of less oxygenation and more stress for the
fetus (61,75,76).
It has been reported that pregnant women with asthma have a 46%
increased risk of delivering a low-birth-weight baby (RR, 1.46; 95%
CI, 1.22-1.75) compared to women without asthma. In addition, the
risk of having an infant which is small for its gestational age is
increased by 22% (RR, 1.22; 95% CI, 1.14-1.31) in women with asthma
(75).
Preterm birth. Pregnancies complicated by
asthma significantly increase the likelihood of preterm birth, a
concerning outcome associated with various maternal and neonatal
health risks. It has been reported that the risk of preterm
delivery is 41% higher in pregnant women with asthma compared to
those without asthma (RR, 1.41; 95% CI, 1.23-1.62) (75). The systemic inflammation
characteristic of uncontrolled asthma exacerbations poses a
significant threat, as it can trigger a cascade of events leading
to the premature onset of labor. Moreover, the hypoxic episodes
frequently experienced during asthma exacerbations further compound
this risk, potentially disrupting the delicate balance necessary
for maintaining the pregnancy to full term. These pathological
processes, driven by the inflammatory response and oxygen
deprivation inherent in uncontrolled asthma, may ultimately
culminate in the untimely initiation of labor, heightening the risk
of preterm birth and its associated complications for both mother
and baby (77-80).
Perinatal morbidity. Infants born to mothers
with asthma face an increased risk of perinatal morbidity,
encompassing various health challenges during the immediate newborn
period. One significant concern is the heightened incidence of
respiratory distress syndrome (RDS), a condition characterized by
breathing difficulties shortly after birth due to underdeveloped
lungs or inadequate surfactant production. Infants born to mothers
with asthma may experience a higher prevalence of RDS due to the
potential impact of maternal asthma on fetal lung development and
function. Additionally, neonates born to mothers with asthma may
require admission to the neonatal intensive care unit (NICU) at a
higher rate than infants born to mothers without asthma. Several
factors, including the increased likelihood of preterm birth
associated with maternal asthma and the potential exacerbation of
respiratory symptoms in newborns exposed to maternal asthma
triggers or allergens during pregnancy, contribute to this
heightened need for NICU admission (81,82).
It has been found that infants born to mothers with
asthma have a 49% higher risk of neonatal mortality (RR, 1.49; 95%
CI, 1.11-2.00) and a 50% higher risk of requiring NICU admission
(RR, 1.50; 95% CI, 1.03-2.20) compared to those born to mothers
without asthma. Additionally, the overall risk of perinatal
mortality, which includes both stillbirth and neonatal death, is
elevated by 25% (RR, 1.25; 95% CI, 1.05-1.50) in this population
(82).
Furthermore, maternal asthma exacerbations during
pregnancy can lead to fetal hypoxia, which may further compromise
the newborn's respiratory function and overall health. The
uncontrolled maternal asthma inflammatory milieu may also
contribute to systemic inflammation in the neonate, potentially
exacerbating respiratory complications and necessitating NICU care
(83).
Respiratory conditions in
offspring
Children born to mothers with asthma are more likely
to develop asthma and other atopic conditions themselves. In the
study by Brew et al (84),
it was reported that children born to mothers with asthma had an
increased risk of developing asthma and other atopic conditions.
Specifically, maternal asthma was associated with a 1.5- to 2-fold
higher likelihood of children developing asthma compared to those
whose mothers did not have asthma (84).
The inflammatory processes that connect maternal and
childhood asthma are complicated. They involve the immune system of
the mother not functioning properly during pregnancy and the
development of the immune system of the child after birth. Maternal
asthma is associated with increased levels of pro-inflammatory
cytokines, such as interleukin (IL)-4, IL-5 and IL-13, which can
cross the placenta and influence fetal immune programming.
Additionally, maternal asthma exacerbations during pregnancy may
lead to the release of inflammatory mediators and oxidative stress
markers, further impacting fetal immune development. These prenatal
exposures can prime the fetal immune system towards a Th2-dominant
phenotype, characterized by heightened allergic responses and
susceptibility to asthma development later in childhood. Moreover,
epigenetic modifications, such as DNA methylation and histone
acetylation, may occur in response to maternal asthma and
contribute to altered gene expression patterns associated with
asthma susceptibility in the offspring (84,85).
Neurodevelopmental and behavioral
issues
Previous studies have suggested an association
between maternal asthma and increased risks of neurodevelopmental
disorders in offspring (86,87).
It has been found that maternal asthma exacerbations during
pregnancy are associated with a 50% increased risk of developing
ASD in children (87).
The association between allergy and asthma with
neurodevelopmental disorders in offspring implicates intricate
mechanisms involving immune dysregulation, inflammatory pathways
and potential disruptions in neurodevelopmental processes. Firstly,
maternal immune dysregulation, commonly observed in individuals
with allergies and asthma, can lead to the release of
pro-inflammatory cytokines and chemokines during pregnancy. These
inflammatory mediators may traverse the placental barrier and exert
direct or indirect effects on fetal neurodevelopment, influencing
processes, such as neuronal migration, synaptogenesis and
myelination. Additionally, maternal allergic responses may trigger
the production of maternal antibodies, including immunoglobulin E
(IgE), which could cross the placenta and interact with fetal
neural tissues, potentially perturbing normal neurodevelopmental
trajectories (88).
Moreover, prenatal exposure to maternal allergic and
asthmatic conditions may contribute to oxidative stress and
systemic inflammation in the developing fetus, further exacerbating
neurodevelopmental vulnerabilities. Oxidative stress markers and
inflammatory cytokines released in response to maternal allergic
reactions could disrupt delicate neurodevelopmental processes,
leading to aberrant synaptic connectivity, altered neurotransmitter
signaling, and neuroinflammation in the offspring (86).
Alterations in neural circuitry and immune balance
could increase the susceptibility of offspring to
neurodevelopmental disorders, including ASD, ADHD and cognitive
deficits (89). The intricate
association between maternal conditions, such as allergies and
asthma and the neurodevelopmental disorders in offspring highlights
the necessity of comprehending the fundamental mechanisms. This
understanding is crucial for devising specific interventions to
reduce these detrimental effects.
Obesity and metabolic syndrome
There is emerging evidence to indicate that children
of asthmatic mothers may have a higher risk of developing obesity
and metabolic syndrome.
In a previous case-control study involving children
aged 6 to 7 years, the researchers aimed to identify factors
associated with asthma and obesity in this age group. They
collected data on asthma symptoms, maternal and childhood factors
and anthropometric measurements. Of the 201 evaluated children,
25.4% displayed asthma symptoms and 37.2% met the classification of
being overweight or obese. The group with asthma symptoms and
overweight/obesity had higher waist circumference, triceps
skinfold, and body mass index compared to those without asthma
symptoms. That study found significant associations between asthma
and overweight/obesity symptoms and maternal factors. The study
specifically identified maternal history of asthma and hypertension
during pregnancy as significant risk factors. Children whose
mothers had a history of asthma were ~3.73-fold more likely to
exhibit asthma and overweight/obesity symptoms, while those whose
mothers experienced hypertension during pregnancy had a 3.29-fold
higher likelihood of displaying these symptoms (72).
5. Recommendations and future
directions
Research gaps
Despite considerable advancements being made in the
understanding and management of asthma during pregnancy,
significant gaps remain, particularly concerning long-term health
impacts and treatment safety. Addressing these gaps is crucial for
enhancing patient care and improving outcomes.
Long-term effects on health
There is a need for longitudinal studies that follow
children born to mothers with asthma into adulthood to
comprehensively assess the long-term health impacts. Such studies
are required to focus on the development of chronic diseases,
mental health outcomes and the overall QoL. The complex interplay
between maternal asthma, environmental factors and genetic
predispositions remains poorly understood and warrants further
investigation (90).
Safety and efficacy of newer
medications
As newer medications and biological treatments
become available for asthma management, their safety and efficacy
during pregnancy require rigorous evaluation. Small cohorts or
post-marketing surveillance reports often limit current data.
Large-scale, randomized controlled trials are necessary to
establish clear safety profiles and dosing guidelines for these
drugs during pregnancy (72).
Research is necessary to determine the
impact of varying degrees of asthma severity on pregnancy
outcomes
This includes understanding how different levels of
disease control may influence both maternal and fetal health and
identifying key interventions that could mitigate the risks
associated with severe asthma (91).
Emerging technologies
The integration of emerging technologies into asthma
management during pregnancy could transform current approaches and
enable more personalized and effective care.
Digital health tools
Digital health tools, including mobile health apps
and wearable devices, provide promising avenues for real-time
monitoring and management of asthma in pregnancy. These tools can
facilitate symptom tracking, medication adherence, and the early
detection of exacerbations. The potential for these technologies to
improve outcomes by enhancing patient engagement and enabling more
dynamic treatment adjustments has been already highlighted
(92).
Telehealth
The expansion of telehealth services can provide
pregnant women with better access to specialized care. In rural or
underserved areas with limited healthcare resources, this is
especially crucial. Telehealth platforms can support regular
consultations with healthcare providers, educational initiatives
and pulmonary function testing at home, thereby improving asthma
management and reducing hospital visits (93,94).
Predictive analytics
Leveraging big data and predictive analytics could
lead to more precise predictions of asthma exacerbations and
treatment responses in pregnant women. These technologies can
analyze vast amounts of data from electronic health records,
environmental sensors, and personal devices to identify patterns
and predict risks, potentially guiding preventative measures and
personalized treatment plans (95,96).
Policy and practice
recommendations
To improve asthma management during pregnancy,
specific policy adjustments and changes in clinical practice are
necessary.
Updated clinical guidelines. There is a need
for regularly updated clinical guidelines that incorporate the
latest research findings and emerging therapies. These guidelines
should provide clear recommendations for managing asthma in
different stages of pregnancy and address the use of new
medications and technologies (97).
Training and education programs. Enhancing
training and education programs for healthcare providers is
essential for ensuring they are equipped with the latest knowledge
and skills to manage asthma in pregnancy effectively. This includes
understanding the pharmacokinetics of asthma medications during
pregnancy, applying new technologies, and interpreting data from
digital health tools (98).
Policy initiatives. Governments and health
organizations need to prioritize funding for asthma research and
technology development, focusing on pregnancy-related issues.
Additionally, policies that improve air quality and reduce
environmental triggers can benefit pregnant women with asthma and
contribute to better overall public health outcomes (99,100). The complex interplays between
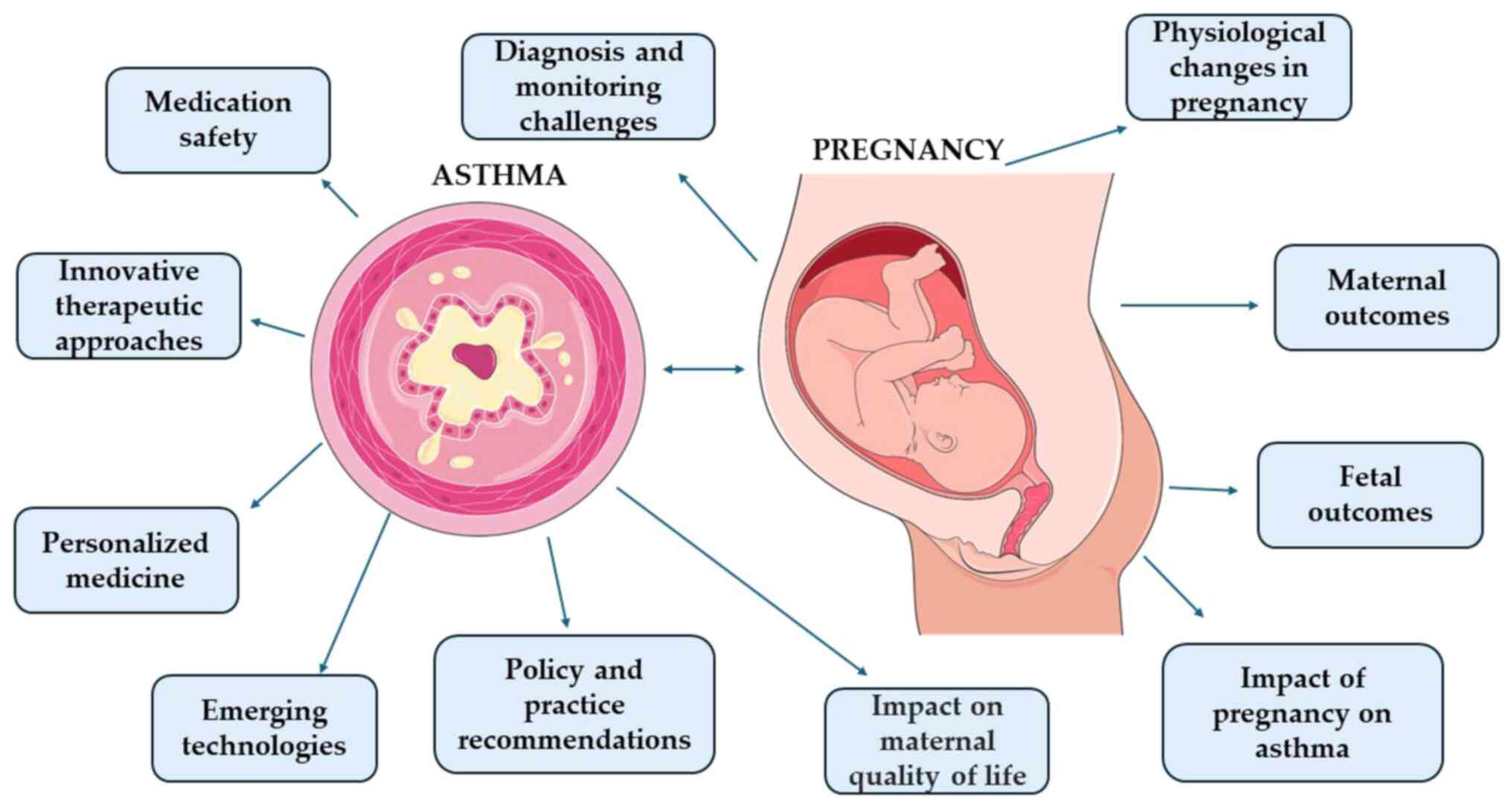
asthma and pregnancy are summarized in Fig. 1.
6. Conclusions
The management of asthma during pregnancy presents
unique challenges that significantly impact both maternal and fetal
health outcomes. Improved pregnancy outcomes are closely associated
with effective asthma control, underscoring the significance of
continuous management and monitoring. Recent advancements in
medication safety have yielded reassuring data on commonly used
asthma treatments during pregnancy, safeguarding both mothers and
fetuses from potential adverse effects. Additionally, innovative
therapeutic approaches, such as digital health tools and
personalized medicine, have shown promising results in improving
asthma management in pregnant patients.
However, despite these advancements, substantial
research gaps remain, particularly concerning the long-term health
implications for children born to mothers with asthma and the
safety and efficacy of newer medications. Addressing these gaps
through rigorous research and integrating findings into clinical
practice is critical for advancing treatment strategies.
Emerging technologies, such as telehealth and
predictive analytics offer exciting opportunities to improve asthma
care during pregnancy, but their integration into routine clinical
practice requires careful consideration and adaptation.
Furthermore, policy adjustments and enhanced training programs for
healthcare professionals are crucial to ensuring the effective
implementation of these new tools and approaches.
In conclusion, ongoing research and adaptation of
clinical practices are vital for meeting the evolving challenges of
managing asthma in pregnant patients effectively. Persistence is
required in efforts to guarantee optimal care for every pregnant
woman with asthma, protecting both the health of the mother and
child.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
AD and VEG conceptualized the present review. AD,
CT, VEG and DAS made substantial contributions to the
interpretation and analysis of data from the literature for
inclusion in the review, and wrote and prepared the draft of the
manuscript. AD and VEG analyzed the data and provided critical
revisions. All authors contributed to manuscript revision, and have
read and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tool Chat
GPT was used to improve the readability and language of the
manuscript, and subsequently, the authors revised and edited the
content produced by the AI tool as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Global Initiative for Asthma (GINA):
Global strategy for asthma management and prevention. GINA,
Fontana, WI, 2020. https://ginasthma.org/2023-gina-main-report/.
|
|
2
|
Centers for Disease Control and Prevention
(CDC): Asthma data, statistics, and surveillance. CDC, Atlanta, GA,
2021. https://www.cdc.gov/asthma/asthmadata.htm#:~:text=The%20current%20asthma%20prevalence%20among,2001%20to%208.0%25%20in%202021.
Accessed May 5, 2024.
|
|
3
|
To T, Stanojevic S, Moores G, Gershon AS,
Bateman ED, Cruz AA and Boulet LP: Global asthma prevalence in
adults: Findings from the cross-sectional world health survey. BMC
Public Health. 12(204)2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Murphy VE, Namazy JA, Powell H, Schatz M,
Chambers C, Attia J and Gibson PG: A meta-analysis of adverse
perinatal outcomes in women with asthma. BJOG. 118:1314–1323.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Popa M, Peltecu G, Gica N, Ciobanu AM,
Botezatu R, Gica C, Steriade A and Panaitescu AM: Asthma in
pregnancy. Review of current literature and recommendations. Mædica
(Bucur). 16:80–87. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Namazy JA, Murphy VE, Powell H, Gibson PG,
Chambers C and Schatz M: Effects of asthma severity, exacerbations
and oral corticosteroids on perinatal outcomes. Eur Respir J.
41:1082–1090. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Schatz M, Dombrowski MP, Wise R, Thom EA,
Landon M, Mabie W, Newman RB, Hauth JC, Lindheimer M, Caritis SN,
et al: Asthma morbidity during pregnancy can be predicted by
severity classification. J Allergy Clin Immunol. 112:283–288.
2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tam A, Morrish D, Wadsworth S, Dorscheid
D, Man SP and Sin DD: The role of female hormones on lung function
in chronic lung diseases. BMC Womens Health. 11(24)2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
To T, Feldman LY, Zhu J and Gershon AS:
Asthma health services utilisation before, during and after
pregnancy: A population-based cohort study. Eur Respir J.
51(1800209)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vyawahare AP, Gaidhane A and Wandile B:
Asthma in pregnancy: A critical review of impact, management, and
outcomes. Cureus. 15(e50094)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Costantine MM: Physiologic and
pharmacokinetic changes in pregnancy. Front Pharmacol.
5(65)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Magon N and Kumar P: Hormones in
pregnancy. Niger Med J. 53:179–183. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
LoMauro A and Aliverti A: Respiratory
physiology of pregnancy: Physiology masterclass. Breathe (Sheff).
11:297–301. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Palmsten K, Bandoli G, Vazquez-Benitez G,
Xi M, Johnson DL, Xu R and Chambers CD: Oral corticosteroid use
during pregnancy and risk of preterm birth. Rheumatology (Oxford).
59:1262–1271. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schatz M, Zeiger RS, Harden K, Hoffman CC,
Chilingar L and Petitti D: The safety of asthma and allergy
medications during pregnancy. J Allergy Clin Immunol. 100:301–306.
1997.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Martel MJ, Rey E, Beauchesne MF, Perreault
S, Lefebvre G, Forget A and Blais L: Use of inhaled corticosteroids
during pregnancy and risk of pregnancy induced hypertension: nested
case-control study. BMJ. 330(230)2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bracken MB, Triche EW, Belanger K, Saftlas
A, Beckett WS and Leaderer BP: Asthma symptoms, severity, and drug
therapy: A prospective study of effects on 2205 pregnancies. Obstet
Gynecol. 102:739–752. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Blais L, Beauchesne MF, Rey E, Malo JL and
Forget A: Use of inhaled corticosteroids during the first trimester
of pregnancy and the risk of congenital malformations among women
with asthma. Thorax. 62:320–328. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bakhireva LN, Jones KL, Schatz M, Johnson
D and Chambers CD: Organization Of Teratology Information Services
Research Group. Asthma medication use in pregnancy and fetal
growth. J Allergy Clin Immunol. 116:503–509. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Otsuka H, Narushima M and Suzuki H:
Assessment of inhaled corticosteroid therapy for asthma treatment
during pregnancy. Allergol Int. 54:381–386. 2005.
|
|
21
|
Rahimi R, Nikfar S and Abdollahi M:
Meta-analysis finds use of inhaled corticosteroids during pregnancy
safe: A systematic meta-analysis review. Hum Exp Toxicol.
25:447–452. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cossette B, Forget A, Beauchesne MF, Rey
É, Lemière C, Larivée P, Battista MC and Blais L: Impact of
maternal use of asthma-controller therapy on perinatal outcomes.
Thorax. 68:724–730. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Eltonsy S, Forget A and Blais L:
Beta2-agonists use during pregnancy and the risk of congenital
malformations. Birth Defects Res A Clin Mol Teratol. 91:937–947.
2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lao TT and Huengsburg M: Labour and
delivery in mothers with asthma. Eur J Obstet Gynecol Reprod Biol.
35:183–190. 1990.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lin S, Munsie JP, Herdt-Losavio ML, Bell
E, Druschel C, Romitti PA and Olney R: National Birth Defects
Prevention Study. Maternal asthma medication use and the risk of
gastroschisis. Am J Epidemiol. 168:73–79. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Clifton VL, Rennie N and Murphy VE: Effect
of inhaled glucocorticoid treatment on placental
11beta-hydroxysteroid dehydrogenase type 2 activity and neonatal
birthweight in pregnancies complicated by asthma. Aust N Z J Obstet
Gynaecol. 46:136–140. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bakhireva LN, Jones KL, Schatz M,
Klonoff-Cohen HS, Johnson D, Slymen DJ and Chambers CD:
Organization of Teratology Information Specialists Collaborative
Research Group. Safety of leukotriene receptor antagonists in
pregnancy. J Allergy Clin Immunol. 119:618–625. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sarkar M, Koren G, Kalra S, Ying A,
Smorlesi C, De Santis M, Diav-Citrin O, Avgil M, Lavigne SV,
Berkovich M and Einarson A: Montelukast use during pregnancy: A
multicentre, prospective, comparative study of infant outcomes. Eur
J Clin Pharmacol. 65:1259–1264. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Koren G, Sarkar M and Einarson A: Safety
of using montelukast during pregnancy. Can Fam Physician.
56:881–882. 2010.PubMed/NCBI
|
|
30
|
Cavero-Carbonell C, Vinkel-Hansen A,
Rabanque-Hernández MJ, Martos C and Garne E: Fetal exposure to
montelukast and congenital anomalies: A population-based study in
Denmark. Birth Defects Res. 109:452–459. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hatakeyama S, Goto M, Yamamoto A, Ogura J,
Watanabe N, Tsutsumi S, Yakuwa N, Yamane R, Nagase S, Takahashi K,
et al: The safety of pranlukast and montelukast during the first
trimester of pregnancy: A prospective, two-centered cohort study in
Japan. Congenit Anom (Kyoto). 62:161–168. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tsai HJ, Wu CH, Chang YH and Yao TC: Use
of leukotriene-receptor antagonists during pregnancy and risk of
neuropsychiatric events in offspring. JAMA Netw Open.
6(e231934)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Namazy JA, Cabana MD, Scheuerle AE, Thorp
JM Jr, Chen H, Carrigan G, Wang Y, Veith J and Andrews EB: The
Xolair pregnancy registry (EXPECT): The safety of omalizumab use
during pregnancy. J Allergy Clin Immunol. 135:407–412.
2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Namazy JA, Blais L, Andrews EB, Scheuerle
AE, Cabana MD, Thorp JM, Umetsu DT, Veith JH, Sun D, Kaufman DG, et
al: Pregnancy outcomes in the omalizumab pregnancy registry and a
disease-matched comparator cohort. J Allergy Clin Immunol.
145:528–536.e1. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gemicioğlu B, Yalçin AD, Havlucu Y,
Karakaya G, Özdemir L, Keren M, Bavbek S, Ediger D, Oğuzülgen İK,
Özşeker ZF and Yorgancıoğlu AA: Country-based report: The safety of
omalizumab treatment in pregnant patients with asthma. Turk J Med
Sci. 51:2516–2523. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Shakuntulla F and Chiarella SE: Safety of
biologics for atopic diseases during pregnancy. J Allergy Clin
Immunol Pract. 10:3149–3155. 2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Murphy VE: Managing asthma in pregnancy.
Breathe (Sheff). 11:258–267. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lim AS, Stewart K, Abramson MJ, Walker SP,
Smith CL and George J: Multidisciplinary approach to management of
maternal asthma (MAMMA): A randomized controlled trial. Chest.
145:1046–1054. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zairina E, Abramson MJ, McDonald CF, Li J,
Dharmasiri T, Stewart K, Walker SP, Paul E and George J: Study
protocol for a randomised controlled trial evaluating the efficacy
of a telehealth program-management of asthma with supportive
telehealth of respiratory function in pregnancy (MASTERY©). BMC
Pulm Med. 15(84)2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Almasi S, Shahbodaghi A and Asadi F:
Efficacy of telemedicine for the management of asthma: A systematic
review. Tanaffos. 21:132–145. 2022.PubMed/NCBI
|
|
41
|
Labor S, Dalbello Tir AM, Plavec D, Juric
I, Roglic M, Pavkov Vukelic J and Labor M: What is safe
enough-asthma in pregnancy-a review of current literature and
recommendations. Asthma Res Pract. 4(11)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Maier M: Personalized medicine-a tradition
in general practice! Eur J Gen Pract. 25:63–64. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lim C and Priefer R: Pharmacogenomics and
pediatric asthmatic medications. J Respir. 2:25–43. 2022.
|
|
44
|
Ferrante G, Fasola S, Malizia V, Licari A,
Cilluffo G, Piacentini G and La Grutta S: Pharmacogenomics: A step
forward precision medicine in childhood asthma. Genes (Basel).
13(599)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
García-Menaya JM, Cordobés-Durán C,
García-Martín E and Agúndez JAG: Pharmacogenetic factors affecting
asthma treatment response. Potential implications for drug therapy.
Front Pharmacol. 10(520)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Stoodley I, Williams L, Thompson C, Scott
H and Wood L: Evidence for lifestyle interventions in asthma.
Breathe (Sheff). 15:e50–e61. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Gautier C and Charpin D: Environmental
triggers and avoidance in the management of asthma. J Asthma
Allergy. 10:47–56. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Nelson HS: Allergen and irritant control:
Importance and implementation. Clin Cornerstone. 1:57–68.
1998.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zacharasiewicz A: Maternal smoking in
pregnancy and its influence on childhood asthma. ERJ Open Res.
2:00042–2016. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Diamanti A, Papadakis S, Schoretsaniti S,
Rovina N, Vivilaki V, Gratziou C and Katsaounou PA: Smoking
cessation in pregnancy: An update for maternity care practitioners.
Tob Induc Dis. 17(57)2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Delakovia T, Sarantaki A, Lykeridou K,
Katsaounou P and Diamanti A: Attitudes and knowledge of midwives
about smoking cessation perinatally. Eur J Midwifery. 7 (Suppl
1)(A130)2023.
|
|
52
|
Georgakopoulou VE and Diamanti A:
Artificial intelligence for smoking cessation in pregnancy. Cureus.
16(e63732)2024.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Vlachou M, Kirkou G, Vivilaki V,
Katsaounou P, Georgakopoulou VE and Diamanti A: Smoking cessation
intervention in puerperium: A systematic review and meta-analysis.
Pneumon. 37(33)2024.
|
|
54
|
Taskou C, Sarantaki A, Beloukas A,
Georgakopoulou VΕ, Daskalakis G, Papalexis P and Lykeridou A:
Knowledge and attitudes of healthcare professionals regarding
perinatal influenza vaccination during the COVID-19 pandemic.
Vaccines (Basel). 11(168)2023.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Marshall H, McMillan M, Andrews RM,
Macartney K and Edwards K: Vaccines in pregnancy: The dual benefit
for pregnant women and infants. Hum Vaccin Immunother. 12:848–856.
2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Murphy VE, Powell H, Wark PAB and Gibson
PG: A prospective study of respiratory viral infection in pregnant
women with and without asthma. Chest. 144:420–427. 2013.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Litonjua AA, Carey VJ, Laranjo N,
Harshfield BJ, McElrath TF, O'Connor GT, Sandel M, Iverson RE Jr,
Lee-Paritz A, Strunk RC, et al: Effect of prenatal supplementation
with vitamin D on asthma or recurrent wheezing in offspring by age
3 years: The VDAART randomized clinical trial. JAMA. 315:362–370.
2016.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Scott HA, Gibson PG, Garg ML, Pretto JJ,
Morgan PJ, Callister R and Wood LG: Dietary restriction and
exercise improve airway inflammation and clinical outcomes in
overweight and obese asthma: A randomized trial. Clin Exp Allergy.
43:36–49. 2013.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Rupani H, Fong WCG, Kyyaly A and
Kurukulaaratchy RJ: Recent insights into the management of
inflammation in asthma. J Inflamm Res. 14:4371–4397.
2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Bonham CA, Patterson KC and Strek ME:
Asthma outcomes and management during pregnancy. Chest.
153:515–527. 2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Abdullah K, Zhu J, Gershon A, Dell S and
To T: Effect of asthma exacerbation during pregnancy in women with
asthma: A population-based cohort study. Eur Respir J.
55(1901335)2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Wang M, He W, Li M, Li F, Jiang L, Wang J,
Wang H, Liu X, Yang K and Qiu J: Maternal asthma and the risk of
hypertensive disorders of pregnancy: A systematic review and
meta-analysis of cohort studies. Hypertens Pregnancy. 39:12–24.
2020.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Mirzakhani H, Carey VJ, McElrath TF, Qiu
W, Hollis BW, O'Connor GT, Zeiger RS, Bacharier L, Litonjua AA and
Weiss ST: Impact of preeclampsia on the relationship between
maternal asthma and offspring asthma: an observation from the
VDAART clinical trial. Am J Respir Crit Care Med. 199:32–42.
2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Rejnö G, Lundholm C, Gong T, Larsson K,
Saltvedt S and Almqvist C: Asthma during pregnancy in a
population-based study-pregnancy complications and adverse
perinatal outcomes. PLoS One. 9(e104755)2014.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Kim S, Kim J, Park SY, Um HY, Kim K, Kim
Y, Park Y, Baek S, Yoon SY, Kwon HS, et al: Effect of pregnancy in
asthma on health care use and perinatal outcomes. J Allergy Clin
Immunol. 136:1215–1223.e1-e6. 2015.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Baghlaf H, Spence AR, Czuzoj-Shulman N and
Abenhaim HA: Pregnancy outcomes among women with asthma. J Matern
Fetal Neonatal Med. 32:1325–1331. 2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Mendola P, Laughon SK, Männistö TI,
Leishear K, Reddy UM, Chen Z and Zhang J: Obstetric complications
among US women with asthma. Am J Obstet Gynecol. 208:127.e1–8.
2013.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Majoor CJ, Kamphuisen PW, Zwinderman AH,
Ten Brinke A, Amelink M, Rijssenbeek-Nouwens L, Sterk PJ, Büller HR
and Bel EH: Risk of deep vein thrombosis and pulmonary embolism in
asthma. Eur Respir J. 42:655–661. 2013.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Schatz M, Harden K, Forsythe A, Chilingar
L, Hoffman C, Sperling W and Zeiger RS: The course of asthma during
pregnancy, post partum, and with successive pregnancies: A
prospective analysis. J Allergy Clin Immunol. 81:509–517.
1988.PubMed/NCBI
|
|
70
|
Stenius-Aarniala BS, Hedman J and Teramo
KA: Acute asthma during pregnancy. Thorax. 51:411–414.
1996.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Han VX, Patel S, Jones HF, Nielsen TC,
Mohammad SS, Hofer MJ, Gold W, Brilot F, Lain SJ, Nassar N and Dale
RC: Maternal acute and chronic inflammation in pregnancy is
associated with common neurodevelopmental disorders: A systematic
review. Transl Psychiatry. 11(71)2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Silva BBD, Silva JD, Traebert JL and
Schlindwein AD: Maternal and early childhood factors associated
with asthma and obesity in children aged 6 to 7 years: A case
control study. Einstein (Sao Paulo). 20(eAO5609)2022.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Fazel N, Kundi M, Jensen-Jarolim E,
Pali-Schöll IM, Kazemzadeh A, Esmaily H, Abdizadeh MF, Akbarzadeh
R, Ahmadi R and Jabbari H: Quality of life and asthma control in
pregnant women with asthma. BMC Pulm Med. 21(415)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Powell H, McCaffery K, Murphy VE, Hensley
MJ, Clifton VL, Giles W and Gibson PG: Psychosocial outcomes are
related to asthma control and quality of life in pregnant women
with asthma. J Asthma. 48:1032–1040. 2011.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Giles W and Murphy V: Asthma in pregnancy:
A review. Obstet Med. 6:58–63. 2013.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Goldie MH and Brightling CE: Asthma in
pregnancy. Obstetrician Gynaecologist. 15:241–245. 2013.
|
|
77
|
Blais L, Kettani FZ, Forget A, Beauchesne
MF and Lemière C: Asthma exacerbations during the first trimester
of pregnancy and congenital malformations: Revisiting the
association in a large representative cohort. Thorax. 70:647–652.
2015.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Vieira AC, Pité H and Morais-Almeida M:
Asthma and pregnancy in the 2020 decade: Still a matter of concern.
J Matern Fetal Neonatal Med. 35:6498–6504. 2022.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Hanania NA and Belfort MA: Acute asthma in
pregnancy. Crit Care Med. 33 (10 Suppl):S319–S324. 2005.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Lao TT and Annie Hui SY: The obstetric
aspects of maternal asthma. Best Pract Res Clin Obstet Gynaecol. 85
(Pt A):57–69. 2022.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Mendola P, Männistö TI, Leishear K, Reddy
UM, Chen Z and Laughon SK: Neonatal health of infants born to
mothers with asthma. J Allergy Clin Immunol. 133:85–90.e1.
2014.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Murphy VE, Wang G, Namazy JA, Powell H,
Gibson PG, Chambers C and Schatz M: The risk of congenital
malformations, perinatal mortality, and neonatal hospitalisation
among pregnant women with asthma: A systematic review and
meta-analysis. BJOG. 120:812–822. 2013.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Källén B, Rydhstroem H and Aberg A: Asthma
during pregnancy-a population based study. Eur J Epidemiol.
16:167–171. 2000.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Brew BK, Allen CW, Toelle BG and Marks GB:
Systematic review and meta-analysis investigating breast feeding
and childhood wheezing illness. Paediatr Perinat Epidemiol.
25:507–518. 2011.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Lebold KM, Jacoby DB and Drake MG:
Inflammatory mechanisms linking maternal and childhood asthma. J
Leukoc Biol. 108:113–121. 2020.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Tamayo JM, Rose D, Church JS, Schwartzer
JJ and Ashwood P: Maternal allergic asthma induces prenatal
neuroinflammation. Brain Sci. 12(1041)2022.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Murphy VE, Gibson P, Talbot PI and Clifton
VL: Severe asthma exacerbations during pregnancy. Obstet Gynecol.
106 (5 Pt 1):1046–1054. 2005.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Burgess L, McCaffery K, Powell H, Murphy
VE, Gibson PG and Turner RM: The influence of asthma control on
psychosocial outcomes for pregnant women with asthma. J Asthma.
10:1013–1019. 2015.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Cookson H, Granell R, Joinson C,
Ben-Shlomo Y and Henderson AJ: Mothers' anxiety during pregnancy is
associated with asthma in their children. J Allergy Clin Immunol.
123:847–853.e11. 2009.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Fletcher JM, Green JC and Neidell MJ: Long
term effects of childhood asthma on adult health. J Health Econ.
29:377–387. 2010.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Wang H, Li N and Huang H: Asthma in
pregnancy: Pathophysiology, diagnosis, whole-course management, and
medication safety. Can Respir J. 2020(9046842)2020.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Tinschert P, Jakob R, Barata F, Kramer JN
and Kowatsch T: The potential of mobile apps for improving asthma
self-management: A review of publicly available and well-adopted
asthma apps. JMIR Mhealth Uhealth. 5(e113)2017.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Hawkins SS: Telehealth in the prenatal and
postpartum periods. J Obstet Gynecol Neonatal Nurs. 52:264–275.
2023.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Morrison D, Wyke S, Agur K, Cameron EJ,
Docking RI, Mackenzie AM, McConnachie A, Raghuvir V, Thomson NC and
Mair FS: Digital asthma self-management interventions: A systematic
review. J Med Internet Res. 16(e51)2014.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Molfino NA, Turcatel G and Riskin D:
Machine learning approaches to predict asthma exacerbations: A
narrative review. Adv Ther. 41:534–552. 2024.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Xiong S, Chen W, Jia X, Jia Y and Liu C:
Machine learning for prediction of asthma exacerbations among
asthmatic patients: A systematic review and meta-analysis. BMC Pulm
Med. 23(278)2023.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Expert Panel Working Group of the National
Heart, Lung and Blood Institute (NHLBI) administered and
coordinated National Asthma Education and Prevention Program
Coordinating Committee (NAEPPCC). Cloutier MM, Baptist AP, Blake
KV, Brooks EG, Bryant-Stephens T, DiMango E, Dixon AE, Elward KS,
Hartert T, et al: 2020 focused updates to the asthma management
guidelines: A report from the National Asthma Education and
Prevention Program Coordinating Committee Expert Panel Working
Group. J Allergy Clin Immunol. 146:1217–1270. 2020.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Yadav P, Jaiswal A, Patel A, Reddy LS and
Sindhu AA: A comprehensive review on asthma challenges in
pregnancy: Exploring first trimester exacerbations and the spectrum
of congenital anomalies. Cureus. 15(e49849)2023.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Tiotiu AI, Novakova P, Nedeva D,
Chong-Neto HJ, Novakova S, Steiropoulos P and Kowal K: Impact of
air pollution on asthma outcomes. Int J Environ Res Public Health.
17(6212)2020.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Kelly FJ, Mudway IS and Fussell JC: Air
pollution and asthma: Critical targets for effective action. Pulm
Ther. 7:9–24. 2021.PubMed/NCBI View Article : Google Scholar
|