Introduction
Thyroid cancer is the most common malignancy of the
endocrine system worldwide, and its global incidence has been
steadily rising in recent decades (1). Papillary thyroid carcinoma (PTC), the
most frequent subtype of thyroid cancer, accounts for 80-85% of
thyroid cancers in the population (2). The majority of patients with PTC
respond well to existing therapeutic strategies, with a 10-year
survival of >90% (3).
Nevertheless, recurrence and distant metastasis have always been
two major causes of death in PTC (4). Thus, there is an urgent need to
thoroughly understand PTC pathogenesis and develop effective
therapeutic strategies against the malignant progression of
PTC.
Formononetin (FMNT; PubChem CID: 5280378), an
isoflavonoid isolated from Astragalus membranaceus and
Spatholobus suberectus, possesses numerous pharmacological
effects, such as anti-inflammatory, anti-oxidant and anticancer
properties (5). Previous studies
have reported that FMNT can act as a novel anti-tumorigenic agent
to induce cell growth inhibition and cell cycle arrest, promote
cell apoptosis, inhibit metastasis and reduce angiogenesis in a
panel of solid tumors including breast (6-8),
colorectal (9), gastric (10) and lung cancer (11). A comprehensive grasp of cellular
processes and molecular signaling pathways involved in
FMNT-medicated antitumor activities is of great urgency.
Polycomb group (PcG) complexes, have been reported
to act as epigenetic regulatory complexes and to be dysregulated in
certain cancers, such as breast cancer, prostate cancer, and
hepatocellular carcinoma, and to participate in tumorigenesis and
tumor progression (12). The
chromobox (CBX) family proteins, canonical components of PcG
complexes, can regulate tumorigenesis and tumor progression by
inhibiting the cell differentiation and self-renewal of cancer stem
cells (13). CBX4, also known as
polycomb 2, is both a SUMO E3 ligase and a transcriptional
regulator involved in cell cycle regulation and DNA damage repair
(14,15). Previous studies have described the
tumor-promoting effect of CBX4 in malignant tumors, such3 as
hepatocellular carcinoma (16),
lung cancer (17), gastric cancer
(18), clear cell renal cell
carcinoma (19) and breast cancer
(20). However, the prognostic
value and biological function of CBX4 in the malignant progression
of PTC remain unclear.
In the present study, the proliferation, clone
formation, migration, invasion, EMT, angiogenesis and stemness of
PTC cells were evaluated, so as to demonstrate the anti-tumorigenic
effect of FMNT and the tumor-promoting effect of CBX4 in the
malignant progression of PTC. Moreover, the study attempted to
expound the association between FMNT and CBX4 and to identify the
potential molecular mechanism.
Materials and methods
Cell culture
The human PTC TPC-1 and normal human thyroid
Nthy-ori3-1 cell lines were purchased from Cobioer Co., Ltd. TPC-1
cells were maintained in Dulbecco's modified Eagle's medium (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin solution (MilliporeSigma) at 37˚C in a
humidified environment with 5% CO2. Nthy-ori3-1 cells
were maintained in Roswell Park Memorial Institute-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
and 1% penicillin-streptomycin solution at 37˚C in a humidified
environment with 5% CO2.
Cell treatment
Nthy-ori3-1 cells and TPC-1 cells were exposed to 0,
10, 30 or, 100 µM FMNT for 24 h at 37˚C.
Cell transfection
CBX4 overexpression plasmid (oe-CBX4) was
established by inserting the CBX4 gene into a pcDNA3.1 vector
(Shanghai GenePharma Co., Ltd.), whereas an empty vector served as
the negative control (oe-NC). Cells were transfected, when they
reached ~85% confluence with 5 µg/ml oe-CBX4 and oe-NC using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37˚C for 48 h strictly according to the
manufacturer's guidelines. Cells were collected 48 h post
transfection for use in subsequent experiments.
Bioinformatics analysis
CBX4 expression in thyroid cancer tissues was
analyzed using the Encyclopedia of RNA Interactomes (ENCORI;
http://rna.sysu.edu.cn/encori/mirTarPathways.php)
database.
Molecular docking
The 3-D structure of CBX4 (PDB ID: 5EPL) was
obtained from the protein data bank (PDB) database (https://www.rcsb.org/). Molecular docking was
conducted using AutoDockTools 4.2 software (https://autodock.scripps.edu/) and the 3D diagrams of
molecular docking models were visualized using PyMol software
(version 3.0; https://pymol.org/).
Cell Counting Kit-8 (CCK-8) assay
The viability of TPC-1 or Nthy-ori3-1 cells was
determined using the CCK-8 assay. The cells (5x103
cells/well) were inoculated into 96-well plates for 24 h of
incubation and then exposed to 0, 10, 30 or, 100 µM FMNT for 24 h.
10 µl CCK-8 reagent (Beyotime Institute of Biotechnology) was added
into each well for a further 4 h incubation at 37˚C. The microplate
reader (Bio-Rad Laboratories, Inc.) recorded the optical density at
450 nm.
5-ethynyl-2'-deoxyuridine (EdU)
staining
The proliferation of TPC-1 cells was determined
using the BeyoClick™ EdU Cell Proliferation Kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. The cells were incubated with EdU reagent
for 2 h at 37˚C, fixed in 4% paraformaldehyde for 15 min,
permeabilized in 0.3% Triton X-100 for 10 min and subsequently
incubated with the BeyoClick reaction solution in darkness for 30
min. Representative images of EdU-positive cells were captured
using a fluorescence microscope (magnification, x200).
Colony formation assay
The clone-forming ability of TPC-1 cells was
determined by employing colony formation assays. The cells were
digested with 0.25% trypsin, resuspended in complete medium and
inoculated (500 cells/well) into 6-well plates for further culture,
and the medium was changed every two days. The cells were incubated
for 14 days at 37˚C, fixed with 4% paraformaldehyde at room
temperature for 30 min and stained with 0.1% crystal violet at room
temperature for 10 min. Images of visible colonies (≥50 cells) were
captured using a digital camera (magnification, x1).
Spheroid formation assay
TPC-1 cells (5x103/well) seeded in 6-well
ultra-low attachment plates were maintained in serum-free medium
supplemented with 20 ng/ml epidermal growth factor (EGF), 20 ng/ml
basic fibroblast growth factor, 20 µl/ml B27 supplement and 1%
penicillin-streptomycin in a humidified atmosphere of 5%
CO2 at 37˚C. After incubation for 7-10 days, tumor
spheroids (diameter >100 µm) were counted and image using a
light microscope (magnification, x200).
Wound healing assay
The migratory capability of TPC-1 cells was assessed
using a wound healing assay. The cells (1x106
cells/well) seeded in 6-well plates were cultured to ~95%
confluence. A sterile 200-µl pipette tip was used to vertically
scratch the cells to create the ‘wound’ and the detached cells were
removed by washing twice with PBS. The cells were then supplemented
with fresh serum-free medium for incubation for 24 h at 37˚C.
Images of the wound area at 0 and 24 h after wounding were captured
using a light microscope (magnification, x100). The migration rate
was calculated according to the width of the wounds measured using
Image J software (version 1.52; National Institutes of Health).
Transwell assay
The invasive capability of TPC-1 cells was
determined by employing Transwell assay. The cells were collected,
resuspended in fresh serum-free medium and placed (2x104
cells/well) in the upper layer of Transwell chamber precoated with
Matrigel at 37˚C for 30 min. The lower layer of Transwell chamber
was supplemented with the complete medium as a chemoattractant.
After 24 h of incubation, invasive cells in the lower chamber were
fixed with 4% paraformaldehyde at room temperature for 30 min,
stained with 0.1% crystal violet at room temperature for 10 min,
images were captured using a light microscope and the number of
cells was quantified using Image J software (version 1.52; National
Institutes of Health) (magnification, x100).
Tube formation assay
The conditioned media (CM) of TPC-1 cells was
collected post 24-h incubation. Human umbilical vein endothelial
cells (HUVECs) (2x104 cells/well; iCell-h110; Cellverse
Bioscience Technology Co., Ltd.) seeded in 96-well plates precoated
with Matrigel at 37˚C for 30 min were cultured in CM for 24 h and
images of tube formation were captured using a light microscope
(magnification, x200).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from TPC-1 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) was reversed transcribed into complementary DNA (cDNA) using
a cDNA Synthesis kit (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Afterwards, qPCR was
performed using SYBR Premix Ex Taq reagents (Takara Bio, Inc.) on
an ABI 7500 quantitative PCR instrument (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The thermocycling conditions used were as
follows: 95˚C for 10 min, followed by 40 cycles of 95˚C for 15 sec
and 60˚C for 1 min. The following primers were used to amplify the
target genes: CBX4 forward (F), 5'-CTGGTGAAATGGAGAGGC-3' and
reverse (R), 5'-GAACGACGGGCAAAGGTAGG-3'; VEGF F,
5'-ATCTTCAAGCCATCCTGTGTGC-3' and R, 5'-CAAGGCCCACAGGGATTTTC-3';
VEGFR2 F, 5'-GGAACCTCACTATCCGCAGAGT-3' and R,
5'-CCAAGTTCGTCTTTTCCTGGGC-3'; and GAPDH F,
5'-GCACCGTCAAGGCTGAGAAC-3' and R: 5'-ATGGTGGTGAAGACGCCAGT-3'. GAPDH
served as the endogenous control and the expression levels of CBX4,
VEGF and VEGFR2 were determined using the 2-ΔΔCq method
(21).
Western blot assay
Total protein extracted from TPC-1 cells with the
application of RIPA lysis buffer (Beyotime Institute of
Biotechnology) was centrifuged at 12,000 x g for 15 min at 4˚C and
protein concentration was determined using the BCA method. A total
of 30 µg/lane of protein samples were separated by 10% SDS-PAGE and
then transferred onto PVDF membranes. After blocking in 5% non-fat
milk for 1 h at 37˚C, membranes were probed with primary antibodies
[anti-CBX4 (1:1,000; cat. no. ab4189; Abcam), anti-NANOG (1:1,000;
cat. no. ab109250; Abcam), anti-OCT4 (1:1,000; cat. no. ab19857;
Abcam), anti-CD133 (1:2,000; cat. no. ab222782; Abcam), anti-MMP2
(1:1,000; cat. no. ab92536; Abcam), anti-MMP9 (1:1,000; cat. no.
ab76003; Abcam), anti-E-cadherin (1:1,000; cat. no. ab40772;
Abcam), anti-N-cadherin (1:5,000; cat. no. ab76011; Abcam) and
anti-Vimentin (1:1,000; cat. no. ab92547; Abcam)] overnight at 4˚C
and subsequently incubated with HRP-conjugated secondary antibodies
(1:20,000, cat. no. ab6721; Abcam) for 1 h at 37˚C. Protein signals
were developed using an electrochemiluminescence (ECL) kit
(Beyotime Institute of Biotechnology) and protein band intensities
were analyzed using Image J software (version 1.52; National
Institutes of Health).
Statistical analysis
Data from three independent repeats were expressed
as the mean ± SD. One-way analysis of variance followed by Tukey's
post hoc test was employed for analyses of multiple groups and
unpaired Student's t-test was employed for analyses of two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
FMNT treatment suppresses the
proliferative and clone-forming abilities of PTC cells
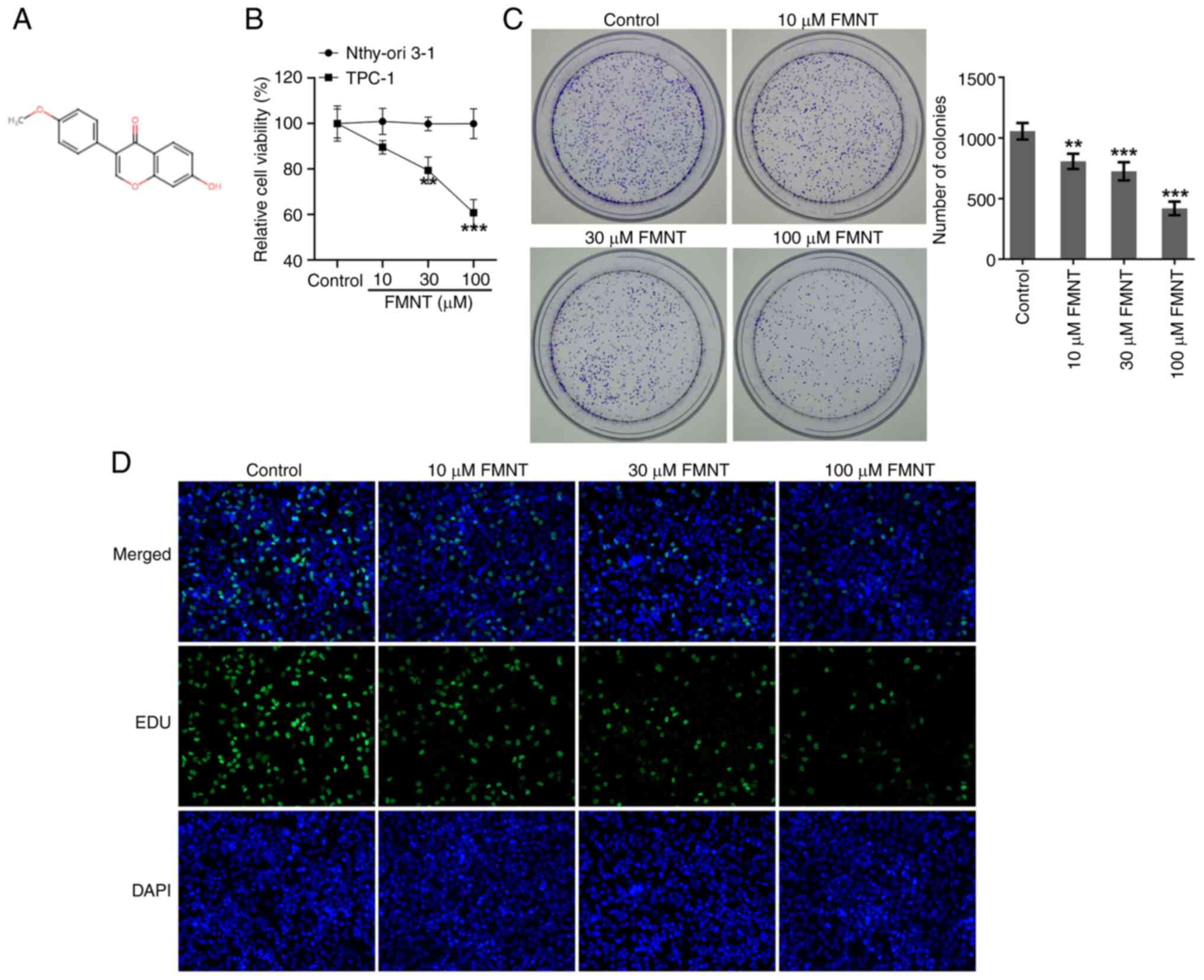
FMNT is an isoflavonoid isolated from Astragalus
membranaceus and Spatholobus suberectus (Fig. 1A). Human TPC-1 and normal human
thyroid Nthy-ori3-1 cell lines were treated with 0, 10, 30 or 100
µM FMNT, for 24 h. The CCK-8 assay revealed that FMNT treatment had
no apparent influence on the viability of Nthy-ori3-1 cells while
it significantly reduced the viability of TPC-1 cells in a
concentration-dependent manner (Fig.
1B). The colony formation assay revealed that FMNT treatment
inhibited the clone-forming ability of TPC-1 cells in a
concentration-dependent manner (Fig.
1C). EdU staining demonstrated that FMNT treatment
dose-dependently decreased the proportion of EdU-positive cells,
which indicated that FMNT treatment repressed the proliferation of
TPC-1 cells in a dose-dependent manner (Fig. 1D).
FMNT treatment represses the
migration, invasion, EMT and angiogenesis of PTC cells
As demonstrated by the wound healing and Transwell
assays, FMNT treatment suppressed the migratory and invasive
capacities of TPC-1 cells in a dose-dependent manner (Fig. 2A and B). MMPs are able to degrade the
extracellular matrix (ECM), facilitating the metastasis of
malignant tumor (22). It was
observed that FMNT treatment also dose-dependently decreased the
expression levels of MMP2 and MMP9 (Fig. 2C). Tumors derive metastatic ability
through EMT (23). The levels of
EMT-associated biomarkers (E-cadherin, N-cadherin and Vimentin)
were detected. FMNT treatment dose-dependently elevated E-cadherin
expression and reduced N-cadherin and Vimentin expression levels,
suppressing EMT of TPC-1 cells (Fig.
2D). Angiogenesis is important for nutritional provision in
tumor growth and metastasis (24).
Tube formation and angiogenesis-associated biomarkers (VEGF and
VEGFR2) were detected to evaluate the angiogenesis ability of
HUVECs. FMNT treatment dose-dependently inhibited tube formation
and decreased VEGF and VEGFR2 levels, indicating that FMNT
treatment suppressed angiogenesis in a dose-dependent manner
(Fig. 2E-G). In summary, FMNT
treatment could suppress PTC metastasis.
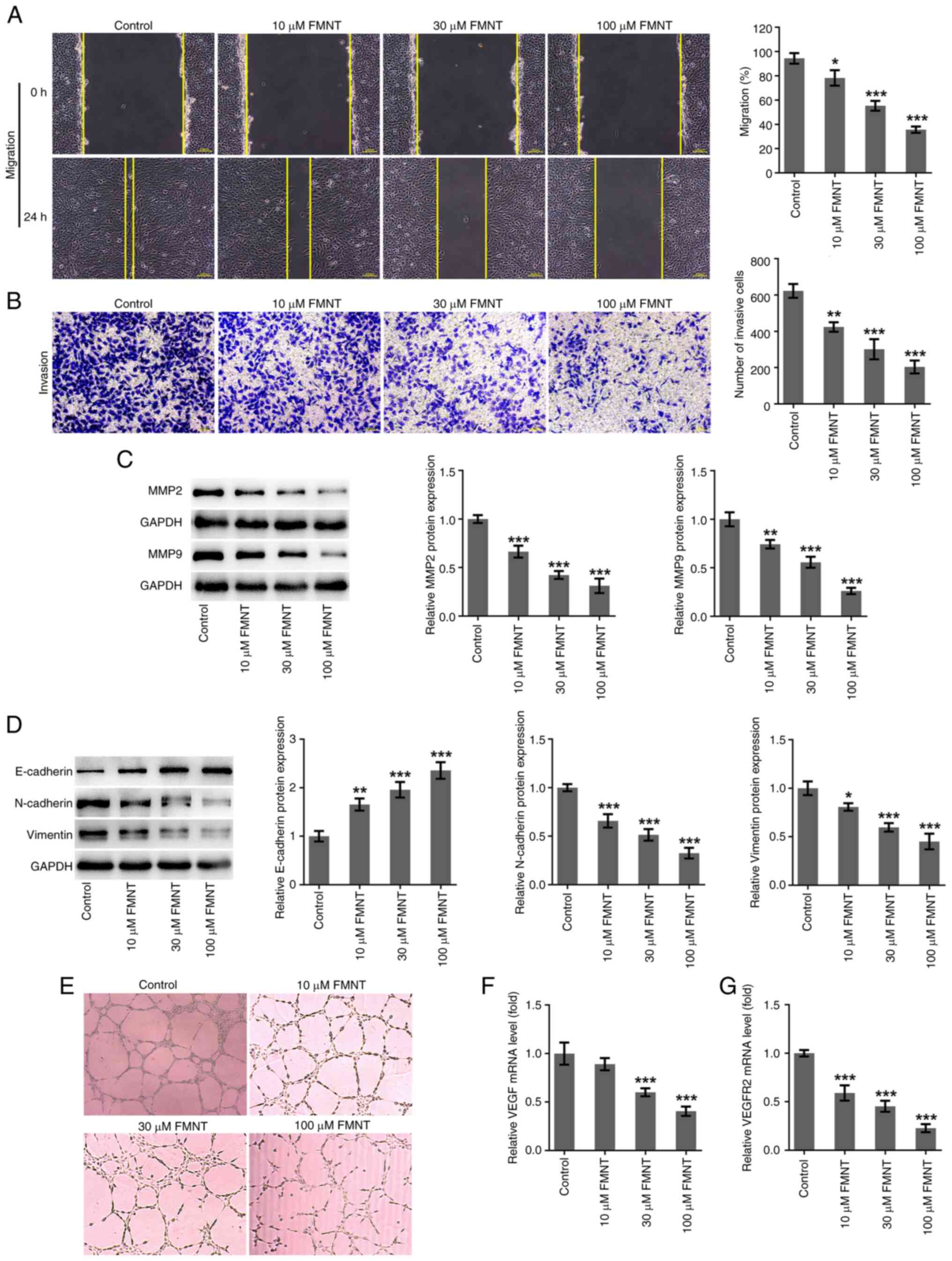 | Figure 2FMNT treatment represses the
migration, invasion, EMT and angiogenesis of papillary thyroid
carcinoma cells. TPC-1 cells were treated with 0, 10, 30 and 100 µM
FMNT for 24 h. (A) The migratory ability of TPC-1 cells was
evaluated by wound healing assay (magnification, x100). (B) The
invasive ability of TPC-1 cells was evaluated by Transwell assay
(magnification, x100). (C) Expression levels of MMP2 and MMP9 in
TPC-1 cells were determined by western blot analysis. (D)
Expression levels of E-cadherin, N-cadherin and Vimentin in TPC-1
cells were determined by western blotting. (E) HUVECs were
incubated with the conditioned media of TPC-1 cells at 37˚C for 24
h. In vitro angiogenesis of HUVECs was evaluated using a
tube formation assay (magnification, x200). (F) VEGF and (G) VEGFR2
levels in TPC-1 cells were determined by reverse
transcription-quantitative PCR. *P<0.05,
**P<0.01 and ***P<0.001 vs. the Control
group. FMNT, formononetin; HUVECs, human umbilical vein endothelial
cells. |
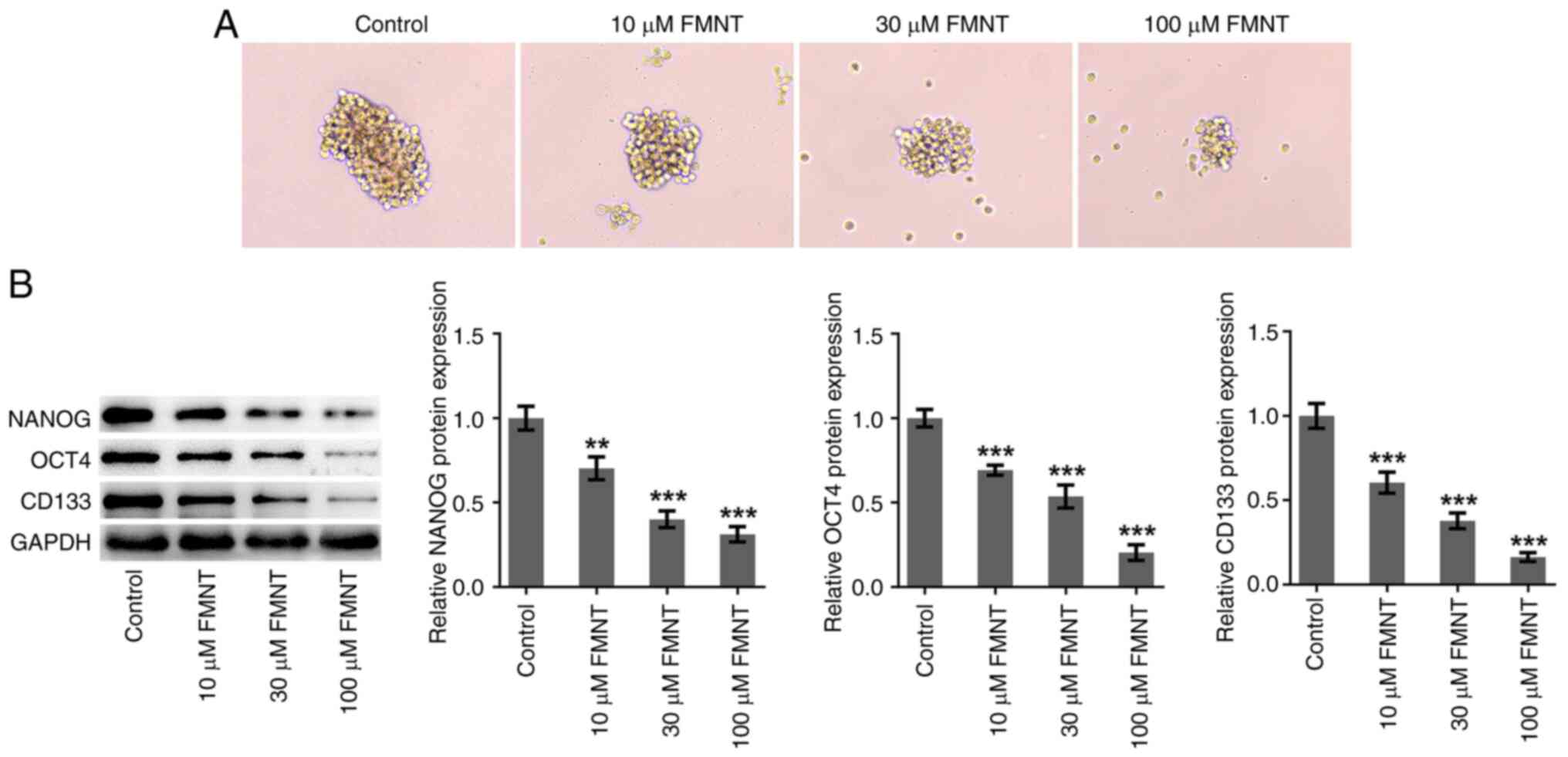
FMNT treatment inhibits stemness
characteristics of PTC cells
Sphere formation and stemness-associated biomarkers
(NANOG, OCT4 and CD133) were detected to evaluate PTC cell
stemness. FMNT treatment dose-dependently inhibited sphere
formation and reduced the expression levels of NANOG, OCT4 and
CD133, indicating that FMNT treatment repressed the stemness of
TPC-1 cells in a dose-dependent manner (Fig. 3).
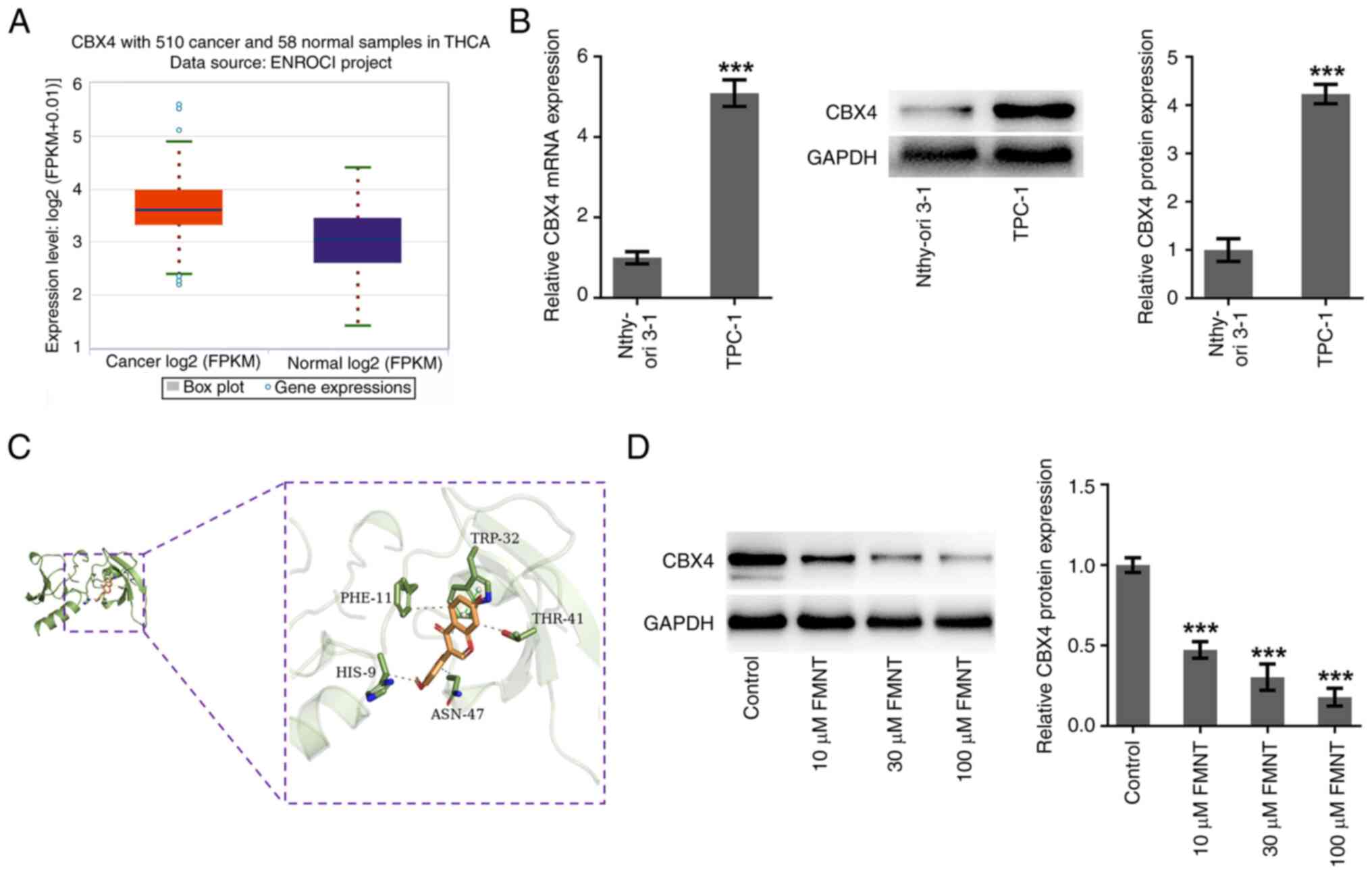
FMNT targets and downregulates CBX4
expression
Expression of CBX4 in thyroid cancer tissues was
explored using the ENCORI database (http://rna.sysu.edu.cn/encori/mirTarPathways.php).
ENCORI data indicated that CBX4 expression was upregulated in
thyroid cancer tissues in comparison with that in normal tissues
(Fig. 4A). Meanwhile, differences
in the expression levels of CBX4 in TPC-1 cells and Nthy-ori3-1
cells were assessed using RT-qPCR and western blot analysis. In
comparison with those in Nthy-ori3-1 cells, CBX4 mRNA and protein
levels were significantly upregulated in TPC-1 cells (Fig. 4B). Furthermore, molecular docking
was performed to explore the compound-protein binding potential
between FMNT and CBX4 and the result demonstrated that FMNT could
interact with CBX4 at the sites of TRP:32, PHE:11, HIS:9, ASN:47
and THR:41 (Fig. 4C). Which
indicated that CBX4 served as a downstream target of FMNT.
Moreover, it was also demonstrated that FMNT treatment
downregulated CBX4 expression in TPC-1 cells in a dose-dependent
manner (Fig. 4D).
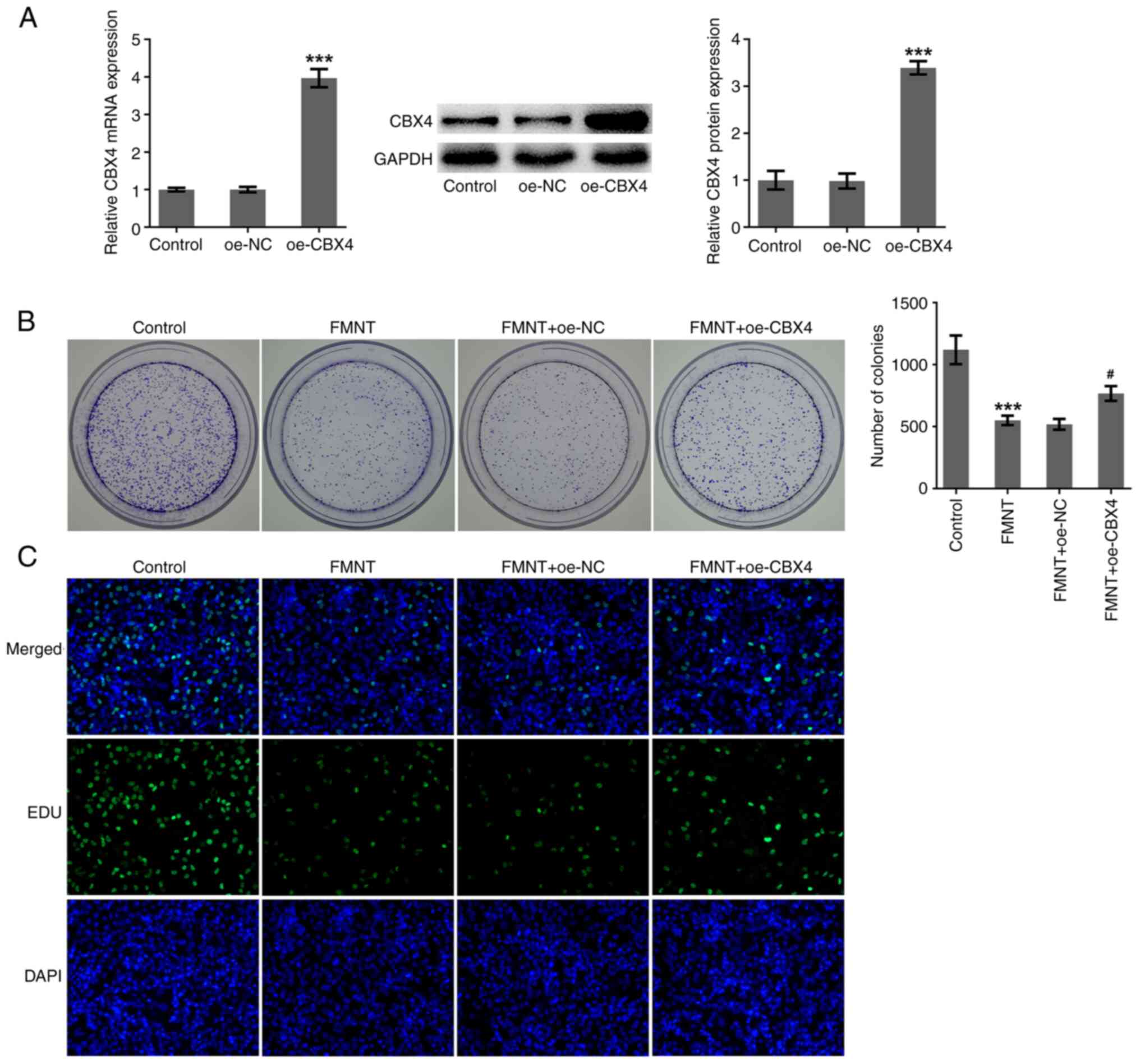
FMNT treatment suppresses the
proliferative and clone-forming abilities of PTC cells by
downregulating CBX4 expression
Whether CBX4 was involved in the anticarcinogenic
effects of FMNT against PTC was further investigated. oe-CBX4 was
introduced into TPC-1 cells to upregulate CBX4 expression and
transfection efficacy was checked using RT-qPCR and western blot
analysis (Fig. 5A). FMNT treatment
significantly inhibited the clone-forming ability of TPC-1 cells,
which was partially reversed by CBX4 overexpression (Fig. 5B). Upregulation of CBX4 increased
the proportion of EdU-positive cells, indicating that the
suppressive effect of FMNT on the proliferation of TPC-1 cells was
abolished by CBX4 overexpression (Fig.
5C). In conclusion, FMNT treatment may suppress the
proliferative and clone-forming abilities of PTC cells depending on
downregulation of CBX4.
FMNT treatment represses the
migration, invasion, EMT and angiogenesis of PTC cells by
downregulating CBX4 expression
FMNT treatment significantly suppressed the
migration and invasion of TPC-1 cells, which was partially reversed
by CBX4 overexpression (Fig. 6A
and B). Moreover, upregulation of
CBX4 elevated the protein expression levels of MMP2 and MMP9,
indicating that the suppressive effect of FMNT on the ECM-degrading
function of MMPs was abolished by CBX4 overexpression (Fig. 6C). Upregulation of CBX4
significantly reduced E-cadherin expression and elevated N-cadherin
and Vimentin expression levels, indicating that the suppressive
effect of FMNT on EMT was abolished by CBX4 overexpression
(Fig. 6D). In addition, FMNT
treatment inhibited tube formation and significantly decreased VEGF
and VEGFR2 mRNA expression levels, which was partially reversed by
CBX4 overexpression (Fig. 6E-G).
Upregulation of CBX4 abolished the suppressive effect of FMNT on
angiogenesis. In summary, FMNT treatment may repress PTC metastasis
depending on downregulation of CBX4.
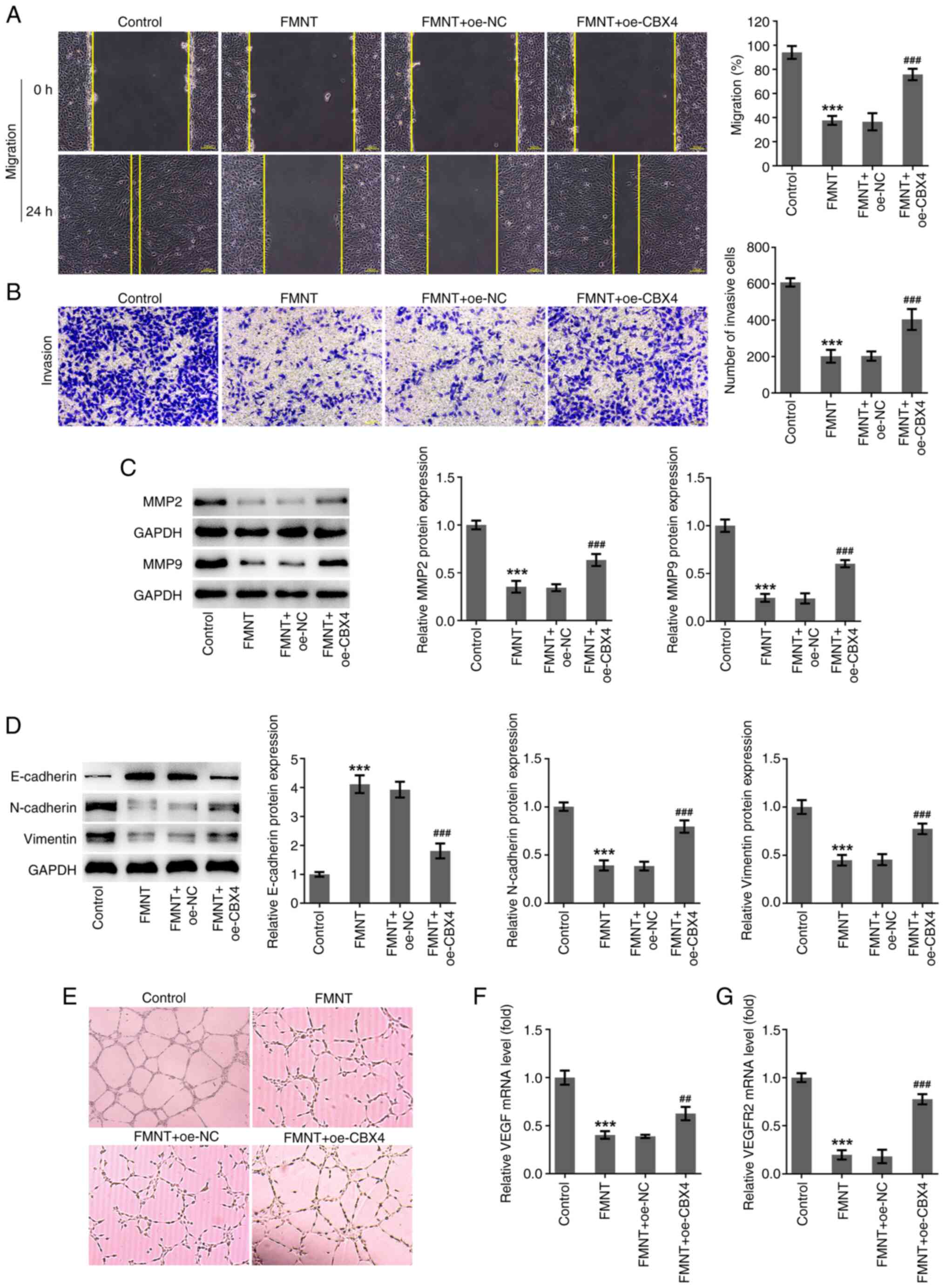 | Figure 6FMNT treatment represses the
migration, invasion, EMT and angiogenesis of papillary thyroid
carcinoma cells by downregulating CBX4 expression. FMNT-treated
TPC-1 cells were transfected with oe-CBX4 or oe-NC. (A) The
migratory ability of TPC-1 cells was evaluated by wound healing
assay (magnification, x100). (B) The invasive ability of TPC-1
cells was evaluated using a Transwell assay (magnification, x100).
(C) Expression levels of MMP2 and MMP9 in TPC-1 cells were
determined by western blotting. (D) Expression levels of
E-cadherin, N-cadherin and Vimentin in TPC-1 cells were determined
by western blot analysis. (E) HUVECs were incubated with the CM of
TPC-1 cells at 37˚C for 24 h. In vitro angiogenesis of
HUVECs was evaluated by tube formation assay (magnification, x200).
(F and G) VEGF and VEGFR2 levels in TPC-1 cells were determined by
reverse transcription-quantitative PCR. ***P<0.001
vs. the Control group; ##P<0.01 and
###P<0.001 vs. FMNT + oe-NC. FMNT, formononetin;
CBX4, chromobox homolog 4; oe, overexpression; NC, negative
control; HUVECs, human umbilical vein endothelial cells. |
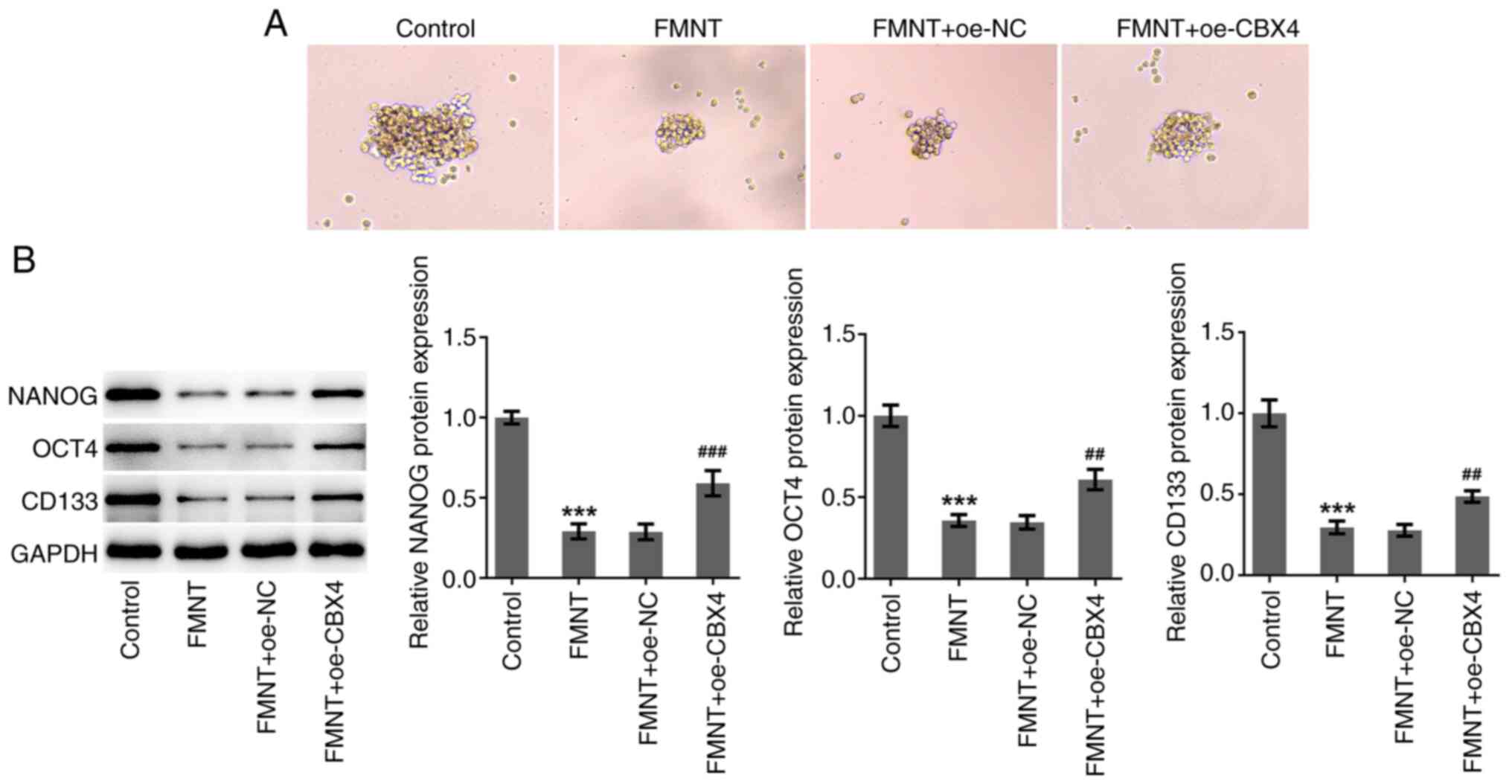
FMNT treatment inhibits stemness
characteristics of PTC cells by downregulating CBX4 expression
FMNT treatment inhibited sphere formation and
significantly reduced the protein expression levels of NANOG, OCT4
and CD133, which was partially reversed by CBX4 overexpression
(Fig. 7). Upregulation of CBX4
abolished the suppressive effect of FMNT on PTC cell stemness. In
conclusion, FMNT treatment may inhibit the stemness characteristics
of PTC cells depending on downregulation of CBX4.
Discussion
Although the treatment options available for PTC
diagnosed at an early stage are effective and achieve comparatively
improved prognosis and clinical outcome in comparison with those
for metastasized and relapsed forms of PTC, there are more
challenges in treating metastasized and relapsed forms of PTC
(25). The present study
investigated the anticarcinogenic effects of FMNT in the malignant
progression of PTC as well as identifying the intrinsic molecular
mechanism. Based on the preliminary results, FMNT was innovatively
described by the authors as an anti-tumorigenic agent in PTC via
downregulation of CBX4.
The combinational use of FMNT and metformin can
induce cell growth inhibition and apoptosis of breast cancer cells
by mediating the ERK1/2 signaling pathway (6). FMNT can impair the proliferative,
migratory and invasive capabilities of human breast cancer cells
via blockade of PI3K/Akt signaling (7,8).
FMNT can exert anti-colorectal cancer effects through suppression
of cellular proliferation and regulation of cancer-related
metabolic pathways (9). FMNT can
inhibit the growth and aggressiveness of gastric cancer cells in
vitro and in vivo by downregulating microRNA
(miR)-542-5p expression (10).
Novel hybrids of FMNT and podophyllotoxin can suppress the growth,
migration and invasion of lung cancer cells (11). In the present study, it was
demonstrated that FMNT suppressed the proliferation, clone
formation, migration, invasion, EMT, angiogenesis and stemness of
PTC cells.
Higher expression levels of CBX4 in thyroid cancer
tissues were assessed using the ENCORI database. In the present
study, it was also demonstrated that CBX4 was highly expressed in
human PTC cell line TPC-1 in comparison with normal human thyroid
cell line Nthy-ori3-1. Importantly, molecular docking confirmed the
compound-protein binding potential between FMNT and CBX4. The
binding stability of FMNT and CBX4 was enhanced by five hydrophobic
interactions, observed at TRP:32, PHE:11, HIS:9, ASN:47 and THR:41.
In addition, FMNT formed hydrophobic interactions by π-π stacking
at the active site residues with TRP:32. Therefore, the inhibition
of CBX4 by FMNT might be attributed to this unique binding pattern.
Additionally, it was shown that FMNT treatment dose-dependently
downregulated CBX4 expression in TPC-1 cells. Based on the
aforementioned results, it was hypothesized that the
anticarcinogenic effects of FMNT against PTC may be implicated in
suppression of CBX4. In hepatocellular carcinoma patients, CBX4
expression is closely associated with tumor size and pathologic
differentiation and patients who have a higher level of CBX4 in
cytoplasm suffer from a shorter overall survival and
recurrence-free survival (16).
CBX4 can promote proliferation and metastasis via regulation of
BMI-1 in lung cancer (17). CBX4
can promote gastric cancer progression and stemness via activating
CDC20(26). CBX4 can
transcriptionally suppress KLF6 via interaction with HDAC1 to exert
oncogenic activities in clear cell renal cell carcinoma (19). CBX4 overexpression can reverse the
suppressive effects of miR-515-5p on the proliferation, migration
and invasion of human breast cancer cells (20). In the present study, it was
demonstrated that upregulation of CBX4 partially abolished the
suppressive effects of FMNT on the proliferation, clone formation,
migration, invasion, EMT, angiogenesis and stemness of PTC cells.
In view of the aforementioned results, it was hypothesized that
FMNT might exert antitumor activity in PTC through multiple-target
therapy rather than single-target therapy. Molecular docking also
demonstrated the compound-protein binding potential between FMNT
and stabilin-2 (SATB2), FMNT and estrogen receptor α (ESR1). SATB2
and ESR1 have been considered as the potential oncogenes. Whether
the anticarcinogenic effects of FMNT against PTC are implicated in
suppression of SATB2 and ESR1 will be the highlights in the
following research.
Collectively, FMNT can act as an anti-tumorigenic
agent in PTC via suppression of CBX4. The results of the present
study are beneficial to the development of promising agents and
effective therapeutic targets for PTC. The current study has
certain limitations; only the TCP-1 cell line derived from male PTC
patients was used. Considering that the absolute number of female
PTC patients is more than twice that of male PTC patients, presents
a limitation to use of these results. In future studies, another
cell line derived from female PTC patients should be further
examined to enhance the persuasiveness of the conclusions in the
present research. Moreover, clinical analysis should be conducted
in the future to support the present findings and to excavate the
predictive values of FMNT.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HY contributed to the conception, study design,
experimental operation, manuscript writing and critical review. JQ
contributed to experimental operation, data collection, data
analysis and manuscript writing. HG contributed to experimental
operation, data collection and manuscript writing. YZ contributed
to the conception, study design, manuscript writing and critical
revision. All authors read and approved the final version of the
manuscript. HY and YZ confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang W, Ruan X, Li Y, Zhi J, Hu L, Hou X,
Shi X, Wang X, Wang J, Ma W, et al: KDM1A promotes thyroid cancer
progression and maintains stemness through the Wnt/β-catenin
signaling pathway. Theranostics. 12:1500–1517. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American thyroid association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American thyroid association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Davies L and Welch HG: Current thyroid
cancer trends in the United States. JAMA Otolaryngol Head Neck
Surg. 140:317–322. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jiang D, Rasul A, Batool R, Sarfraz I,
Hussain G, Mateen Tahir M, Qin T, Selamoglu Z, Ali M, Li J and Li
X: Potential anticancer properties and mechanisms of action of
formononetin. Biomed Res Int. 2019(5854315)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xin M, Wang Y, Ren Q and Guo Y:
Formononetin and metformin act synergistically to inhibit growth of
MCF-7 breast cancer cells in vitro. Biomed Pharmacother.
109:2084–2089. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen J, Zeng J, Xin M, Huang W and Chen X:
Formononetin induces cell cycle arrest of human breast cancer cells
via IGF1/PI3K/Akt pathways in vitro and in vivo. Horm Metab Res.
43:681–686. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhou R, Xu L, Ye M, Liao M, Du H and Chen
H: Formononetin inhibits migration and invasion of MDA-MB-231 and
4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through
PI3K/AKT signaling pathways. Horm Metab Res. 46:753–760.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang L, Gong Y, Wang S and Gao F:
Anti-colorectal cancer mechanisms of formononetin identified by
network pharmacological approach. Med Sci Monit. 25:7709–7714.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang WS and Zhao CS: Formononetin exhibits
anticancer activity in gastric carcinoma cell and regulating
miR-542-5p. Kaohsiung J Med Sci. 37:215–225. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang C, Xie Q, Zeng X, Tao N, Xu Y, Chen
Y, Wang J and Zhang L: Novel hybrids of podophyllotoxin and
formononetin inhibit the growth, migration and invasion of lung
cancer cells. Bioorg Chem. 85:445–454. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chan HL and Morey L: Emerging Roles for
polycomb-group proteins in stem cells and cancer. Trends Biochem
Sci. 44:688–700. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Klauke K, Radulović V, Broekhuis M,
Weersing E, Zwart E, Olthof S, Ritsema M, Bruggeman S, Wu X, Helin
K, et al: Polycomb Cbx family members mediate the balance between
haematopoietic stem cell self-renewal and differentiation. Nat Cell
Biol. 15:353–362. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Ismail IH, Gagné JP, Caron MC, McDonald D,
Xu Z, Masson JY, Poirier GG and Hendzel MJ: CBX4-mediated SUMO
modification regulates BMI1 recruitment at sites of DNA damage.
Nucleic Acids Res. 40:5497–5510. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pan Y, Li Q, Cao Z and Zhao S: The SUMO E3
ligase CBX4 is identified as a poor prognostic marker of gastric
cancer through multipronged OMIC analyses. Genes Dis. 8:827–837.
2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang B, Tang J, Liao D, Wang G, Zhang M,
Sang Y, Cao J, Wu Y, Zhang R, Li S, et al: Chromobox homolog 4 is
correlated with prognosis and tumor cell growth in hepatocellular
carcinoma. Ann Surg Oncol. 20 (Suppl 3):S684–S692. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hu C, Zhang Q, Tang Q, Zhou H, Liu W,
Huang J, Liu Y, Wang Q, Zhang J, Zhou M, et al: CBX4 promotes the
proliferation and metastasis via regulating BMI-1 in lung cancer. J
Cell Mol Med. 24:618–631. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fang X and Pan A: MiR-507 inhibits the
progression of gastric carcinoma via targeting CBX4-mediated
activation of Wnt/β-catenin and HIF-1α pathways. Clin Transl Oncol.
24:2021–2028. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jiang N, Niu G, Pan YH, Pan W, Zhang MF,
Zhang CZ and Shen H: CBX4 transcriptionally suppresses KLF6 via
interaction with HDAC1 to exert oncogenic activities in clear cell
renal cell carcinoma. EBioMedicine. 53(102692)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wen LJ, Wang YS and Tan PY: miR-515-5p
inhibits the proliferation, migration and invasion of human breast
cancer cells by targeting CBX4. Exp Ther Med.
22(1328)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Winer A, Adams S and Mignatti P: Matrix
metalloproteinase inhibitors in cancer therapy: Turning past
failures into future successes. Mol Cancer Ther. 17:1147–1155.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Aiello NM and Kang Y: Context-dependent
EMT programs in cancer metastasis. J Exp Med. 216:1016–1026.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Paduch R: The role of lymphangiogenesis
and angiogenesis in tumor metastasis. Cell Oncol (Dordr).
39:397–410. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Khan AQ, Ahmed EI, Elareer N, Fathima H,
Prabhu KS, Siveen KS, Kulinski M, Azizi F, Dermime S, Ahmad A, et
al: Curcumin-mediated apoptotic cell death in papillary thyroid
cancer and cancer stem-like cells through targeting of the
JAK/STAT3 signaling pathway. Int J Mol Sci. 21(438)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li W, Chen H, Wang Z, Liu J, Lei X and
Chen W: Chromobox 4 (CBX4) promotes tumor progression and stemness
via activating CDC20 in gastric cancer. J Gastrointest Oncol.
13:1058–1072. 2022.PubMed/NCBI View Article : Google Scholar
|