Introduction
Harlequin syndrome (HS), initially reported by Lance
et al in 1988, is a rare autonomic disorder caused by
hemifacial cutaneous sympathetic denervation (1,2,3). It
is characterized by semi-facial sweating and discoloration, sharply
demarcated at the midline with normal ocular sympathetic
innervation (3). It is mainly
idiopathic in nature, often triggered by exercise, emotions or
exposure to heat. HS is used to describe the transient hemi body
flushing often observed in premature neonates due to immaturity of
the hypothalamic centers and vasomotor instability (5). Congenital cases represent ~6% of all
the patients with HS (3,6). However, it can also be a
manifestation of underlying cervical and upper thoracic pathologies
resulting in the compression of these sympathetic fibers or
iatrogenic from the surgical or anesthetic procedures around the
neck. The present study revealed, to the best of the authors'
knowledge, the first case of an oncologic patient with HS as a
result of pleural metastasectomy with video-assisted thoracic
surgery (VATS).
Case report
A 28-year-old woman with a history of metastatic
osteosarcoma was presented in Attikon University Hospital (Athens,
Greece) with 5 days of worsening dyspnea, in September 2023.
Computed tomography (CT) revealed new metastatic lung lesions. She
had received neoadjuvant methotrexate, doxorubicin and cisplatin
(MAP) (7) in October 2020 and
undergone surgical removal of the tumoral mass of the right
proximal humerus in February 2021. Histopathological examination
from the lesion was suggestive of low-grade osteosarcoma (mdm2
amplified). Afterwards the female patient completed adjuvant
treatment with MAP and Mifamurtide. In July 2022 contrast-enhanced
CT thorax revealed new pleural nodules located mainly on left upper
lobe. Pleural biopsy confirmed disease progression. After receiving
three cycles of high dose ifosfamide (8) in September 2022, progression was
reported with pleural metastatic lesions and clinical worsening.
VATS metastasectomy was decided by the multidisciplinary team and
the female patient underwent partial left pneumonectomy,
pericardiectomy and pleurectomy in January 2023. The female patient
was then enrolled in phase IV clinical trial and had already
received three cycles of cabozatinib when presented in Attikon
University Hospital (Athens, Greece) with new metastatic lung
lesions and new onset dyspnea in September 2023.
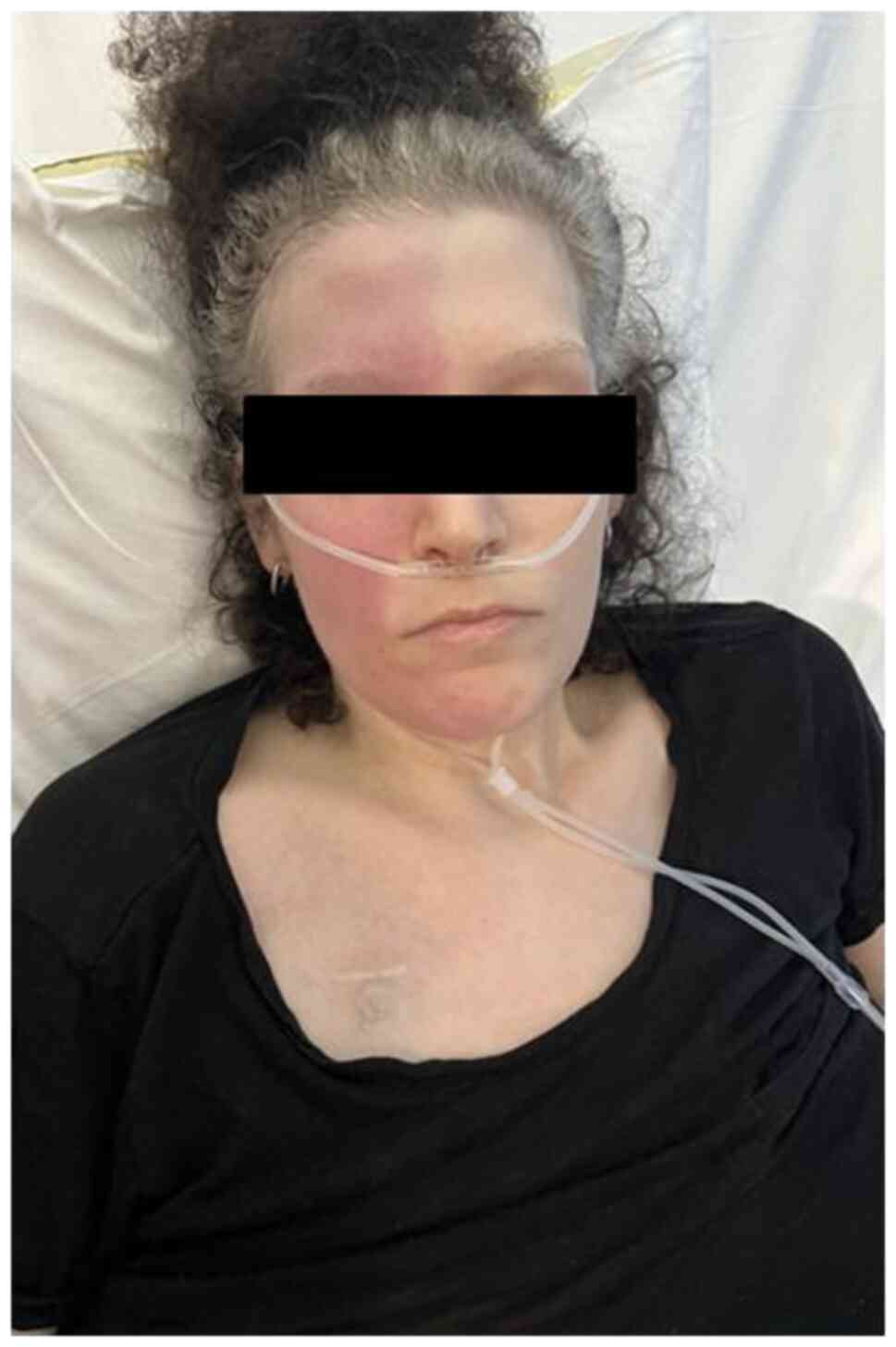
Following emotional distress, on the third day of
admission, the female patient presented with right-sided facial
flushing and profuse sweating with the left side of the face
remaining pale and dry (Fig. 1).
The symptoms were resolved spontaneously after 15 min. The physical
examination did not reveal any neurological alterations. No
ophthalmological abnormalities were observed. Dermatological
examination at rest revealed no abnormalities. Routine laboratory
studies and carotid artery ultrasonography did not reveal any
pathological findings. Pre-operative and post-operative
contrast-enhanced CT images illustrated the anatomical region where
the iatrogenic injury may have occurred and the diagnosis of
iatrogenic HS was considered (Fig.
2A-C) (9). The patient
succumbed after 2 days and did not experience any further episodes
of Harlequin syndrome prior to their passing.
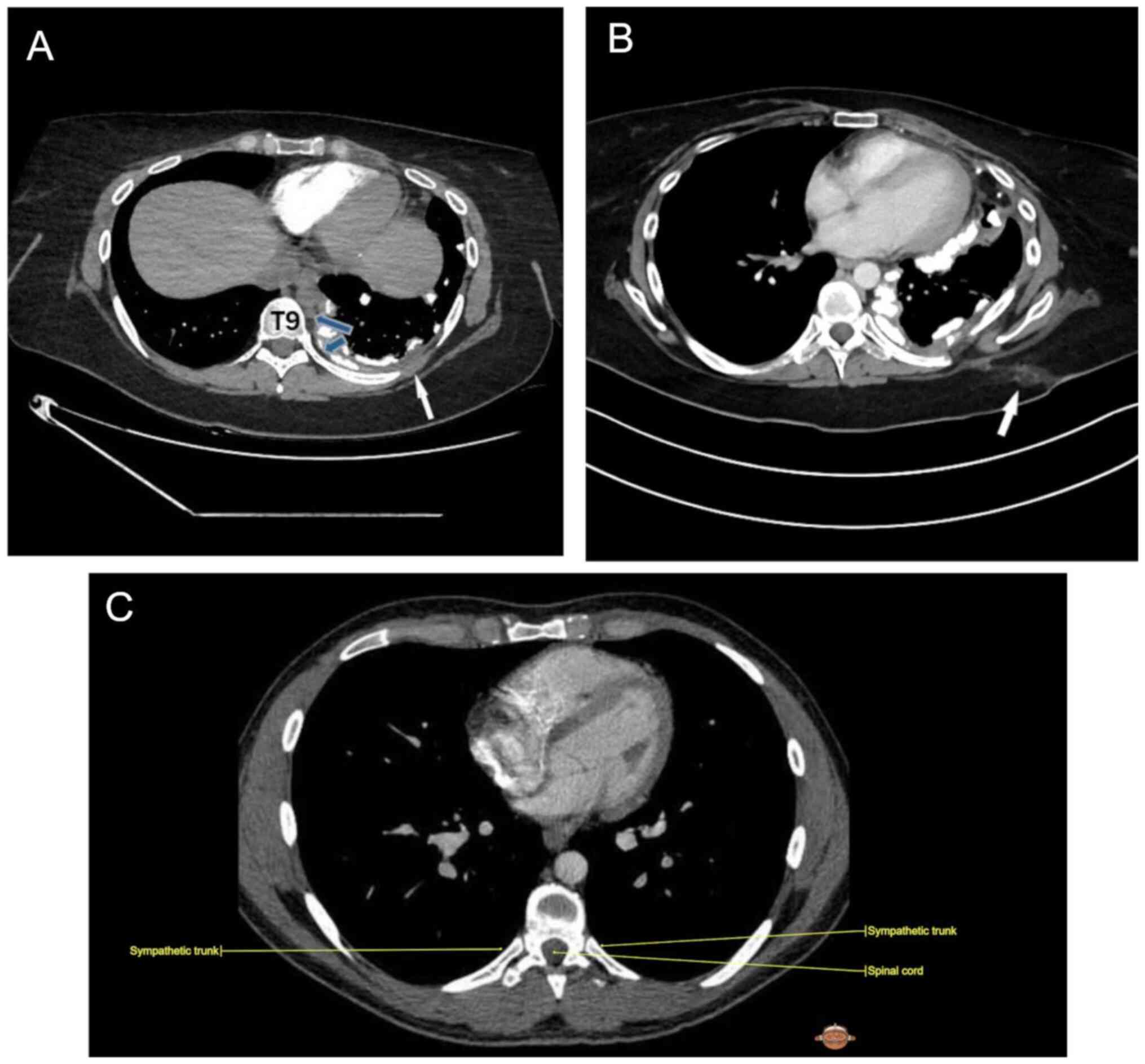 | Figure 2(A) Pre-operative contrast-enhanced
CT image, axial section soft tissue window, demonstrates
circumferential and nodular pleural thickening (white arrow)
predominantly along the left lateral pleural space including
mediastinum pleural surface along with extensive calcifications,
representing osteosarcoma pleural metastasis; blue arrows reveal
sympathetic chain. (B) Post-operative contrast-enhanced CT image,
axial section soft tissue window, demonstrates post-operative
changes in the subcutaneous fat of the left hemithorax (arrow) in
8th to 9th intercostal space. (C) Anatomic image of sympathetic
track in 8th intercostal place; Courtesy of IMAIOS ©️ ‘Micheau A,
Hoa D, e-Anatomy, www.imaios.com, DOI: 10.37019/e-anatomy’. CT,
computed tomography. |
Discussion
The 28-year-old female patient represented an
example of probable iatrogenic HS. Localization of the lesion in HS
must be based on both the patient's clinical history and her
constellation of symptoms.
The pathology can manifest at any point along the
sympathetic outflow to the face. The second neuron (preganglionic
fibers) leaves the spinal cord at T2-T3 and synapse with the third
neuron (post-ganglionic fibers) in the superior cervical ganglion.
Post-ganglionic fibers that supply the medial forehead and nose
travel with the internal carotid artery, while fibers for other
facial areas and the neck travel with the external carotid artery
(10).
HS is caused by the unilateral blockade of the T2-T3
fibers carrying sudomotor and vasomotor supply to the face. VATS
pleurectomy can rarely cause damage to the thoracic sympathetic
chain (11). However, the possible
mechanism of damage in the present case might be the utilization of
diathermy or mechanical disruption (12). This method has been historically
employed for treating palmar and axillary hyperhidrosis by
interrupting the upper thoracic sympathetic chain (13).
Regarding causes of HS, ~54.6% of reported cases
have idiopathic etiology, while in 45.4% of cases, the
HS-associated symptomatology occurred secondarily, concurring with
other autonomic nervous system impairments or iatrogenic (14). In adults, a majority of HS cases
lack a discernible medical cause, with one-third attributed to
expanding neoplasms or iatrogenic damage (15).
The iatrogenic origin of HS has been documented in
several cases. Fringeli et al (16) reported HS in a 55-year-old female
patient with large median and right paramedian disc hernia-induced
right C7 radiculopathy following C6-C7 fusion discectomy and
additional anterior spondylodesis. Visible HS-suggestive symptoms
on the right side of the face (flushing and excessive sweating),
and contralateral facial anhidrosis, miosis and ptosis, were noted
similar to the first case reported (3). Similarly, Sullivan et al
(17) noted HS symptoms in a
female patient post-T3 erector spinae plane block for radical
mastectomy and axillary dissection, while Burlacu and Buggy
described HS and Horner syndrome coexisting after left mastectomy
and immediate latissimus dorsi reconstruction (18). Post-operative malignancies have
also been linked to HS, such as in a case involving a 72-year-old
man with squamous non-small cell lung cancer, where axillary and
supraclavicular lymph node enlargement exerted compressive actions
on nearby vascular structures, including carotid artery, the
internal jugular vein and subclavian artery (19,20).
Another possible iatrogenic cause of HS has been reported by Van
Slycke et al (21) in a
74-year-old woman who underwent compressive retrosternal goiter
thyroidectomy. Post-operation, patients reported recurring sudden
left-sided facial flushing and sweating triggered by emotional
changes, physical exertion, or heat. In some cases, HS could fully
remit with no further relapses.
Treatment of HS typically depends on the severity of
symptoms and the underlying cause. For most patients, HS is benign
and does not require intervention. In cases where HS persists,
treatment strategies are often tailored to the underlying condition
or predisposing factor, especially if symptoms cause significant
distress or social embarrassment. Stellate ganglion block (22), botulinum toxin injections to
regulate sweating and other symptoms (23), surgery or radiation to remove a
tumor or lesion (24) have been
employed to manage symptoms of HS. The 28-year-old female patient
of the present case report did not have oculomotor changes (ptosis
or miosis), or upper extremity sudomotor or vasomotor changes. This
indicates that T1 and T4 remained unaffected, thereby locating the
sympathetic injury to the second or third thoracic segments of the
spinal cord. Since this incident happened 8 months post left
pleurectomy this indicates an iatrogenic HS. The present study
reports, to the best of the authors' knowledge, the first case of a
patient with HS as a result of pleural metastasectomy. HS case
reports published in PubMed (https://pubmed.ncbi.nlm.nih.gov/) are summarized in
Table I.
 | Table IHS case reports in PubMed
database. |
Table I
HS case reports in PubMed
database.
| First author/s,
year | Title | (Refs.) |
|---|
| Sharma et
al, 2023 | HS and autonomic
seizures - a rare association | (25) |
| Navickaitė et
al, 2023 |
Sarcoidosis-associated sensory
ganglionopathy and HS: A case report | (26) |
| Karam et al,
2023 | HS during
peripheral cardiopulmonary bypass in a patient with an obstructing
tracheal schwannoma: A case report | (27) |
| Melka et al,
2023 | Idiopathic HS in a
patient from Ethiopia: A case report | (28) |
| Giunta et
al, 2023 | Management of HS
Under ECPELLA support: A report of two cases and a proposed
approach | (29) |
| Hylton et
al, 2022 | HS as a
complication of epidural anesthesia in an infant: Do adjunct
medications play a role? | (30) |
| Dalldorf et
al, 2022 | HS following
regional liposomal bupivacaine use in a partial sternectomy | (31) |
| Yan et al,
2022 | HS induced by
intraspinal analgesia in patients with advanced cancer: a case
report | (32) |
| Korbi et al,
2022 | Harlequin syndrome:
An asymmetric face | (33) |
| Persson et
al, 2022 | HS associated with
thoracic epidural anesthesia | (34) |
| Schultz et
al, 2020 | HS following
microwave ablation in a child with a symptomatic paraspinal
mass | (35) |
| Wagner et
al, 2019 | HS after
thoracoscopic repair of a child with tracheoesophageal fistula | (12) |
| Tanaka et
al, 2019 | Cardiac sympathetic
hyperactivity of lung cancer-associated HS | (36) |
| Elboukhar et
al, 2019 | Idiopathic HS: A
case report and literature review | (37) |
| Pasrija et
al, 2019 | HS during
venoarterial extracorporeal membrane oxygenation | (38) |
| Hans-Bittner et
al, 2018 | Do you know this
syndrome? HS | (39) |
| Lee et al,
2017 | HS and Horner
syndrome after neck schwannoma excision in a pediatric patient | (40) |
| Lefevre et
al, 2017 | Development of HS
following placement of thoracic epidural anesthesia in a pediatric
patient undergoing Nuss procedure | (41) |
| Jeon et al,
2017 | HS following
resection of mediastinal ganglioneuroma | (42) |
| Algahtani et
al, 2017 | Idiopathic HS
manifesting during exercise: A case report and review of the
literature | (43) |
| Al Hanshi et
al, 2017 | A case study of HS
in VA-ECMO | (44) |
| Kim et al,
2016 | A pediatric case of
idiopathic HS | (45) |
| Naqvi et al,
2016 | HS with
contralateral anhidrosis after an upper chest gunshot wound | (46) |
| Jung et al,
2015 | Iatrogenic HS: A
new case | (47) |
| Emsley et
al, 2013 | Post-exertional HS
with spontaneous improvement | (48) |
| Breunig et
al, 2012 | HS in childhood -
Case report | (49) |
| Pradeep et
al, 2011 | HS in a case of
toxic goitre: A rare association | (50) |
| Moon et al,
2005 | HS with crossed
sympathetic deficit of the face and arm | (51) |
| Fallon et
al, 2005 | HS in two
athletes | (52) |
| Lombardi et
al, 2004 | HS: An association
with overlap parasomnia | (53) |
| Swan et al,
2003 | Iatrogenic HS | (54) |
| Corbett et
al, 1999 | HS | (55) |
| Noda et al,
1991 | HS due to superior
mediastinal neurinoma | (56) |
| Lance et al,
1988 | HS: The sudden
onset of unilateral flushing and sweating | (3) |
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MM and AK conceptualized the present study,
performed the methodology, and wrote/prepared the original draft.
AP, IK, PE, EE contributed to interpretation of the data. MK, AB,
NG, EZ, KM contributed to acquisition of data. AP supervised the
present study. AP, IK, PE, EE, MK, AB, NG, EZ and KM wrote,
reviewed and edited the manuscript. All authors read and approved
the final version of the manuscript. MM and AK confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Written informed consent was obtained from the legal
guardian for patient participation in the present study.
Patient consent for publication
Patient characteristics have been anonymized in
compliance with ethical standards. Written informed consent was
obtained from the next of kin for the publication of any associated
images or patient data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cheshire WP Jr and Low PA: Harlequin
syndrome: Still only half understood. J Neuroophthalmol.
28:169–170. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bremner F and Smith S: Pupillographic
findings in 39 consecutive cases of harlequin syndrome. J
Neuroophthalmol. 28:171–177. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lance JW, Drummond PD, Gandevia SC and
Morris JG: Harlequin syndrome: The sudden onset of unilateral
flushing and sweating. J Neurol Neurosurg Psychiatry. 51:635–642.
1988.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tascilar N, Tekin NS, Erdem Z, Alpay A and
Emre U: Unnoticed dysautonomic syndrome of the face: Harlequin
syndrome. Auton Neurosci. 137:1–9. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Drummond PD and Finch PM: Reflex control
of facial flushing during body heating in man. Brain. 112 (Pt
5):1351–1358. 1989.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Drummond PD and Edis RH: Loss of facial
sweating and flushing in Holmes-Adie syndrome. Neurology.
40:847–849. 1990.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yu D, Zhang S, Feng A, Xu D, Zhu Q, Mao Y,
Zhao Y, Lv Y, Han C, Liu R and Tian Y: Methotrexate, doxorubicin,
and cisplatinum regimen is still the preferred option for
osteosarcoma chemotherapy: A meta-analysis and clinical
observation. Medicine (Baltimore). 98(e15582)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sanfilippo R, Bertulli R, Marrari A,
Fumagalli E, Pilotti S, Morosi C, Messina A, Dei Tos AP, Gronchi A
and Casali PG: High-dose continuous-infusion ifosfamide in advanced
well-differentiated/dedifferentiated liposarcoma. Clin Sarcoma Res.
4(16)2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Micheau A HD. e-Anatomy.
|
|
10
|
Vidal Esteban A, Natera-de Benito D,
Martínez Sánchez D, Reche Sainz A, Rodríguez Díaz MR, Alfaro
Iznaola CM and de Santos Moreno MT: Congenital Harlequin syndrome
as an isolated phenomenon: A case report and review of the
literature. Eur J Paediatr Neurol. 20:426–430. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
van Brakel TJ and Barendregt WB: Wet and
dry hands after video-assisted thoracoscopic pleurectomy. Interact
Cardiovasc Thorac Surg. 16:563–564. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wagner R, Lacher M, Merkenschlager A and
Markel M: Harlequin syndrome after thoracoscopic repair of a child
with tracheoesophageal fistula (TEF). European J Pediatr Surg Rep.
7:e63–e65. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sugimura H, Spratt EH, Compeau CG, Kattail
D and Shargall Y: Thoracoscopic sympathetic clipping for
hyperhidrosis: long-term results and reversibility. J Thorac
Cardiovasc Surg. 137:1370–6; discussion 1376-7. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mavroudis I, Balmus IM, Ciobica A, Luca
AC, Chowdhury R, Iordache AC, Gorgan DL and Radu I: Mini-review on
the harlequin syndrome-a rare dysautonomic manifestation requiring
attention. Medicina (Kaunas). 58(938)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Willaert WI, Scheltinga MR, Steenhuisen SF
and Hiel JA: Harlequin syndrome: Two new cases and a management
proposal. Acta Neurol Belg. 109:214–220. 2009.PubMed/NCBI
|
|
16
|
Fringeli Y, Humm AM, Ansorge A and
Maestretti G: Harlequin sign concomitant with Horner syndrome after
anterior cervical discectomy: A case of intrusion into the cervical
sympathetic system. J Neurosurg Spine. 26:684–687. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sullivan TR, Kanda P, Gagne S and Costache
I: Harlequin Syndrome associated with erector spinae plane block.
Anesthesiology. 131(665)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Burlacu CL and Buggy DJ: Coexisting
harlequin and Horner syndromes after high thoracic paravertebral
anaesthesia. Br J Anaesth. 95:822–824. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Biondi A, Persiani R, Zoccali M, Rausei S,
Cananzi F and D'Ugo D: Harlequin syndrome. Ann Thorac Surg.
88(304)2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tubbs RS, Salter G, Wellons JC III and
Oakes WJ: Blood supply of the human cervical sympathetic chain and
ganglia. Eur J Morphol. 40:283–288. 2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Van Slycke S, Stockman A, Dionigi G,
Carette R, Gillardin JP, Brusselaers N and Vermeersch H: Harlequin
syndrome after thyroidectomy for compressive retrosternal goiter.
case report and review of the literature. Acta Chir Belg.
114:212–214. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Reddy H, Fatah S, Gulve A and Carmichael
AJ: Novel management of harlequin syndrome with stellate ganglion
block. Br J Dermatol. 169:954–956. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Manhães RK, Spitz M and Vasconcellos LF:
Botulinum toxin for treatment of Harlequin syndrome. Parkinsonism
Relat Disord. 23:112–113. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kirk F, Crathern K and Stroebel A:
Video-assisted thoracoscopic sympathectomy for Harlequin syndrome.
Eur J Cardiothorac Surg. 63(ezac577)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sharma B, Sharma P, Ahuja I and Pemawat A:
Harlequin syndrome and autonomic seizures-A rare association. Ann
Indian Acad Neurol. 26:839–841. 2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Navickaitė I, Ališauskienė M, Petrauskienė
S and Žemgulytė G: Sarcoidosis-associated sensory ganglionopathy
and harlequin syndrome: A case report. Medicina (Kaunas).
59(1495)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Karam C, Abou Nafeh N, Aouad MT,
Siddik-Sayyid S, Kaddoum R, Zeeni C, Anka S, Shaya B and Khalili A:
Harlequin syndrome during peripheral cardiopulmonary bypass in a
patient with an obstructing tracheal schwannoma: A case report.
Clin Case Rep. 11(e7509)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Melka D and Zebenigus M: Idiopathic
harlequin syndrome in a patient from Ethiopia: A case report.
Ethiop J Health Sci. 33:383–386. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Giunta M, Recchia EG, Capuano P, Toscano
A, Attisani M, Rinaldi M and Brazzi L: Management of harlequin
syndrome under ECPELLA support: A report of two cases and a
proposed approach. Ann Card Anaesth. 26:97–101. 2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hylton JRE: Harlequin syndrome as a
complication of epidural anaesthesia in an infant: Do adjunct
medications play a role? Indian J Anaesth. 66:669–672.
2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dalldorf DA, Hart A, Grant SA and Teeter
EG: Harlequin syndrome following regional liposomal bupivacaine use
in a partial sternectomy. Cureus. 14(e28005)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yan R, Dang C and Yuan W: Harlequin
syndrome induced by intraspinal analgesia in patients with advanced
cancer: A case report. Transl Cancer Res. 11:2457–2461.
2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Korbi M, Boumaiza S, Achour A, Belhadjali
H and Zili J: Harlequin syndrome: An asymmetric face. Clin Case
Rep. 10(e05833)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Persson RM, Tellnes K, Hoven H, Haaverstad
R and Svendsen ØS: Harlequin syndrome associated with thoracic
epidural anaesthesia. Anaesth Rep.
10(10.1002/anr3.12144)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Schultz L, Mackarey A, Oh C and Kent P:
Harlequin syndrome following microwave ablation in a child with a
symptomatic paraspinal mass. BMJ Case Rep.
13(e232700)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tanaka Y and Satomi K: Cardiac sympathetic
hyperactivity of lung cancer-associated harlequin syndrome. JMA J.
2:190–191. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Elboukhari K, Baybay H, Elloudi S, Douhi Z
and Mernissi FZ: Idiopathic harlequin syndrome: A case report and
literature review. Pan Afr Med J. 33(141)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pasrija C, Bedeir K, Jeudy J and Kon ZN:
Harlequin syndrome during venoarterial extracorporeal membrane
oxygenation. Radiol Cardiothorac Imaging. 1(e190031)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hans-Bittner NR, Bittner GC and Hans Filho
G: Do you know this syndrome? Harlequin syndrome. An Bras Dermatol.
93:585–586. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lee DH, Seong JY, Yoon TM, Lee JK and Lim
SC: Harlequin syndrome and Horner syndrome after neck schwannoma
excision in a pediatric patient: A case report. Medicine
(Baltimore). 96(e8548)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lefevre A and Schnepper G: Development of
harlequin syndrome following placement of thoracic epidural
anesthesia in a pediatric patient undergoing Nuss procedure. Clin
Case Rep. 5:1523–1525. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Jeon YJ, Son J and Cho JH: Harlequin
syndrome following resection of mediastinal ganglioneuroma. Korean
J Thorac Cardiovasc Surg. 50:130–132. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Algahtani H, Shirah B, Algahtani R and
Alkahtani A: Idiopathic harlequin syndrome manifesting during
exercise: A case report and review of the literature. Case Rep Med.
2017(5342593)2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Al Hanshi SAM and Al Othmani F: A case
study of Harlequin syndrome in VA-ECMO. Qatar Med J.
2017(39)2017.
|
|
45
|
Kim JY, Lee MS, Kim SY, Kim HJ, Lee SJ,
You CW, Kim JS and Kang JH: A pediatric case of idiopathic
Harlequin syndrome. Korean J Pediatr. 59 (Suppl 1):S125–S128.
2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Naqvi SY, Zainal A, Flood SP and Kinniry
P: Harlequin syndrome with contralateral anhidrosis after an upper
chest gunshot wound. BMJ Case Rep.
2016(bcr2016216931)2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Jung JM, Lee MH, Won CH, Chang SE, Lee MW,
Choi JH and Moon KC: Iatrogenic harlequin syndrome: A new case. Ann
Dermatol. 27:101–102. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Emsley HC: Postexertional harlequin
syndrome with spontaneous improvement. BMJ Case Rep.
2013(bcr2013200516)2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Breunig Jde A, Hartmann M, Freire CF and
de Almeida HL Jr: Harlequin syndrome in childhood-case report. An
Bras Dermatol. 87:907–909. 2012.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Pradeep PV, Benede AK, Harshita SS and
Jayashree B: Harlequin syndrome in a case of toxic goitre: A rare
association. Case Rep Med. 2011(293076)2011.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Moon SY, Shin DI, Park SH and Kim JS:
Harlequin syndrome with crossed sympathetic deficit of the face and
arm. J Korean Med Sci. 20:329–330. 2005.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Fallon KE and May JJ: Harlequin syndrome
in two athletes. Br J Sports Med. 39(e1)2005.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Lombardi C, Vetrugno R, Provini F, Plazzi
G, Pierangeli G, Coccagna G, Lugaresi E, Montagna P and Cortelli P:
Harlequin syndrome: An association with overlap parasomnia. J
Neurol Neurosurg Psychiatry. 75:341–342. 2004.PubMed/NCBI
|
|
54
|
Swan MC, Nicolaou M and Paes TR:
Iatrogenic harlequin syndrome. Postgrad Med J.
79(278)2003.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Corbett M and Abernethy DA: Harlequin
syndrome. J Neurol Neurosurg Psychiatry. 66(544)1999.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Noda S: Harlequin syndrome due to superior
mediastinal neurinoma. J Neurol Neurosurg Psychiatry.
54(744)1991.PubMed/NCBI View Article : Google Scholar
|