Introduction
Valvular heart disease (VHD) represents a prevalent
cardiovascular condition, impacting >100 million individuals
globally (1). Primarily stemming
from rheumatic fever and degenerative valves, VHD manifests as an
anomaly in the heart valve structure, disrupting cardiac
hemodynamics and diminishing the well-being of affected
individuals, thereby posing a substantial risk to their lives
(2). Despite a global reduction in
the incidence of rheumatic heart disease, it remains a significant
health concern in low- to middle-income nations (3). Patients afflicted by this condition
often experience a compromised quality of life and face elevated
risks of heart failure, stroke and arrhythmia. Surgery to implant
artificial heart valves has significantly improved the quality of
life for patients and plays a crucial role in treating advanced
cardiac valve diseases. With ~30 million heart valves inserted
worldwide each year, the replacement of heart valves with
prosthetic valves, whether mechanical or bioprosthetic, is an
effective solution for severe VHD (4). Individuals who undergo heart valve
surgery require long-term anticoagulation therapy to prevent
embolic events. Despite the development of new oral anticoagulants
that address certain limitations of warfarin therapy, warfarin
remains the standard of care for patients with prosthetic heart
valves (5).
Warfarin, an established oral vitamin K antagonist,
is one of the oldest and most commonly prescribed coagulation
inhibitors used in anticoagulant therapy (6). Recognized for its efficacy, safety
and cost-effectiveness, it is on the World Health Organization's
list of essential medicines (7).
Warfarin is widely used to prevent various thromboembolic events
and conditions, including prosthetic heart valve replacement,
atrial fibrillation, deep venous thrombosis and pulmonary embolism
(8). However, monitoring warfarin
in a clinical setting poses challenges due to its narrow
therapeutic window and heightened sensitivity to drug interactions
(9). A notable limitation in
warfarin-treated patients is the considerable inter-individual
variability in the required dose to reach the target anticoagulant
effect (10). To gauge the
anticoagulant effects of warfarin, clinicians rely on the index of
anticoagulant activity, represented by the international normalized
ratio (INR), to attain the desired target range.
The duration taken to attain the effective INR
threshold is an essential assessment of anticoagulant performance,
leading to the development of numerous warfarin dose prediction
models aimed at reaching this stability promptly (11,12).
In clinical settings, the patient's response to warfarin exhibits
significant variability, with factors such as the initial warfarin
dose, interacting drugs, age, liver disease and dietary vitamin K
intake all known to influence the prothrombin response (13). Swiftly reaching the therapeutic INR
range for warfarin holds potential benefits, including minimizing
the length of heparin bridging and reducing treatment costs
(14). Conversely, prolonging the
time to reach the therapeutic INR range may extend hospital length
of stay (LOS) and elevate the risk of cardiovascular complications,
particularly in the starting treatment phase. Therefore, it is
crucial to investigate the factors related to rapidly reaching the
effective INR threshold to expedite the attainment of the
therapeutic INR range in patients with VHD (4,7).
With this objective, the present study aimed to assess multiple
factors that could impact the prompt achievement of the effective
INR threshold in warfarin treatment and evaluate its safety in
clinical applications.
Patients and methods
Study design and subjects
The retrospective cohort study involved 201
inpatients undergoing heart valve replacement or repair and
receiving warfarin prescriptions. Among them, 167 inpatients were
first-time users. The enrolment spanned from July 2022 to June 2023
at the First Affiliated Hospital of Zhengzhou University
(Zhengzhou, China). Clinical data were collected from an electronic
medical records database, focusing on patients who met the
following inclusion criteria: i) Age ≥18 years; ii) initiation of
oral warfarin therapy with no prior history of warfarin use within
1 month before admission; iii) maintenance of an INR range between
1.5 and 2.5 prior to discharge. Exclusion criteria comprised the
following: i) Patients who switched anticoagulant medications and
ceased warfarin before discharge; ii) individuals with an INR
exceeding 1.3 before commencing warfarin; iii) patients who did not
survive until discharge. A cardiac examination of the participants
was conducted using color Doppler ultrasonography. The present
study was reviewed and approved by the Ethics Committee of the
First Affiliated Hospital of Zhengzhou University (Zhengzhou,
China).
Data collection and definition
Patient data were gathered, encompassing basic
demographics, laboratory results and clinical information obtained
during the hospital stay. Basic demographics included gender, age,
weight and height. Laboratory results encompassed baseline levels
of the creatinine clearance rate, bilirubin, alanine
aminotransferase and aspartate aminotransferase, at the start of
warfarin therapy, along with the fluctuations in the INR observed
throughout hospitalization. Clinical information encompassed the
indications for warfarin use, comorbidities (including coronary
heart disease, congestive heart failure, diabetes mellitus, stroke
and hypertension), alcohol consumption and smoking history,
concurrent medications, initial warfarin dose, LOS at the hospital
post-medication and adverse effects. Indications for warfarin
treatment and comorbidities were derived from the diagnoses
recorded at admission and discharge. The initial warfarin dose was
categorized as either low or high, with 3 mg/day serving as the
cutoff point. Post-medication hospital LOS was calculated as the
duration from the commencement of warfarin administration to the
day of discharge. The body mass index (BMI) was determined by
dividing weight in kilograms by the square of height in meters and
was classified into two categories: i) low-BMI (<24 kg/m²); and
ii) high-BMI (≥24 kg/m²). Liver dysfunction was characterized by
exceeding three times the upper limit of normal in aspartate
aminotransferase or alanine aminotransferase levels (or surpassing
twice the upper limit of normal for bilirubin levels). A creatinine
clearance rate <30 ml/min was used as the criterion for
determining renal insufficiency.
The time to reach the therapeutic target INR was
identified as the day when the INR level of 1.8 was attained
following the start of warfarin treatment during the hospital stay.
The patients were categorized into slowly reaching the target INR
range group (>7 days) and rapidly reaching the target INR range
group (≤7 days) based on the mean time to reach the treatment
target INR (1.5-2.5) (7). Adverse
outcomes included laboratory-related complications and bleeding
episodes (major or non-major). Major bleeding was characterized as
a fatal or symptomatic bleeding episode necessitating the
transfusion of at least 2 units of red blood cells, a drop in
hemoglobin by ≥2 g/dl, or bleeding in critical areas (intraocular,
intracranial, pericardial, retroperitoneal, intraspinal,
intra-articular or intracompartmental syndrome). Non-major bleeding
was characterized as a clinically relevant bleeding that was not
classified as major. A laboratory adverse event was defined as an
instance in which inpatients had an INR level ≥4.
Statistical analysis
Continuous variables are presented as the mean ±
standard deviation or median [interquartile range (IQR)], while
categorical variables are expressed as n (%). Statistical
significance was assessed using chi-square tests for categorical
variables and Mann-Whitney U-tests for continuous variables that
were not normally distributed. Factors associated with a low
initial dose of warfarin were assessed using both univariate
analysis and multivariable logistic regression model analysis.
Variables with a significance level of ≤0.20 in the univariate
analysis were retained in the final multivariable model. For each
variable in the model, the odds ratio (OR) and corresponding 95%
confidence interval (CI) were provided. Data analyses were
performed using SPSS software (version 21.0; IBM Corp.). P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient characteristics
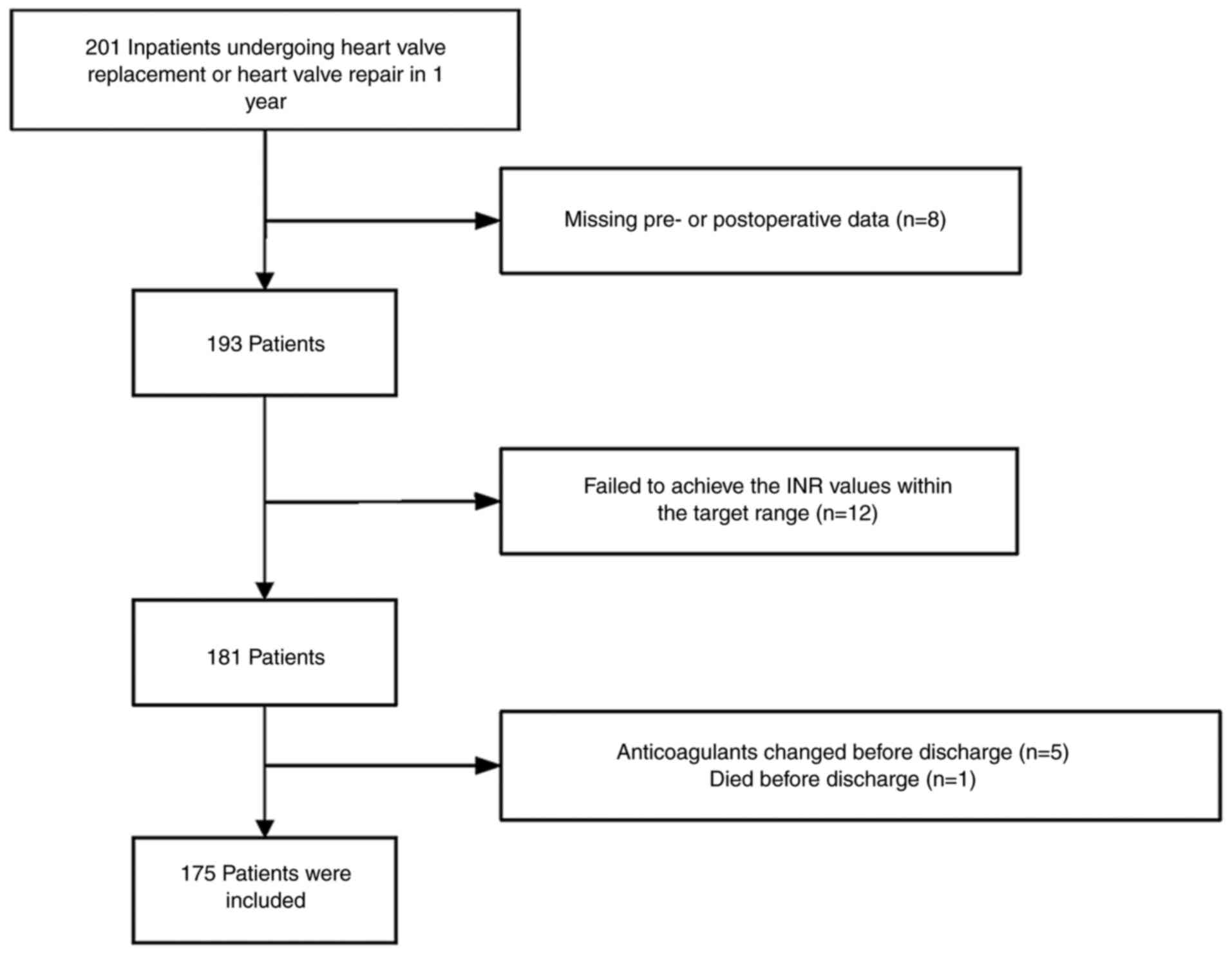
As depicted in Fig.
1, the selection process, guided by the inclusion and exclusion
criteria, involved the recording of preoperative baseline clinical
data for 201 patients diagnosed with VHD undergoing valve
replacement or repair, with primary indications for warfarin
treatment episodes. Out of these, 12 patients did not attain the
INR values within the target range (1.5-2.5) before discharge. In
addition, 8 patients were excluded due to incomplete pre- or
postoperative data. Those who changed anticoagulants before
discharge (5 cases) and individuals who did not survive until
discharge (1 case of severe pneumonia) were also excluded. Among
the patients receiving warfarin, a total of 175 patients (87.1%)
met the eligibility criteria, achieved the target INR range and
were included in the reported results.
In the present study, the main indications for
warfarin were VHD necessitating heart valve surgery, encompassing
both heart valve replacement and heart valve repair. Among the
patients, 36.6, 6.3 and 21.1% underwent mitral valve, tricuspid
valve and aortic valve replacement or repair, respectively (data
not shown). In addition, 29.1 and 6.9% of the patients underwent
double and triple valve procedures (data not shown). All 175
subjects were divided into two groups based on the time taken to
reach the therapeutic INR target, using a cutoff of 7 days. Among
those receiving warfarin, the mean time to reach the therapeutic
target postoperatively was 9.8 days, covering a period of 3-28 days
(data not shown). As indicated in Table I, the median duration of
hospitalization after medication was notably reduced in the group
rapidly reaching the INR target [9 days (IQR, 8-11 days)] compared
to the group reaching it more slowly [13 days (IQR, 10-17 days)]
(P=0.001).
 | Table IPatient characteristics for different
reaching the target international normalized ratio range
groups. |
Table I
Patient characteristics for different
reaching the target international normalized ratio range
groups.
| Variable | Totals (n=175) | Slowly reaching the
target INR range group (n=78) | Rapidly reaching the
target INR range group (n=97) | P-value |
|---|
| Male sex | 90 (51.4) | 42 (53.8) | 48 (49.4) | 0.648 |
| Age >65 years | 71 (40.6) | 25 (32.1) | 46 (47.4) | 0.045 |
| Body mass index
<24 kg/m2 | 89 (50.9) | 32 (41.0) | 57 (58.8) | 0.020 |
| Smoking history | 52 (29.7) | 30 (38.5) | 22 (22.7) | 0.023 |
| Drinking history | 59 (33.7) | 22 (28.2) | 37 (38.1) | 0.167 |
| Indications:
Thrombosis prophylaxis Comorbidities | 34 (19.4) | 14 (17.9) | 20 (20.6) | 0.657 |
| Hypertension | 69 (39.4) | 28 (35.9) | 41 (42.3) | 0.391 |
| Diabetes
mellitus | 21 (12.0) | 11 (14.1) | 10 (10.3) | 0.443 |
| Stroke | 7 (4.0) | 3 (3.8) | 4 (4.1) | 0.187 |
| Congestive heart
failure | 56 (32.0) | 25 (32.1) | 31 (32.0) | 0.990 |
| Coronary heart
disease | 45 (25.7) | 22 (28.2) | 23 (23.7) | 0.290 |
| Liver
dysfunction | 21 (12.0) | 8 (10.3) | 13 (13.4) | 0.524 |
| Renal
insufficiency | 19 (10.9) | 10 (12.8) | 9 (9.3) | 0.454 |
| Concomitant statins
or antimicrobials | 88 (50.3) | 37 (47.4) | 51 (52.6) | 0.499 |
| Initial dose of
warfarin ≥3 mg/day | 137 (78.3) | 54 (59.2) | 83 (85.6) | 0.009 |
| Post-medication
hospital length of stay, days | 12 (9-14) | 13 (10-17) | 9 (8-11) | 0.001 |
Factors influencing the rapidly
reaching INR threshold for warfarin management following heart
valve surgery
As indicated in Table
I, the study participants had a mean age of 52.7±12.3 years,
with 85 (48.6%) being women. Univariate analyses revealed no
significant differences in gender between the group slowly reaching
the INR target and the group rapidly reaching the INR target
(P>0.1). Similarly, there were no statistically significant
differences in comorbid conditions (coronary heart disease,
congestive heart failure and hypertension), hepatic dysfunction, or
concurrent use of statins or antimicrobials between the two groups
(P>0.05). Having a BMI <24 kg/m2 (P=0.020), a
smoking history (P=0.023) and an initial warfarin dose ≥3 mg/day
(P=0.009) were identified as separate factors influencing the rapid
achievement of the target INR level for warfarin management.
Factors associated with a reduced
initial dose of warfarin
The warfarin doses administered to 175 patients
post-operation were examined. Among the patients, 85.1% (149 cases)
received an initial warfarin dose of 3 mg, while 14.9% (26 cases)
were prescribed an initial dose of <3 mg. As indicated in
Table II, multivariable analyses
showed no significant differences in features such as gender and
age between the group with a low initial dose and the group with a
high initial dose. Additional factors with significance values of
P≤0.05 were incorporated into the final multivariable framework. A
total of six variables were independently related to a reduced
initial dose of warfarin, including drinking history (OR, 0.100;
95% CI, 0.016-0.645; P=0.016), thrombosis prophylaxis (OR, 6.885;
95% CI, 1.944-24.386; P=0.003), liver dysfunction (OR, 10.13; 95%
CI, 2.533-40.51; P=0.001), renal insufficiency (OR, 4.714; 95% CI,
1.349-16.467; P=0.015), BMI (OR, 3.644; 95% CI, 1.084-12.253;
P=0.037) and antimicrobials (OR, 4.141; 95% CI, 1.152-14.885;
P=0.029).
 | Table IIFactors associated with the low
initial dose of warfarin in the multivariate analysis. |
Table II
Factors associated with the low
initial dose of warfarin in the multivariate analysis.
| Variable | OR (95% CI) | P-value |
|---|
| Female sex | 1.399
(0.345-5.683) | 0.638 |
| Age, >65
years | 2.067
(0.548-7.788) | 0.284 |
| Drinking history | 0.100
(0.016-0.645) | 0.016 |
| Smoking history | 0.735
(0.132-4.097) | 0.726 |
| Diabetes | 1.358
(0.265-6.961) | 0.714 |
| Indications:
Thrombosis prophylaxis | 6.885
(1.944-24.386) | 0.003 |
| Liver
dysfunction | 10.13
(2.533-40.51) | 0.001 |
| Renal
insufficiency | 4.714
(1.349-16.467) | 0.015 |
| BMI <24
kg/m2 | 3.644
(1.084-12.253) | 0.037 |
| Antimicrobials | 4.141
(1.152-14.885) | 0.029 |
Warfarin management reduces the risk
of adverse events
Bleeding events were assessed as a secondary outcome
to compare differences between the groups. Out of the 175
participants, 23 (13.1%) experienced confirmed non-major bleeding
events. The locations of non-major bleeding included wounds (15
cases), ecchymosis (2 cases), the gastrointestinal tract (2 cases),
occult blood in the stool (2 cases) and microscopic hematuria (2
cases). No major bleeding events were documented across the entire
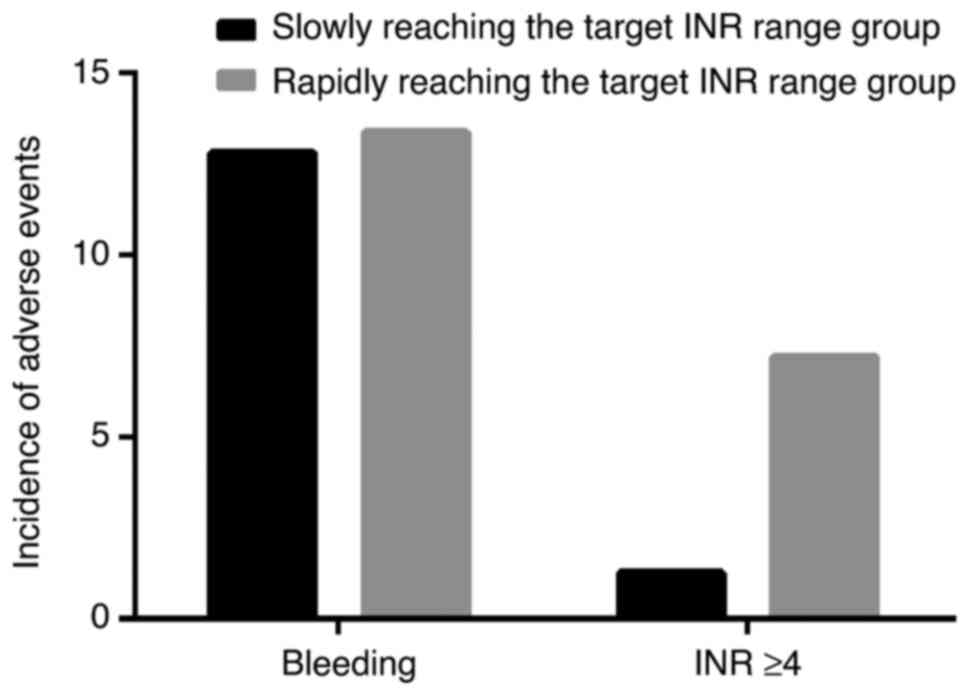
study cohort. As depicted in Fig.
2, those slowly and rapidly reaching the target INR range
showed no significant differences in bleeding incidents (12.8 vs.
13.4%, P>0.05). During hospitalization for warfarin management,
the occurrence of elevated INR levels (≥4) was observed in 8
patients (4.6%), with a higher occurrence in 7 patients rapidly
reaching the target INR range compared with those reaching it
slowly (7.7 vs. 2.1%, P=0.045).
Discussion
The current study provided a comprehensive analysis
of potential confounding factors influencing the rapid achievement
of the therapeutic INR target in patients undergoing heart valve
surgery and receiving conventional warfarin management. Notably,
the present findings indicated that individuals aged >65 years,
with a BMI <24 kg/m2, no smoking history and a
starting warfarin dosage >3 mg/day tended to reach the
therapeutic INR target rapidly. Furthermore, reaching the INR
target quickly was associated with a significant reduction in the
post-medication hospital LOS. This outcome aligns with findings
from previous research (11-14).
The current results suggest that individuals with a higher BMI may
necessitate an increased initial warfarin dose vs. the conventional
treatment to reach the therapeutic INR more promptly. It is
noteworthy that the BMI has not been factored into determining
starting warfarin dosing decisions in current recommendations
(12).
Furthermore, the present study underscored that
being elderly (>65 years of age) stands out as a separate
prognostic factor of rapidly reaching the INR target during
warfarin management. While a limited number of studies demonstrated
that age can considerably affect the warfarin maintenance dose
needed to achieve a stable therapeutic INR, there is a scarcity of
research exploring the impact of age in determining the warfarin
starting dose requirements for expeditious attainment of the
therapeutic INR (14-18).
The present research contributes data indicating that patients aged
≤65 years old may benefit from commencing warfarin with a higher
initial dose, facilitating a more rapid achievement of therapeutic
levels and consequently expediting the onset of anticoagulant
effects.
Similar to findings in other studies (19-21),
the current research highlights that a high warfarin starting dose
emerges as a separate prognostic factor for rapidly reaching the
INR threshold during warfarin management in patients undergoing
heart valve surgery. Consequently, the present study delved into an
analysis of situations in which physicians opted for a low initial
warfarin dose. The present findings revealed that patients
prescribed low initial warfarin doses were more likely to have a
drinking history, liver dysfunction, renal insufficiency,
concomitant use of antimicrobials and a criterion for
thromboprophylaxis in comparison with patients administered higher
starting doses of warfarin. Existing guidelines recommend smaller
starting doses for aged patients, those experiencing liver
dysfunction, heart failure or an increased bleeding risk (22). However, the present results
suggested that the warfarin dosing strategies in clinical practices
may not completely align with these guidelines.
While statistical significance was not reached in
the present cohort, possibly due to its modest size, there is
suggestive evidence that severe renal insufficiency could be a
factor associated with the rapid achievement of the INR target in
warfarin therapy. Other factors explored in previous studies, such
as diabetes mellitus and no smoking history, have been specifically
linked to the time required to reach the therapeutic INR (18,23).
Importantly, the present study suggested that factors including
gender, comorbid conditions, justifications for warfarin treatment
and coexisting treatments demonstrated no significant impact on the
rapid achievement of optimal INR levels for warfarin treatment.
The primary complications associated with warfarin
treatment encompass bleeding and overdosing on anticoagulants
(24,25). Consequently, the current study
aimed to assess the safety of promptly reaching the INR following
the commencement of warfarin medication, gauged by the frequency of
bleeding events and INR values ≥4. The present findings revealed
that the rapid achievement of the INR target with warfarin was not
associated with an elevated risk of bleeding events. However, it
was associated with an increased occurrence of INR values ≥4,
suggesting that patients who swiftly attained the desired INR range
were more prone to experiencing an elevated risk of an excessively
high INR. Given the strong link between an excessively high INR and
associated bleeding risk during warfarin administration (7,18),
it is advisable to vigilantly track INR measurements in patients
reaching the therapeutic INR rapidly.
The present study possesses certain limitations.
Firstly, it is a retrospective study conducted at a single medical
center with a relatively small sample size, which restricted the
ability to examine all potential influencing factors thoroughly.
Furthermore, while the influence of genetic factors on the time
required to reach the therapeutic INR range during warfarin therapy
is well-documented, the present authors could not include any
genetic data in the present analysis due to limitations in genetic
testing availability at the hospital. It is worth noting that
genetic testing is not routinely performed in several healthcare
settings. In clinical practice, physicians often adjust the
starting warfarin dose according to individual patient factors. In
addition, a limitation of the present study is the lack of further
evaluation, including potential additional in vitro and
in vivo validation, which would provide a more comprehensive
understanding of the findings. Therefore, despite these
limitations, these analyses continue to have clinical
significance.
The findings of the present study suggested a need
for prospective research involving varied warfarin starting doses
across various patient populations. Such studies would be
instrumental in identifying the most effective warfarin management
strategies for reaching the INR threshold rapidly.
The current study assessed the factors influencing
the rapid achievement of the target INR threshold for inpatients
undergoing heart valve surgery and receiving conventional warfarin
management. The results indicated that patients aged >65 years,
those with a BMI <24 kg/m2 and those prescribed a
starting warfarin dose ≥3 mg/day had a higher likelihood of rapidly
attaining the therapeutic INR. Conversely, patients with a BMI ≥24
kg/m2 may require a higher warfarin starting dose. To
enhance safety, it is recommended to implement more stringent INR
tracking and make effective adjustments to the warfarin dose,
particularly for patients reaching an INR ≥1.8 within a week of
initiating warfarin therapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HH and AY: Conception and design of the study,
methodology, manuscript writing-editing. HH, AY, XH and YX: Design
of the methodology and contribution to data acquisition. XH and LY:
Statistical analyses. HH and AY drafted and revised the manuscript.
HH and AY checked and confirm the authenticity of all the data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
This study was reviewed and approved by the Ethics
Committee of the First Affiliated Hospital of Zhengzhou University
(approval no. 2024-KY-0597).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
El-Andari R, Bozso SJ, Kang JJH, Bedard
AMA, Adams C, Wang W and Nagendran J: Heart valve surgery and the
obesity paradox: A systematic review. Clin Obes.
12(e12506)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Moreira JL, Barletta PHAAS and Baucia JA:
Morbidity and Mortality in Patients Undergoing Mitral Valve
Replacement at a Cardiovascular Surgery Referral Service: A
Retrospective Analysis. Braz J Cardiovasc Surg. 36:183–191.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kumar RK, Antunes MJ, Beaton A, Mirabel M,
Nkomo VT, Okello E, Regmi PR, Reményi B, Sliwa-Hähnle K, Zühlke LJ,
et al: Contemporary diagnosis and management of rheumatic heart
disease: Implications for closing the gap: A scientific statement
from the American heart association. Circulation. 142:e337–e357.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sun JC, Davidson MJ, Lamy A and Eikelboom
JW: Antithrombotic management of patients with prosthetic heart
valves: Current evidence and future trends. Lancet. 374:565–576.
2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tsao CW, Aday AW, Almarzooq ZI, Alonso A,
Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP,
Commodore-Mensah Y, et al: Heart disease and stroke statistics-2022
update: A report from the American heart association. Circulation.
145:e153–e639. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wardrop D and Keeling D: The story of the
discovery of heparin and warfarin. Br J Haematol. 141:757–763.
2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Khoshnam-Rad N and Kargar M: Is
warfarin-induced rapid rise in INR post-cardiac surgery sssociated
with increased bleeding risk? Ann Pharmacother. 55:135–136.
2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang CL, Wu VC, Kuo CF, Chu PH, Tseng HJ,
Wen MS and Chang SH: Efficacy and safety of non-vitamin K
antagonist oral anticoagulants in atrial fibrillation patients with
impaired liver function: A retrospective cohort study. J Am Heart
Assoc. 7(e009263)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhu Z, Li Y, Meng X, Han J, Li Y, Liu K,
Shen J, Qin Y and Zhang H: New warfarin anticoagulation management
model after heart valve surgery: Rationale and design of a
prospective, multicentre, randomised trial to compare an
internet-based warfarin anticoagulation management model with the
traditional warfarin management model. BMJ Open.
9(e032949)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Byambajav T, Waki T, Miura K and
Tanaka-Mizuno S: Association between adherence to warfarin and
thrombotic events in patients with antiphospholipid syndrome in
Japan: A claims-based retrospective cohort study. Pharmacoepidemiol
Drug Saf. 31:149–157. 2022.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Kohsaka S, Katada J, Saito K, Jenkins A,
Li B, Mardekian J and Terayama Y: Safety and effectiveness of
non-vitamin K oral anticoagulants versus warfarin in real-world
patients with non-valvular atrial fibrillation: A retrospective
analysis of contemporary Japanese administrative claims data. Open
Heart. 7(e001232)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Soyombo BM, Taylor A, Gillard C, Wilson C
and Bailey Wheeler J: Impact of body mass index on 90-day warfarin
requirements: A retrospective chart review. Ther Adv Cardiovasc
Dis. 15(17539447211012803)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jiang S, Lv M, Zeng Z, Fang Z, Chen M,
Qian J, Wu T, Chen W and Zhang J: Efficacy and safety of app-based
remote warfarin management during COVID-19-related lockdown: A
retrospective cohort study. J Thromb Thrombolysis. 54:20–28.
2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bixby AL, Shaikh SA, Naik AS, Cotiguala L,
McMurry K, Samaniego-Picota MD, Marshall VD and Park JM: Safety and
efficacy of direct-acting oral anticoagulants versus warfarin in
kidney transplant recipients: A retrospective single-center cohort
study. Transpl Int. 33:740–751. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wong CS, Batchelor K, Bua J and Newall F:
Safety and efficacy of warfarin in paediatric patients with
prosthetic cardiac valves: A retrospective audit. Thromb Res.
128:331–334. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pritchard ER, Murillo JR Jr, Putney D and
Hobaugh EC: Single-center, retrospective evaluation of safety and
efficacy of direct oral anticoagulants versus low-molecular-weight
heparin and vitamin K antagonist in patients with cancer. J Oncol
Pharm Pract. 25:52–59. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jones K, Pham C, Aguilar C and Sheth S:
Retrospective review on the safety and efficacy of direct oral
anticoagulants compared with warfarin in patients with cirrhosis.
Fed Pract. 37:479–485. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Yang W, Ma J, Hu W, Dai H and Xu H:
Associated factors and safety of the rapidly achieving first
therapeutic target of warfarin in hospitalized patients: A
retrospective cohort study. Int J Clin Pharm. 44:939–946.
2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Margolis AR, Porter AL, Staresinic CE and
Ray CA: Impact of an extended International Normalized Ratio
follow-up interval on healthcare use among veteran patients on
stable warfarin doses. Am J Health Syst Pharm. 76:1848–1852.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sridharan K, Banny RA and Husain A:
Evaluation of stable doses of warfarin in a patient cohort. Drug
Res (Stuttg). 70:570–575. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ohshima A, Koyama T, Ogawa A, Zamami Y,
Tanaka HY, Kitamura Y, Sendo T, Hinotsu S, Miller MW and Kano MR:
Oral anticoagulants usage in Japanese patients aged 18-74 years
with non-valvular atrial fibrillation: A retrospective analysis
based on insurance claims data. Fam Pract. 36:685–692.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Maslin B, Springer E, Zhu R, Kodumudi V
and Vadivelu N: Perioperative Safety of Warfarin Therapy and
Reversal. Curr Drug Saf. 11:149–155. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lip GYH, Keshishian A, Kang A, Luo X,
Atreja N, Zhang Y, Schuler P, Jiang J, Yuce H and Deitelzweig S:
Effectiveness and safety of oral anticoagulants in non-valvular
atrial fibrillation patients with prior bleeding events: A
retrospective analysis of administrative claims databases. J Thromb
Thrombolysis. 54:33–46. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pilotto A, Gallina P, Copetti M, Pilotto
A, Marcato F, Mello AM, Simonato M, Logroscino G, Padovani A,
Ferrucci L, et al: Warfarin treatment and all-cause mortality in
community-dwelling older adults with atrial fibrillation: A
retrospective observational study. J Am Geriatr Soc. 64:1416–1424.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li Y, Liu W, Liu X, Shen H, Hou F, Jin P,
Xi Z and Zhou Y: Warfarin therapy in Chinese patients with atrial
fibrillation treated with percutaneous coronary intervention: A 5
year follow-up retrospective cohort study. Curr Med Res Opin.
35:1777–1783. 2019.PubMed/NCBI View Article : Google Scholar
|