1. Introduction
Ischemic stroke (IS) has amongst the highest
morbidity and disability rates worldwide (1). Thrombolytic therapy, which uses a
tissue plasminogen activator to restore cerebral blood flow, is
currently one of the most effective treatments for IS. However, its
application is limited to a small subset of patients, due to the
restrictive time window for administration (1,2).
During the early stages of IS, collateral circulation is primarily
dependent on the reopening of pre-existing vascular networks. In
the later stages, this process shifts toward angiogenesis. Research
has revealed that angiogenesis typically starts ~3 days
post-stroke, and progresses for 21 days or longer (2). Autopsy studies of brain tissue from
patients with IS, with varying survival durations, revealed a
notable increase in micro-vessel density in the infarcted area
compared with the contralateral hemisphere (3). Furthermore, the extent of
peri-infarct angiogenesis was found to be positively correlated
with survival, survival duration and neurological recovery. These
findings suggested that angiogenesis in the infarcted region
following IS holds a significant therapeutic potential. In mouse
models of middle cerebral artery occlusion (MCAO), proliferating
endothelial cells (ECs) have been observed in the ischemic
penumbra, contributing to an increase in vascular density (4).
Following IS, EC dysfunction occurs first due to the
direct contact between the vascular endothelium and blood in the
artery. ECs are located in the innermost layer of the arterial
wall, and fluid shear stress in the vessel wall primarily affects
ECs. Disturbed blood flow promotes an ECs' response in
atherosclerosis, including dysfunction of both contractile and
diastolic functions of ECs (5).
Ischemic recovery is mediated by neoangiogenesis and requires
interactions between ECs and pericytes to form a stable
microvascular network (6). The
blood-brain barrier (BBB) is primarily constituted by diverse types
of brain ECs. It ensures the transport of specific nutrients from
peripheral circulation to the central nervous system (CNS) while
preventing the entry of harmful substances. The mechanism through
which activation of the Caspase-4/11-GSDMD signaling pathway in
brain ECs results in inflammatory disruption of the BBB has been
identified (7). A cascade of
effects is triggered in ECs following IS, and it is crucial to
examine the generation and development of EC dysfunction following
IS and to investigate how this dysfunction impacts angiogenesis.
The aim of the present review was to summarize and analyze the
mechanisms of EC dysfunction post-IS to provide new insights for IS
prevention and treatment.
2. EC origin and function
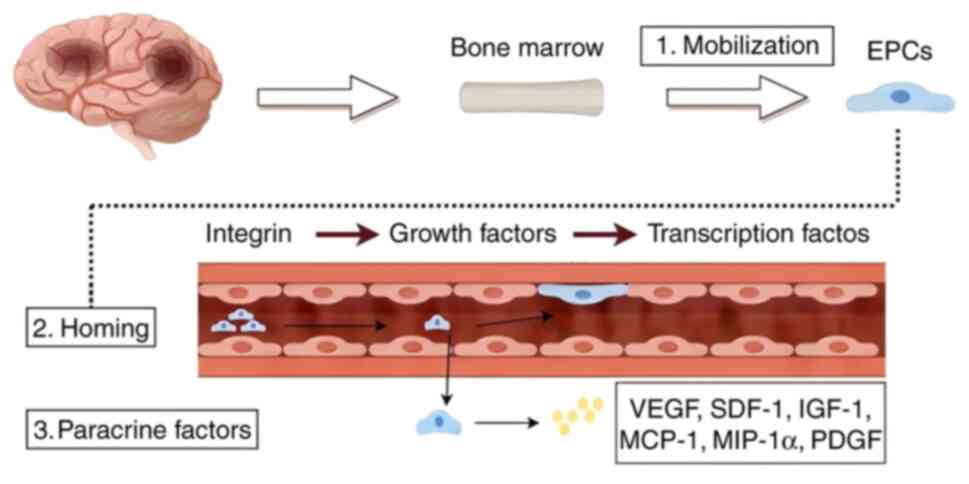
ECs are derived from endothelial progenitor cells
(EPCs), which can either distinctly develop into ECs and
incorporate into the injured vessel or regulate the injury through
paracrine factors acting on surrounding cells and vessels (8).
The differentiation of EPCs into ECs is a multi-step
process that can be divided into three distinct phases (9): i) Integrin-mediated adhesion to the
extracellular matrix (10); ii)
growth factor-driven proliferation and differentiation (11); and iii) the regulation of EC
maturation and stabilization by specific transcription factors
(12). Beyond their role in
differentiating into ECs, EPCs are also known to secrete various
paracrine factors, including vascular endothelial growth factor
(VEGF), stromal cell-derived factor 1 (SDF-1), insulin-like growth
factor 1, active monocyte chemotactic protein 1, macrophage
inflammatory protein-1α and platelet-derived growth factor
(13). These secreted factors can
interact with multiple cell types to facilitate angiogenesis and
promote tissue repair. The origin of ECs and the overall process of
angiogenesis are demonstrated in Fig.
1.
3. EC dysfunction following IS
ECs and hypoxia-inducible factor-1α
(HIF-1α)
Under normal oxygen levels, the proline residue of
the HIF-1α protein binds to Von Hippel-Lindau proteins in the
presence of oxygen, iron (II) and α-ketoglutarate, leading to its
rapid degradation through the ubiquitin-proteasome pathway
(14). By contrast, hypoxic
conditions prevent the hydroxylation of HIF-1α, allowing it to
accumulate, translocate to the nucleus and bind with HIF-1β,
thereby triggering the transcription of genes related to hypoxia,
such as VEGF (15). The brain is
highly sensitive to oxygen and nutrient fluctuations, requiring
efficient blood supply. To support its metabolic needs, the glucose
transporter type 1 (GLUT1) protein is expressed in ECs and neurons,
facilitating glucose transport and sustaining glycolytic processes.
Following IS, neuronal cells in the ischemic penumbra have an
increased demand for nutrients, which cannot be transported without
ECs. HIF-1α activation can maintain redox homeostasis by
facilitating glucose transport and glycolysis. Studies have
revealed that hypoxic preconditioning helps reduce cortical
neuronal loss in rats with traumatic brain injury (16,17).
This protective effect is primarily linked to the upregulation of
HIF-1α, which in turn stimulates the expression of GLUT1 and 3,
enhancing glucose uptake by neurons. The phosphatidylinositol
3-kinase/protein kinase B/mammalian target of rapamycin
(PI3K/AKT/mTOR) signaling pathway plays a crucial role in
vascularization, including the survival, migration and angiogenesis
of ECs. That activation of the PI3K/AKT/mTOR pathway further
stimulates its downstream target, HIF-1α, which regulates the
expression of VEGF, a key factor that promotes vascularization
following IS (18). In addition,
receptors such as the epidermal growth factor receptor, fibroblast
growth factor receptors and ΙL-6 receptor exert neuroprotective
effects against cerebral ischemia by activating the PI3K/AKT/mTOR
pathway, leading to increased levels of HIF-1α (19). HIF-1α, in turn, promotes
angiogenesis in ischemic tissues by increasing VEGF expression
(20). In ECs, the PI3K/AKT
pathway is also involved in angiogenesis through its regulation of
nitric oxide (NO) signaling, which is modulated by endothelial NO
synthase (eNOS). Evidence suggests that NO donors can enhance the
transcriptional activity and expression of HIF-1α, thereby
increasing VEGF mRNA levels (21).
These findings indicated that HIF-1α plays a pivotal role in
regulating EC dysfunction under oxidative stress following IS,
largely through stimulating angiogenesis. The mechanism of HIF-1α
and ECs angiogenesis-related factors is demonstrated in Fig. 2.
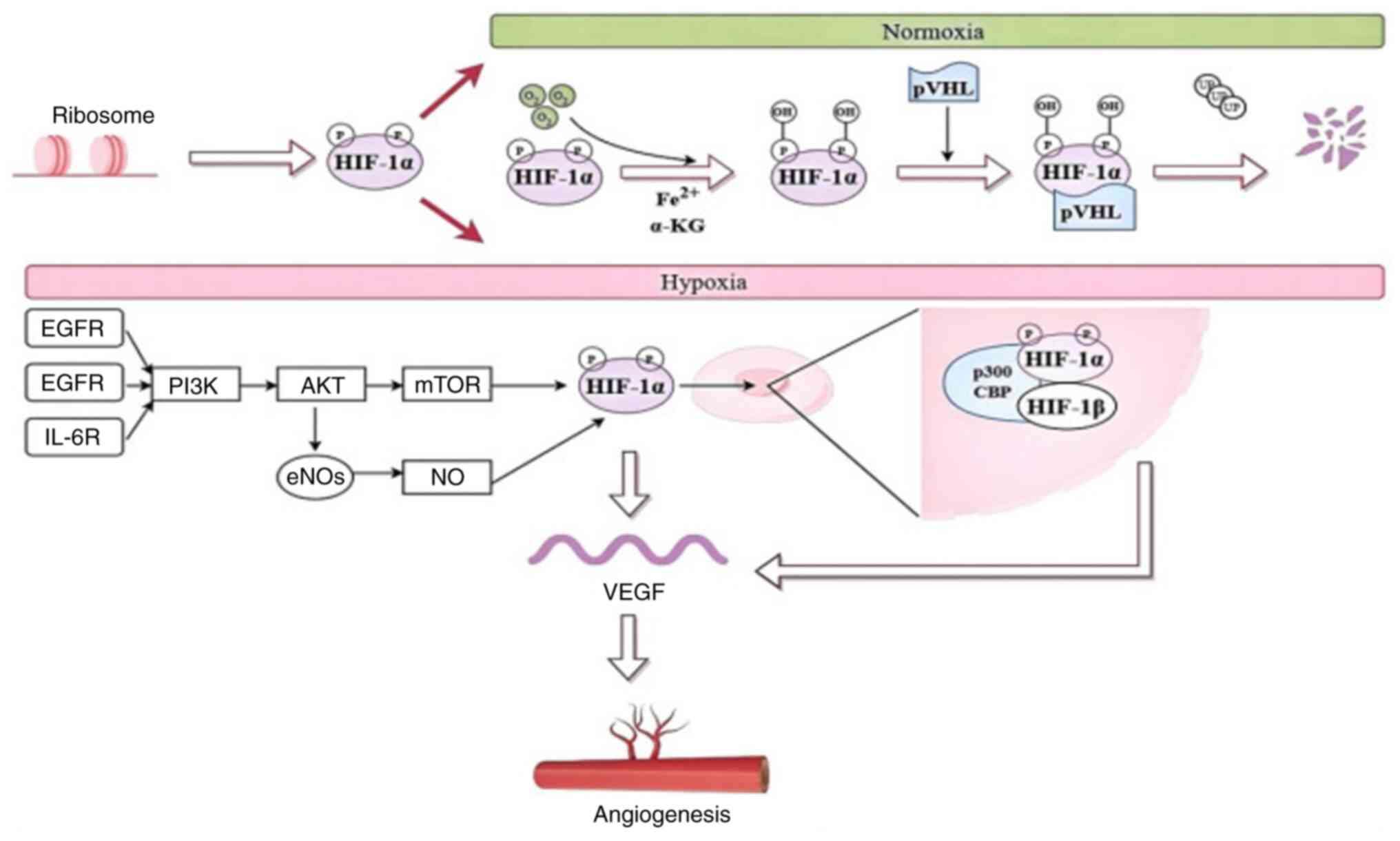 | Figure 2HIF-1α derived from the ribosomes can
be expressed in different ways under normoxic and hypoxic
conditions. Arrows indicate signal propagation downstream. HIF-1α,
hypoxia-inducible factor-1α; Fe2+, ferrous iron; α-KG,
α-ketoglutarate; EGFR, epidermal growth factor receptor, FGFR,
fibroblast growth factor receptors; IL-6R, interleukin-6 receptor;
PI3K/AKT/mTOR, phosphatidylinositol 3/Kinase protein kinase
B/mammalian target of rapamycin; NO, nitric oxide; eNOS,
endothelial NO synthase; VEGF, vascular endothelial growth
factor. |
ECs and inflammatory cytokines
Pro-inflammatory molecules, such as TNF-α, IL-1 and
IL-6, are key contributors to the development of cerebral
infarction. Although these factors exacerbate tissue damage mainly
by promoting inflammatory responses, they can, in some cases,
participate in angiogenesis by modulating angiogenesis-related
signaling pathways.
TNF-α
TNF-α is a cytokine that exerts numerous
pro-inflammatory effects and is pivotal in both pathological and
physiological processes (22).
Following IS, TNF-α is activated within 1 h, reaches its peak
between 6 and 10 h, and typically diminishes within 1 to 2 days
(23). Increased TNF-α exerts both
neuroprotective and neurotoxic effects following IS (23). TNF-α exerts its effects by binding
to two receptors, TNF receptor 1 (TNFR1) and TNFR2, both of which
are involved in hind limb ischemia-induced angiogenesis (24). When TNF-α interacts with TNFR1, it
triggers the expression of pro-erythropoietin (EPO) in cerebral ECs
by increasing the EPO receptor (EPOR) levels. EPO then promotes
angiogenesis by upregulating EPOR, which enhances the activation of
signaling pathways such as VEGF/VEGFR2 and angiopoietin-1/Tie2
(Ang1/Tie2) (25). Furthermore,
TNF-α directly influences ECs to regulate angiogenesis via VEGF
signaling activation. In addition, TNF activates several signaling
pathways, including NF-κB and AKT. NF-κB activation by TNF can
trigger AKT signaling, and both the NF-κB and PI3K/AKT pathways are
crucial for the upregulation of TNF/TNFR1 in EPOR (26). These findings suggested that TNF-α
may play a neuroprotective role by promoting angiogenesis through
EPO and its associated signaling mechanisms.
IL-1. The pro-inflammatory cytokine IL-1
plays a central role in cerebrovascular inflammation following
ischemic injury. After cerebral ischemia, microglia, astrocytes and
ECs release two isoforms of IL-1, IL-1α and IL-1β, which primarily
target ECs and astrocytes (27,28).
IL-1 stimulates the expression of LG3, a C-terminal fragment of
perlecan, in brain cell cultures. Perlecan is a key component of
the basement membrane of ECs in mature tissues, and LG3 has been
identified as a potentially neuroprotective and pro-angiogenic
fragment of the extracellular matrix (29). LG3 can mitigate β-amyloid-induced
neuronal toxicity by binding to the α2β1 integrin (30). In addition, IL-1 promotes the
expression of pentraxin-3 (PTX3), an acute-phase protein involved
in brain repair processes following ischemia. PTX3 is crucial for
mechanisms such as neurogenesis and angiogenesis in the
post-ischemic brain (31). After
14 days of MCAO, VEGF exerts a potent pro-angiogenic effect through
the activation of VEGFR2, which is upregulated after ischemia and
reduced in PTX3 knockout mice (32).
The pro-inflammatory actions of IL-1β are mediated
via its receptor, IL-1R1. The inhibition of IL-1β binding to IL-1R1
can prevent cerebral edema and brain tissue damage in experimental
IS models. IL-1β stimulates the chemokines SDF-1/CXCL-12, which
accumulates at the BBB, promoting leukocyte infiltration into the
CNS, along with microvascular leakage and cerebral edema (33). Studies on the impact of IL-1β on
trans-endothelial electrical resistance in human cerebral
microvasculature have revealed that IL-1β disrupts the endothelial
barrier through the PKC-dependent phosphorylation of PKC-θ and
ZO-1(34). As IL-1β is a key
inflammatory mediator, targeting the inhibition of PKC-θ or ZO-1
phosphorylation offers a potential strategy to prevent BBB
disruption during neuroinflammation (35). In addition, macrophage-derived
IL-1β enhances the expression of the pro-angiogenic isoform
VEGF-A165a, while suppressing the anti-angiogenic VEGF-A165b
through the activation of signal transducer and activator of
transcription-3 (STAT-3) and NF-κB pathways. This shift promotes
VEGF expression and contributes to inflammatory vascularization
(36).
IL-1α has been revealed to be significantly more
potent than IL-1β in activating ECs, as evidenced by the increased
expression of the pro-angiogenic chemokine CXCL-1. In addition,
IL-1α stimulates a strong, concentration-dependent expression of
the angiogenic mediator IL-6(37).
It also promotes EC proliferation, migration and the formation of
tubular structures. These responses can be blocked by the IL-1
receptor antagonist. Thus, while IL-1-driven inflammation can
exacerbate ischemic injury, it also plays a dual role by enhancing
the brain repair mechanisms and supporting functional recovery.
IL-6. IL-6 is a multifunctional cytokine and
an important messenger molecule between leukocytes, vascular ECs
and thin-walled tissue-resident cells, produced by several types of
cells, such as monocytes, macrophages, adipocytes, hematopoietic
cells and ECs (38). The function
of IL-6 is mediated through a unique receptor system consisting of
two functional proteins: An IL-6-specific receptor (IL-6R) and a
second glycoprotein, gp130. Signaling through gp130 is mediated by
two pathways: The JAK-STAT and the Ras-MAPK pathway (39). In the vasculature, IL-6 can act
directly or indirectly on vascular ECs. IL-6 preconditioning
induces VEGF secretion from neural stem cells via STAT3, which
promotes angiogenesis in the ischemic brain (40). A related study (41) found that two days after mild
transient cerebral ischemia, wild-type mice exhibited a significant
early increase in IL-6 gene transcription and protein levels in the
ischemic brain. This was accompanied by an early upregulation of
mRNAs for angiogenesis-related genes, such as VEGFR2 and eNOS. In
mice with MCAO, the increase in circulating VEGF was diminished
following IL-6 knockdown at 48 h (42). Monocyte-derived IL-6 plays a
critical role in programming microglia to repair the damaged brain
vasculature. Cerebrovascular repair was absent in IL-6 knockout
mice or those lacking microglial IL-6Ra expression, but could be
restored through exogenous IL-6 administration (43).
The aforementioned studies suggested that IL-6
promotes angiogenesis following cerebral infarction, thereby
providing long-term histological and functional protection. ECs and
inflammatory cytokines are shown in Fig. 3.
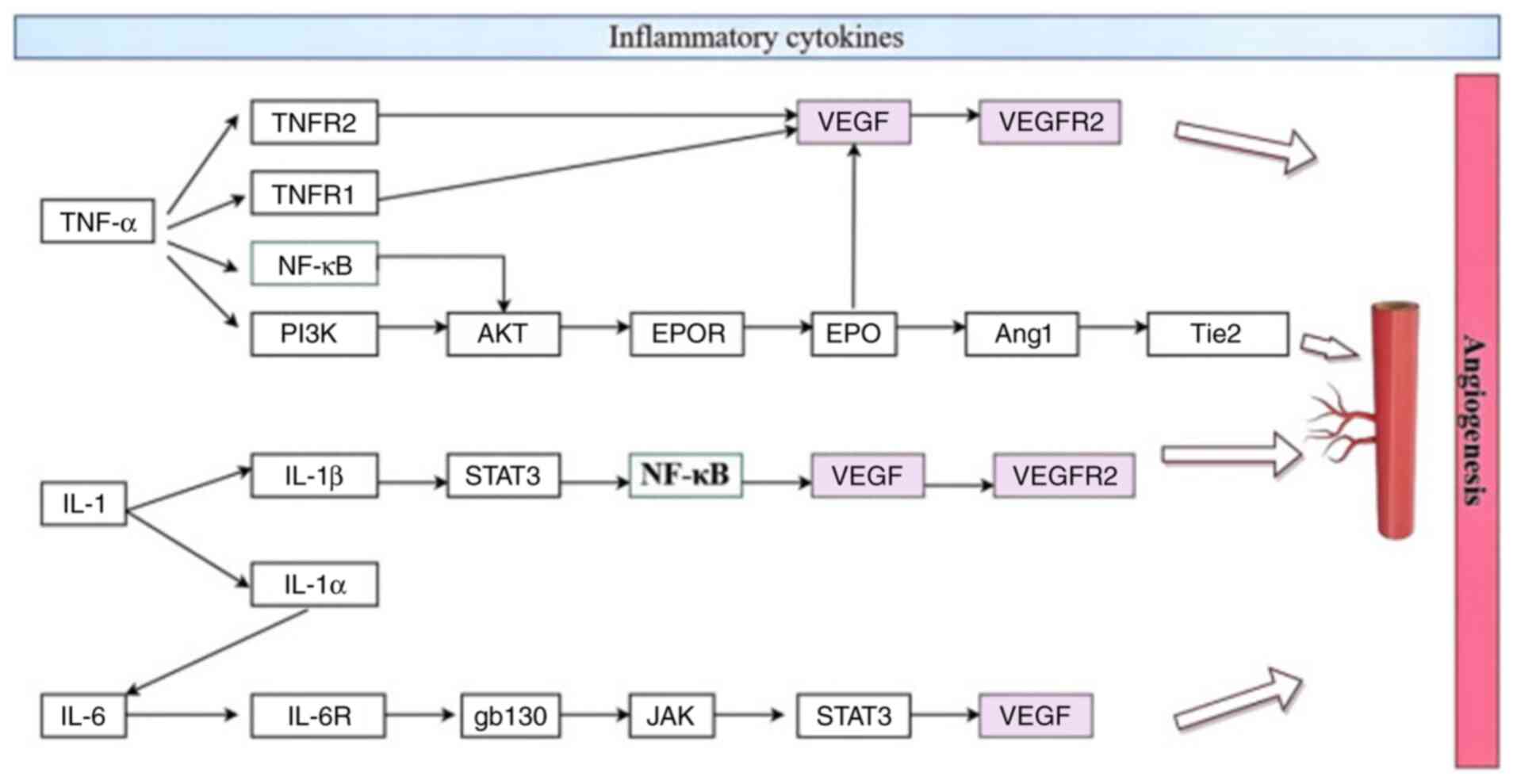 | Figure 3Endothelial cells and inflammatory
cytokines. TNFR1/2, TNF receptor 1/2; PI3K, phosphatidylinositol
3-kinase; AKT, protein kinase B; EPO, erythropoietin; EPOR, EPO
receptor; VEGF, vascular endothelial growth factor; VEGFR2, VEGF
receptor 2; Ang1, angiopoietin-1; Tie2, tyrosine kinase with
immunoglobulin and EPO receptor domains receptor; STAT3, signal
transducer and activator of transcription 3; IL-6R, IL-6 receptor;
gb130, glycoprotein 130; JAK, Janus kinase. |
ECs and adhesion factors
The EC membrane serves as the interface between ECs
and the external environment, facilitating material exchange and
signal transmission. The integrity and dynamic changes of the EC
membrane significantly influence the activity and role of different
adhesion molecules. These molecules, expressed on the ECs membrane,
are critical in vascular biology as they regulate blood flow,
immune cell migration and inflammatory responses. Specifically,
adhesion molecules are vital for leukocyte infiltration into the
CNS. The molecular interactions between ECs and circulating
leukocytes are primarily mediated by three categories of adhesion
molecules: Selectins such as E-, P- and L-selectins, immunoglobulin
superfamily members such as intercellular adhesion molecule-1
(ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) and
integrins.
Selectins
Selectins are membrane-bound glycoproteins with
three main members: E-selectin (CD62E), P-selectin (CD62P) and
L-selectin (CD62L) (44). The
molecular mechanisms of leukocyte infiltration into ischemic
tissues involve these selectins. CD62E, also known as E-selectin,
plays a crucial role in the adhesion of leukocytes to ECs, and its
involvement in the homing and angiogenesis of EPCs has been
experimentally studied. CD62E is synthesized in response to
inflammatory stimuli, such as TNF-α and IL-1, and is expressed on
the EC membrane within hours of stimulation. CD62P or P-selectin,
is found on ECs and platelet granule membranes, and is rapidly
expressed on the outer cell membrane upon activation by factors
such as thrombin or histamine. CD62L is present on lymphocytes,
neutrophils and monocytes, and following cell activation it is
cleaved from the membrane through proteolytic processes.
Immunoglobulin superfamily. The
immunoglobulin superfamily consists of a group of cell surface and
soluble proteins, several of which serve as adhesion molecules. A
total of 5 key members of this family include ICAM-1, ICAM-2,
VCAM-1, platelet endothelial cell adhesion molecule 1 and mucosal
vascular addressin in cell adhesion molecule 1. Among these, ICAM-1
and VCAM-1 are some of the most extensively studied adhesion
molecules in the context of IS. The expression of these EC adhesion
molecules is a crucial step in the recruitment of circulating
leukocytes to areas of inflammation. Hypoxic conditions and
cytokines, such as IL-1β and TNF-α, significantly enhance the
expression of these molecules (45).
The role of ICAM-1 in regulating cerebral leukocyte
recruitment during neuroinflammation and ischemia has been clearly
established through studies involving ICAM-1 knockout mice and the
use of ICAM-1 blocking antibodies (46). In vivo research in mice has
revealed that TNF-α triggers the upregulation of both ICAM-1 and
VCAM-1. The expression levels of these molecules begin to rise
between 2 and 5 h after brain injury, peak between 5 and 9 h, and
remain elevated above baseline levels for at least 24 h (47). Of note, the increases in ICAM-1 and
VCAM-1 expression are dose-dependent, with a significant
upregulation at 5 µg/kg and a maximal increase observed at doses
between 10-25 µg/kg.
Correlative studies have revealed that VEGF
increases VCAM-1 and ICAM-1 protein levels and promotes leukocyte
adhesion in an NF-κB-dependent manner (48). ICAM-1 expression influences
VEGF-A-induced eNOS activity and angiogenesis by regulating
endothelial glutathione levels (49). Studies also have revealed that both
VCAM-1 and integrin α4 (ITGA4) are upregulated by TNF-α in ECs.
sVCAM-1-induced angiogenesis is facilitated by the upregulation of
VEGF through the p38 MAPK/FAK signaling pathway. The sVCAM-1/ITGA4
pathway may play a role in inflammatory angiogenesis (50).
The aforementioned findings suggested that
inflammatory cytokines promote the increase in the expression of
ICAM-1 and VCAM-1, which enhances the interaction between ECs and
circulating leukocytes, thereby promoting angiogenesis following
IS.
Integrins. Integrins are heterodimeric
proteins made up of distinct α-subunits paired with common
β-subunits. The β-subunits are categorized into three subfamilies:
β1, β2 and β3 integrins. Integrins in the β1 subfamily play a key
role in connecting to the extracellular matrix, binding to
collagen, laminin and fibronectin. By contrast, β2 integrins are
crucial for mediating the adhesion between leukocytes and ECs. β3
integrins, also referred to as integrin αvβ3 or cytokines, are
involved in blood clot formation and stabilization. These integrins
are vital for regulating processes such as hematopoiesis, leukocyte
recruitment and inflammatory responses (51).
Brain ECs demonstrate minimal expression of adhesion
molecules, which prevents peripheral immune cells from crossing
into the CNS (25). β2 Integrin, a
key integrin subunit, plays a significant role in directing EPCs to
areas of active vascular angiogenesis. Known as a
leukocyte-specific receptor, β2 integrin interacts with various
ICAM family members and polysaccharides (52).
The impact of neuroinflammatory mechanisms (whether
detrimental or protective) depends on the timing following cerebral
ischemia. Inflammation may exacerbate ischemic injury in the early
stages of IS, whereas the inflammatory response may protect neurons
by promoting neurogenesis, angiogenesis and neuroplasticity in the
later stages. Integrins such as α5β1 and αvβ3, play a crucial role
in regulating the processes of angiogenesis and inflammation
following cerebral ischemia (53).
The Ang1/Tie2 signaling pathway and the integrin α5β1 interact with
ECs following IS, promoting angiogenesis (54). VEGF upregulation by α5β1 and αvβ3,
and their ligand-endo-ligand proteins in the ischemic penumbra,
stimulate EC proliferation. TNF-α or FGF-2-induced angiogenesis is
dependent on αvβ3, while VEGF and TGF-α-induced angiogenesis is
dependent on αvβ5, suggesting that αv integrins play a key role in
potent angiogenesis (55).
Adhesion molecules on ECs play a crucial role in
maintaining vascular health and function. Targeting these molecules
to modulate their expression can reduce inflammation and improve EC
function. For example, the development of small-molecule inhibitors
targeting ICAM-1 or VCAM-1 may help suppress intravascular
inflammation and reduce cerebrovascular lesions (56). The development of therapies that
promote EC repair and regeneration may help to improve the function
of the vascular endothelium and provide a new direction in the
treatment of cerebrovascular diseases. For example, stem cell
therapy (57) or gene therapy
(58) is used to promote
endothelial repair and restore the normal function of blood
vessels. The association between ECs and adhesion factors is
demonstrated in Fig. 4.
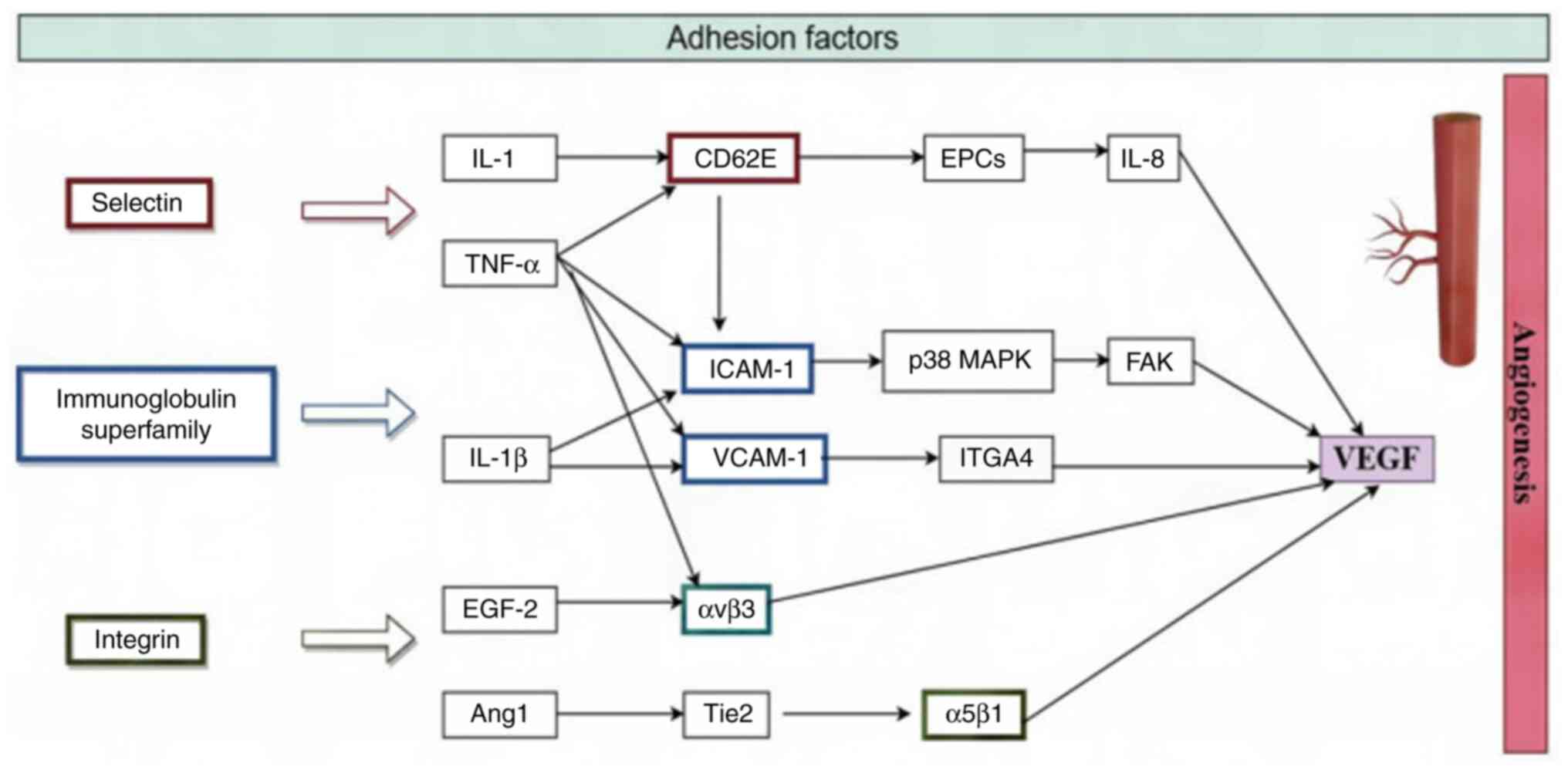 | Figure 4Endothelial cells and adhesion
factors. CD62E, e-selectin; EPCs, endothelial progenitor cells;
ICAM-1, intercellular adhesion molecule 1; p38 MAPK, p38
mitogen-activated protein kinase; FAK, focal adhesion kinase;
VCAM-1, vascular cell adhesion molecule 1; ITGA4, Integrin α4;
FGF-2, fibroblast growth factor 2; αvβ3, Integrin αvβ3; Ang1,
Angiopoietin-1; Tie2, tyrosine kinase with immunoglobulin and EPO
receptor domains receptor; α5β1, Integrin α 5β1; VEGF, vascular
endothelial growth factor. |
4. Conclusions
Following IS, ECs trigger a cascade of reactions,
including oxidative stress and inflammation. Promoting angiogenesis
can help alleviate the ischemic and hypoxic conditions in the
affected brain tissue.
Next, the clinical translational applications which
are related to the aforementioned cytokines will be discussed. For
HIF-1α, related studies have revealed that Dan-Deng-Tong-Nao soft
gel capsules promote angiogenesis to safeguard brain tissue against
IS and exert beneficial effects in brain microvascular ECs by
activating the HIF-1α/VEGFA/NOTCH1 signaling pathway (57-59).
Chinese patent medicines have significant advantages in the
long-term treatment of stroke, due to their versatility and
multi-target nature (59). Tissue
kallikrein (TK) has emerged as a promising neuroprotective agent in
IS. Research in preclinical models has revealed that TK
supplementation activates the PI3K/AKT signaling pathway by
enhancing the expression of bradykinin receptor 2 during the
ischemic phase. This activation promotes the nuclear translocation
of HIF-1α, which in turn boosts the expression of VEGF and eNOS,
strengthening the neurovascular unit. In addition, TK suppresses
the activation of the kallikrein-kinin system triggered by
reperfusion injury, effectively reducing inflammation, oxidative
stress production and endothelial barrier dysfunction (60). The treatment of endothelial barrier
dysfunction by Dan-Deng-Tong-Nao soft gel capsules and TK warrants
continued exploration in the future.
Inflammatory cytokines such as TNF, IL-1 and IL-6
play a crucial role in tissue damage during stroke, making them
important targets for IS treatment. Studies have revealed that
these cytokines significantly contribute to stroke-induced injury
(61). Specifically, TNF-α and
IL-1β can trigger the expression of procoagulant molecules, such as
tissue factor and plasminogen activator inhibitor-1, by activating
the NF-κB and AP-1 signaling pathways (61). Hexahydrocurcumin (HHC), a major
metabolite of curcumin, has been found to improve hypertensive and
vascular remodeling in Nω-Amino-L-arginine methyl ester (L-NAME)
-induced rats (62). TGF-β plays a
pivotal role in inflammatory responses by promoting fibrogenesis, a
key process in vascular remodeling. Matrix metalloproteinases
(MMPs), a family of zinc-dependent enzymes, are involved in
degrading extracellular matrix components such as collagen and
elastin. During vascular formation and remodeling, MMPs act as
inflammatory mediators. Elevated levels of TGF-β1, MMP-2 and MMP-9
have been observed in L-NAME-induced hypertensive rats, indicating
their involvement in the pathogenesis of vascular changes.
HHC treatment has been revealed to reduce the
expression of key proteins involved in vascular remodeling,
including TGF-β1, MMP-9 and collagen type I. In addition, N-myc
downstream-regulated gene 1 (NDRG1), a member of the NDRG family,
plays a crucial role in cell differentiation, proliferation and
stress responses. NDRG1 is a key mediator in regulating endothelial
inflammation, thrombotic responses and vascular remodeling. NDRG1
knockdown using lentivirus-based NDRG1 shRNA significantly reduces
the expression of cytokines, chemokines and adhesion molecules
induced by IL-1β and TNF-α (63).
Given this, the strategic use of HHC at appropriate times, combined
with targeted regulation of NDRG1, holds promise as a therapeutic
approach for modulating endothelial inflammation, thrombotic
responses and angiogenesis in the context of IS.
Studies on adhesion molecules indicate that
gingerol, the active compound in fresh ginger, undergoes
dehydration to form 6-shogaol in dried ginger rhizomes. 6-Shogaol
has been revealed to reduce the levels of cell adhesion molecules,
particularly in lipopolysaccharide-activated ECs. It notably
decreased the expression of ICAM-1, VCAM-1 and E-selectin on the
endothelial surface in response to TNF stimulation. Furthermore,
mRNA analysis demonstrated that higher concentrations of 6-shogaol
led to a concentration-dependent reduction in the gene expression
of ICAM-1, VCAM-1 and E-selectin. Therefore, identifying components
with the opposite effect to gingerol may present a new direction
for future clinical research aimed at promoting the regulation of
EC adhesion molecules and enhancing angiogenesis following IS
(64).
In the present study, from the perspective of EC
dysfunction, the angiogenesis pathways associated with ECs
following IS were summarized. The exploration of these mechanisms
can provide intervention strategies for future treatment following
IS. The importance of HIF-1α in EC function under hypoxic
conditions was emphasized, particularly in promoting angiogenesis
and improving cerebral blood supply, both of which are crucial in
the management and avoidance of cerebral ischemia.
Inflammatory cytokines and adhesion factors affect
the function and angiogenesis of ECs after IS through distinct
mechanisms and signaling pathways, thereby playing a significant
role in the pathological processes of cerebral ischemia. However,
there remains much to explore regarding the dysfunction of ECs
after IS.
For example, from the perspective of inflammatory
factors, it is important to continue investigating the expression
patterns and mechanisms of action of inflammatory cytokines,
including TNF-α, IL-1 and IL-6, after IS and how they regulate
angiogenesis by affecting ECs. This includes exploring how these
cytokines activate receptors on ECs and their temporal dynamics
during inflammation and repair.
From the perspective of adhesion factors, it is
necessary to investigate changes in the expression of adhesion
factors, such as selectins, the immunoglobulin superfamily, and
integrins, after IS, and how they influence leukocyte recruitment
and EC function. Exploring the specific roles of these molecules in
angiogenesis and repair, as well as how they interact with other
signaling pathways, is critical.
From the perspective of oxidative stress, the impact
of oxidative stress generated by ECs on their function and
angiogenesis after IS should be studied. Understanding how ECs
respond to oxidative stress and whether antioxidant strategies can
improve EC function and promote angiogenesis is essential.
Based on the understanding of these pathways and
molecules, novel therapeutic strategies can be developed to
ameliorate endothelial dysfunction and promote angiogenesis after
IS. These strategies may include small molecule drugs targeting
specific molecules, biologics, gene therapy and traditional Chinese
medicines.
Further translation into clinical applications will
be achieved by translating laboratory discoveries into clinical
practice, incorporating multimodal imaging technology, and
utilizing advanced imaging techniques to monitor the dynamic
process of post-IS angiogenesis. These techniques will also assess
the effects of novel therapeutic interventions. This includes
analyzing clinical trials and patient samples to validate
laboratory results and identify the most promising therapeutic
targets. These explorations will result in a deeper understanding
of EC function and the regulatory mechanisms of angiogenesis after
IS, providing new intervention strategies for the treatment of
IS.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Heilongjiang
Provincial Natural Science Foundation of China (grant no.
LH2022H080).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
RG conceived and designed the study. JLT, GL, XFL
and LM collected the data and performed the literature search. RG
was involved in the writing of the manuscript. SS was responsible
for the revision of the manuscript. All authors have read and
approved the final version of the manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Saini V, Guada L and Yavagal DR: Global
epidemiology of stroke and access to acute ischemic stroke
interventions. Neurology. 97 (Suppl 2):S6–S16. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhu L, Wang M, Liu Y, Fu P, Zhang W, Zhang
H, Roe AW and Xi W: Single-microvessel occlusion produces
lamina-specific microvascular flow vasodynamics and signs of
neurodegenerative change. Cell Rep. 42(112469)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Krupinski J, Kaluza J, Kumar P, Kumar S
and Wang JM: Role of angiogenesis in patients with cerebral
ischemic stroke. Stroke. 25:1794–1798. 1994.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kim JJ, Kim DH, Lee JY, Lee BC, Kang I,
Kook MG, Kong D, Choi SW, Woo HM, Kim DI and Kang KS: cAMP/EPAC
signaling enables ETV2 to induce endothelial cells with high
angiogenesis potential. Mol Ther. 28:466–478. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tamargo IA, Baek KI, Kim Y, Park C and Jo
H: Flow-induced reprogramming of endothelial cells in
atherosclerosis. Nat Rev Cardiol. 20:738–753. 2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cartland SP, Patil MS, Kelland E, Le N,
Boccanfuso L, Stanley CP, Cholan PM, Dona MI, Patrick R, McGrath J,
et al: The generation of stable microvessels in ischemia is
mediated by endothelial cell derived TRAIL. Sci Adv.
10(eadn8760)2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wei C, Jiang W, Wang R, Zhong H, He H, Gao
X, Zhong S, Yu F, Guo Q, Zhang L, et al: Brain endothelial GSDMD
activation mediates inflammatory BBB breakdown. Nature.
629:893–900. 2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yan F, Liu X, Ding H and Zhang W:
Paracrine mechanisms of endothelial progenitor cells in vascular
repair. Acta Histochem. 124(151833)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Oh IY, Yoon CH, Hur J, Kim JH, Kim TY, Lee
CS, Park KW, Chae IH, Oh BH, Park YB and Kim HS: Involvement of
E-selectin in recruitment of endothelial progenitor cells and
angiogenesis in ischemic muscle. Blood. 110:3891–3899.
2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Resnikoff HA and Schwarzbauer JE:
Increased basal fibronectin is sufficient to promote excess
endothelial cell matrix assembly causing localized barrier
dysfunction. Mol Biol Cell. 35(ar120)2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hildbrand P, Cirulli V, Prinsen RC, Smith
KA, Torbett BE, Salomon DR and Crisa L: The role of angiopoietins
in the development of endothelial cells from cord blood CD34+
progenitors. Blood. 104:2010–2019. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Heinisch PP, Bello C, Emmert MY, Carrel T,
Dreßen M, Hörer J, Winkler B and Luedi MM: Endothelial progenitor
cells as biomarkers of cardiovascular pathologies: A narrative
review. Cells. 11(1678)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sultan I, Ramste M, Peletier P,
Hemanthakumar KA, Ramanujam D, Tirronen A, von Wright Y, Antila S,
Saharinen P, Eklund L, et al: Contribution of VEGF-B-Induced
endocardial endothelial cell lineage in physiological versus
pathological cardiac hypertrophy. Circ Res. 134:1465–1482.
2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jaakkola P, Mole DR, Tian YM, Wilson MI,
Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji
M, Schofield CJ, et al: Targeting of HIF-alpha to the von
hippel-lindau ubiquitylation complex by O2-regulated prolyl
hydroxylation. Science. 292:468–472. 2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cha S, Kim HG, Jang H, Lee J, Chao T, Baek
NI, Song IS and Lee YM: Steppogenin suppresses tumor growth and
sprouting angiogenesis through inhibition of HIF-1α in tumors and
DLL4 activity in the endothelium. Phytomedicine.
108(154513)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Qin C, Yang S, Chu YH, Zhang H, Pang XW,
Chen L, Zhou LQ, Chen M, Tian DS and Wang W: Signaling pathways
involved in ischemic stroke: Molecular mechanisms and therapeutic
interventions. Signal Transduct Target Ther. 7(215)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen X, Qian W, Zhang Y, Zhao P, Lin X,
Yang S, Zhuge Q and Ni H: Ginsenoside CK cooperates with bone
mesenchymal stem cells to enhance angiogenesis post-stroke via
GLUT1 and HIF-1α/VEGF pathway. Phytother Res. 38:4321–4335.
2024.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Patra K, Jana S, Sarkar A, Mandal DP and
Bhattacharjee S: The inhibition of hypoxia-induced angiogenesis and
metastasis by cinnamaldehyde is mediated by decreasing HIF-1α
protein synthesis via PI3K/Akt pathway. Biofactors. 45:401–415.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen X, Fu K, Lai Y, Dong C, Chen Z, Huang
Y, Li G, Jiang R, Wu H, Wang A, et al: Tetrahydropalmatine:
Orchestrating survival-Regulating autophagy and apoptosis via the
PI3K/AKT/mTOR pathway in perforator flaps. Biomed Pharmacother.
169(115887)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Amin N, Chen S, Ren Q, Tan X, Botchway
BOA, Hu Z, Chen F, Ye S, Du X, Chen Z and Fang M: Hypoxia inducible
factor-1α attenuates ischemic brain damage by modulating
inflammatory response and glial activity. Cells.
10(1359)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jeong H, Choi D, Oh Y, Heo J and Hong J: A
Nanocoating co-localizing nitric oxide and growth factor onto
individual endothelial cells reveals synergistic effects on
angiogenesis. Adv Healthc Mater. 11(e2102095)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wu DM, Liu JP, Liu J, Ge WH, Wu SZ, Zeng
CJ, Liang J, Liu K, Lin Q, Hong XW, et al: Immune pathway
activation in neurons triggers neural damage after stroke. Cell
Rep. 42(113368)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Di Santo C, La Russa D, Greco R, Persico
A, Zanaboni AM, Bagetta G and Amantea D: Characterization of the
involvement of tumour necrosis factor (TNF)-α-stimulated gene 6
(TSG-6) in ischemic brain injury caused by middle cerebral artery
occlusion in mouse. Int J Mol Sci. 24(5800)2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nouri Barkestani M, Shamdani S, Afshar
Bakshloo M, Arouche N, Bambai B, Uzan G and Naserian S: TNFα
priming through its interaction with TNFR2 enhances endothelial
progenitor cell immunosuppressive effect: new hope for their
widespread clinical application. Cell Commun Signal.
19(1)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhao ZA, Yan L, Wen J, Satyanarayanan SK,
Yu F, Lu J, Liu YU and Su H: Cellular and molecular mechanisms in
vascular repair after traumatic brain injury: A narrative review.
Burns Trauma. 11(tkad033)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yu Z, Witman N, Wang W, Li D, Yan B, Deng
M, Wang X, Wang H, Zhou G, Liu W, et al: Cell-mediated delivery of
VEGF modified mRNA enhances blood vessel regeneration and
ameliorates murine critical limb ischemia. J Control Release.
310:103–114. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Luís JP, Simões CJV and Brito RMM: The
therapeutic prospects of targeting IL-1R1 for the modulation of
neuroinflammation in central nervous system disorders. Int J Mol
Sci. 23(1731)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Riddle RB, Jennbacken K, Hansson KM and
Harper MT: Endothelial inflammation and neutrophil transmigration
are modulated by extracellular matrix composition in an
inflammation-on-a-chip model. Sci Rep. 12(6855)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Saini MG and Bix GJ: Oxygen-glucose
deprivation (OGD) and interleukin-1 (IL-1) differentially modulate
cathepsin B/L mediated generation of neuro protective perlecan LG3
by neurons. Brain Res. 1438:65–74. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Parham CL, Shaw C, Auckland LD, Dickeson
SK, Griswold-Prenner I and Bix G: Perlecan Domain V inhibits
amyloid-β induced activation of the α2β1 integrin-mediated
neurotoxic signaling cascade. J Alzheimers Dis. 54:1629–1647.
2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rajkovic I, Wong R, Lemarchand E,
Rivers-Auty J, Rajkovic O, Garlanda C, Allan SM and Pinteaux E:
Pentraxin 3 promotes long-term cerebral blood flow recovery,
angiogenesis, and neuronal survival after stroke. J Mol Med (Berl).
96:1319–1332. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bayat M, Tabrizi R, Saied Salehi M, Karimi
N, Rahimi M, Hooshmandi E, Razavi Moosavi N, Fadakar N and
Borhani-Haghighi A: Association of long non-coding RNA malat1 with
serum levels of interleukin-1 Β and vitamin D in patients with
ischemic stroke. Galen Med J. 12:1–10. 2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Takata F, Nakagawa S, Matsumoto J and
Dohgu S: Blood-brain barrier dysfunction amplifies the development
of neuroinflammation: understanding of cellular events in brain
microvascular endothelial cells for prevention and treatment of BBB
dysfunction. Front Cell Neurosci. 15(661838)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mantsounga CS, Lee C, Neverson J, Sharma
S, Healy A, Berus JM, Parry C, Ceneri NM, López-Giráldez F, Chun
HJ, et al: Macrophage IL-1β promotes arteriogenesis by autocrine
STAT3- and NF-κB-mediated transcription of pro-angiogenic VEGF-A.
Cell Rep. 38(110309)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yamasaki Y, Matsuura N, Shozuhara H,
Onodera H, Itoyama Y and Kogure K: Interleukin-1 as a pathogenetic
mediator of ischemic brain damage in rats. Stroke. 26:676–80;
discussion 681. 1995.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Matys P, Mirończuk A, Starosz A, Grubczak
K, Kochanowicz J, Kułakowska A and Kapica-Topczewska K: Expanding
role of interleukin-1 family cytokines in acute ischemic stroke.
Int J Mol Sci. 25(10515)2024.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Scheller J, Ettich J, Wittich C, Pudewell
S, Floss DM and Rafii P: Exploring the landscape of synthetic
IL-6-type cytokines. FEBS J. 291:2030–2050. 2024.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Akira S, Nishio Y, Inoue M, Wang XJ, Wei
S, Matsusaka T, Yoshida K, Sudo T, Naruto M and Kishimoto T:
Molecular cloning of APRF, a novel IFN-stimulated gene factor 3
p91-related transcription factor involved in the gp130-mediated
signaling pathway. Cell. 77:63–71. 1994.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sakata H, Narasimhan P, Niizuma K, Maier
CM, Wakai T and Chan PH: Interleukin 6-preconditioned neural stem
cells reduce ischaemic injury in stroke mice. Brain. 135:3298–3310.
2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Monsour M, Croci DM, Agazzi S and
Borlongan CV: Contemplating IL-6, a double-edged sword cytokine:
Which side to use for stroke pathology. CNS Neurosci Ther.
29:493–497. 2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Cohen T, Nahari D, Cerem LW, Neufeld G and
Levi BZ: Interleukin 6 induces the expression of vascular
endothelial growth factor. J Biol Chem. 271:736–741.
1996.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ley K: The role of selectins in
inflammation and disease. Trends Mol Med. 9:263–268.
2003.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Choi BR, Johnson KR, Maric D and McGavern
DB: Monocyte-derived IL-6 programs microglia to rebuild damaged
brain vasculature. Nat Immunol. 24:1110–1123. 2023.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Frijns CJ and Kappelle LJ: Inflammatory
cell adhesion molecules in ischemic cerebrovascular disease.
Stroke. 33:2115–2122. 2002.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Cheng D and Wang Y, Li J, Yao Y, Zhang S
and Wang Y: Transcriptomic analysis identifies the S100
calcium-binding protein β subunit (S100B) and intercellular
adhesion molecule-1 (ICAM-1) as potential diagnostic biomarkers for
acute cerebral infarction. Genes Dis. 11:46–48. 2023.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Henninger DD, Panés J, Eppihimer M,
Russell J, Gerritsen M, Anderson DC and Granger DN:
Cytokine-induced VCAM-1 and ICAM-1 expression in different organs
of the mouse. J Immunol. 158:1825–1832. 1997.PubMed/NCBI
|
|
47
|
Kim I, Moon SO, Kim SH, Kim HJ, Koh YS and
Koh GY: Vascular endothelial growth factor expression of
intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion
molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B
activation in endothelial cells. J Biol Chem. 276:7614–7620.
2001.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Rüegg C, Postigo AA, Sikorski EE, Butcher
EC, Pytela R and Erle DJ: Role of integrin alpha 4 beta 7/alpha 4
beta P in lymphocyte adherence to fibronectin and VCAM-1 and in
homotypic cell clustering. J Cell Biol. 117:179–189.
1992.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Langston W, Chidlow JH Jr, Booth BA,
Barlow SC, Lefer DJ, Patel RP and Kevil CG: Regulation of
endothelial glutathione by ICAM-1 governs VEGF-A-mediated eNOS
activity and angiogenesis. Free Radic Biol Med. 42:720–729.
2007.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Nakao S, Kuwano T, Ishibashi T, Kuwano M
and Ono M: Synergistic effect of TNF-alpha in soluble
VCAM-1-induced angiogenesis through alpha 4 integrins. J Immunol.
170:5704–5711. 2003.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ibli SI, Hu J, Looso M, Weigert A, Ratiu
C, Wittig J, Drekolia MK, Tombor L, Randriamboavonjy V, Leisegang
MS, et al: Mapping the endothelial cell S-sulfhydrome highlights
the crucial role of integrin sulfhydration in vascular function.
Circulation. 143:935–948. 2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Bi JJ and Yi L: Effects of integrins and
integrin αvβ3 inhibitor on angiogenesis in cerebral ischemic
stroke. J Huazhong Univ Sci Technolog Med Sci. 34:299–305.
2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Huang Q, Chen B, Wang F, Huang H, Milner R
and Li L: The temporal expression patterns of fibronectin and its
receptors-α5β1 and αvβ3 integrins on blood vessels after cerebral
ischemia. Restor Neurol Neurosci. 33:493–507. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Pang D, Wang L, Dong J, Lai X, Huang Q,
Milner R and Li L: Integrin α5β1-Ang1/Tie2 receptor cross-talk
regulates brain endothelial cell responses following cerebral
ischemia. Exp Mol Med. 50:1–12. 2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zhang X, Wang L, Han Z, Dong J, Pang D, Fu
Y and Li L: KLF4 alleviates cerebral vascular injury by
ameliorating vascular endothelial inflammation and regulating tight
junction protein expression following ischemic stroke. J
Neuroinflammation. 17(107)2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Jeucken KCM, van Rooijen CCN, Kan YY,
Kocken LA, Jongejan A, van Steen ACI, van Buul JD, Olsson HK, van
Hamburg JP and Tas SW: Differential contribution of NF-κB signaling
pathways to CD4+ Memory T cell induced activation of endothelial
cells. Front Immunol. 13(860327)2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Xie Y, Sun Y, Liu Y, Zhao J, Liu Q, Xu J,
Qin Y, He R, Yuan F, Wu T, et al: Targeted delivery of
RGD-CD146+CD271+ human umbilical cord
mesenchymal stem cell-derived exosomes promotes blood-spinal cord
barrier repair after spinal cord injury. ACS Nano. 17:18008–18024.
2023.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lin Y, Gil CH, Banno K, Yokoyama M, Wingo
M, Go E, Prasain N, Liu Y, Hato T, Naito H, et al: ABCG2-expressing
clonal repopulating endothelial cells serve to form and maintain
blood vessels. Circulation. 150:451–465. 2024.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Wang L, Li J, Wang Y, Ge C, Huang Q, Li L,
Wang N, Chen Y, Zhou X, Chang D, et al: Dan-Deng-Tong-Nao softgel
capsule promotes angiogenesis of cerebral microvasculature to
protect cerebral ischemia reperfusion injury via activating
HIF-1α-VEGFA-Notch1 signaling pathway. Phytomedicine.
118(154966)2023.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Ran X, Xu T, Ruan H, Wang X and Zhang Q:
Tissue Kallikrein supplementation in ischemic phase protects the
neurovascular unit and attenuates reperfusion-induced injury in
ischemic stroke. Pharmacol Res. 209(107435)2024.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Peiretti F, Alessi MC, Henry M, Anfosso F,
Juhan-Vague I and Nalbone G: Intracellular calcium mobilization
suppresses the TNF-alpha-stimulated synthesis of PAI-1 in human
endothelial cells. Indications that calcium acts at a translational
level. Arterioscler Thromb Vasc Biol. 17:1550–1560. 1997.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Panthiya L, Tocharus J, Onsa-Ard A,
Chaichompoo W, Suksamrarn A and Tocharus C: Hexahydrocurcumin
ameliorates hypertensive and vascular remodeling in L-NAME-induced
rats. Biochim Biophys Acta Mol Basis Dis.
1868(166317)2022.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Zhang G, Qin Q, Zhang C, Sun X, Kazama K,
Yi B, Cheng F, Guo ZF and Sun J: NDRG1 signaling is essential for
endothelial inflammation and vascular remodeling. Circ Res.
132:306–319. 2023.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Bischoff-Kont I, Primke T, Niebergall LS,
Zech T and Fürst R: Ginger constituent 6-shogaol inhibits
inflammation- and angiogenesis-related cell functions in primary
human endothelial cells. Front Pharmacol. 13(844767)2022.PubMed/NCBI View Article : Google Scholar
|