Introduction
Sunitinib is an oral multi-tyrosine kinase inhibitor
(TKI) used as a first-line treatment of metastatic renal cell
carcinoma (mRCC). It is primarily metabolized by CYP3A4 to an
active metabolite, N-desethyl sunitinib, which has kinase
inhibitory activity similar to that of sunitinib. Sunitinib and
N-desetyl sunitinib contribute to therapeutic efficacy and
the occurrence of adverse events (AEs) (1-6).
Therefore, in clinical practice, the target trough concentration
for sunitinib treatment planning is set at 50-100 ng/ml as the
total sunitinib concentration, which is the sum of sunitinib and
N-desethyl sunitinib concentrations (7,8).
Given that CYP3A4 is also involved in the metabolism of sunitinib
to its inactive metabolites, a combination of drugs that affect
CYP3A4 activity should be considered with caution. On the other
hand, although, a study on drug-drug interactions in rats reported
that the combination of ketoconazole, a CYP3A4 inhibitor, and
sunitinib significantly increased the concentration of sunitinib
(9), the effects of drug
combinations with moderate or weak CYP3A4 inhibitors remain
unclear. Furthermore, while the combination of TKIs may be highly
effective, their efficacy and safety are unexplored and therefore
not recommended. We report the case of a patient undergoing
sunitinib therapy for mRCC who was diagnosed with chronic myeloid
leukemia (CML), and was simultaneously treated with sunitinib and
dasatinib, a multi-TKI with moderate CYP3A4 inhibition (10,11)
for CML.
Case report
A 55-year-old Japanese man visited the Department of
Urology at Gunma University Hospital as an outpatient for treatment
of mRCC. The patient had undergone right nephrectomy for RCC at
another hospital 19 years prior to the outpatient visit, and the
pathology results at that time showed clear cell RCC of the right
kidney, G1>G2. The International mRCC Database Consortium risk
classification at the time of diagnosis indicated a favorable risk.
After nephrectomy, the patient was treated with postoperative
interferon therapy for RCC according to the RCC guidelines in Japan
at that time; however, metastases in the lymph nodes, pancreas, and
liver were observed. Therefore, sunitinib treatment was initiated
four years prior to the outpatient visit. Sunitinib at the Japanese
standard dose of 37.5 mg/day for mRCC. Due to progressive
metastatic growth, the sunitinib dose was increased to 50 mg/day,
thus achieving disease control. Sunitinib was administered in
14-day on/7 day-off cycles. His medical history included mRCC,
diabetes mellitus, Graves' disease, hypertension, and
hyperlipidemia, all of which were controlled with drug therapy
(sitagliptin phosphate hydrate, miglitol, azilsartan, amlodipine
besylate, and atorvastatin calcium hydrate).
During a routine follow-up blood test, an increase
in white blood cell (WBC) and platelet counts (PLT) was observed
(WBC 17.3x103/µl and PLT 601x103/µl).
Cytogenetic analysis identified a 46, XY, t (9; 22) (q34; q11.2)
karyotype (Fig. 1), and genetic
testing using real-time PCR conducted by SRL (Fukuoka, Japan), an
external laboratory with clinical laboratory accreditation,
detected major BCR-ABL fusion mRNA. Fluorescence in situ
hybridization showed 98.0% positivity for the BCR-ABL1 fusion
signal (Fig. 2). Based on these
finding, the patient was diagnosed with chronic-phase CML.
Treatment with dasatinib was initiated to prevent progression to
the acute phase due to rising blast counts. In addition, it was
difficult to reduce or discontinue sunitinib because the disease
had previously progressed with administration of low-dose
sunitinib. At that time, pembrolizumab had not yet been approved,
and while nivolumab had been approved as an alternative, the
clinical team preferred to reserve nivolumab for potential future
use in case sunitinib became ineffective. Given the demonstrated
efficacy of sunitinib at the increased dose, it was decided to
continue sunitinib as the primary treatment for mRCC. After
multidisciplinary discussions between the urology and hematology
teams, it was decided to implement concurrent therapy with
sunitinib and dasatinib. Sunitinib and dasatinib share similar
mechanisms of action, raising concerns about the potential risk of
overlapping toxicities. Furthermore, the moderate CYP3A4 inhibitory
effect of dasatinib posed an additional risk of pharmacokinetic
interactions between the two drugs. Considering these points, the
attending physician and medical staff determined that the risks
could be effectively managed by closely monitoring the patient's
clinical condition and implementing therapeutic drug monitoring
(TDM) of sunitinib, a recognized TDM target drug in Japan, to
ensure safe and effective administration.
At the start of dasatinib administration (day 1),
his height and weight were 172 cm and 68.2 kg, respectively, and
laboratory data were serum creatinine level 1.11 mg/dl, estimated
glomerular filtration rate (eGFR) level 53.7 ml/min/1.73
m2, aspartate aminotransferase level 17 U/IL, alanine
aminotransferase level 15 U/IL, and total bilirubin level 0.4
mg/dl, without detectable abnormalities in renal and hepatic
function. The combination drugs included sunitinib (50 mg/day, 14
days on/7 days off), bifidobacteria powder, heparinoid cream,
sitagliptin phosphate hydrate, miglitol, azilsartan, amlodipine
besylate, atorvastatin calcium hydrate, and esomeprazole magnesium
hydrate, and no change in medications taken in the past month. A
hand-foot skin reaction (HFSR) and diarrhea (both CTCAE grade 1)
occurred on day -30 and were controlled using heparinoid cream and
bifidobacterial powder. No other AEs were detected. The total
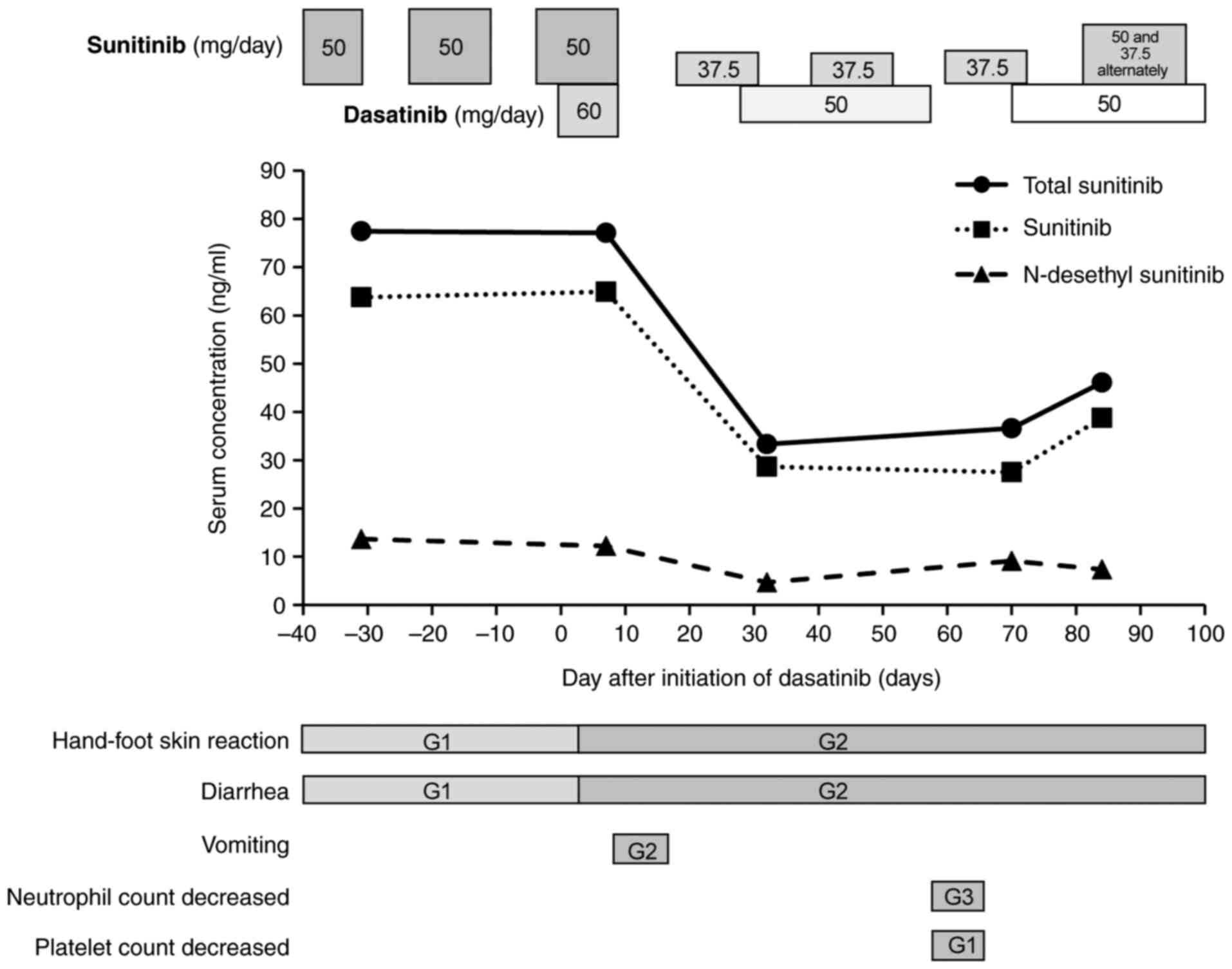
sunitinib trough serum concentration immediately before starting
dasatinib treatment was 77.4 ng/ml (sunitinib: 63.7 ng/ml,
N-desethyl sunitinib: 13.7 ng/ml, Fig. 3).
The initial dose of dasatinib was set at 60 mg/day
(60% dose) at the hematologist's discretion, considering the risk
of worsening of comorbidities such as diabetes mellitus and
abdominal aortic aneurysm, and drug-drug interactions between
sunitinib and dasatinib. After starting dasatinib treatment, the
HFSR worsened to CTCAE grade 2 on day 7, and treatment with
betamethasone butyrate propionate ointment was initiated. Sunitinib
and N-desethyl sunitinib concentrations were measured on day
7, considering the risk of drug-drug interactions between sunitinib
and dasatinib. The total sunitinib trough serum concentration was
77.1 ng/ml (sunitinib: 64.9 ng/ml, N-desethyl sunitinib:
12.2 ng/ml), and no change in sunitinib serum concentration was
observed after starting the dasatinib combination therapy (Fig. 3). CTCAE grade 2 vomiting and grade
2 diarrhea occurred on day 8 and dasatinib treatment was paused on
day 9.
Although vomiting and diarrhea were subsequently
controlled, due to the risk of severe AEs occurring with the
combination of sunitinib and dasatinib, the sunitinib dose was
changed to 37.5 mg/day (one dose reduction level, 14 days on/7 days
off) on day 18 in accordance with the Japanese package insert of
sunitinib. As vomiting and diarrhea were resolved, dasatinib was
resumed on day 28 with a dose reduction of 50 mg/day (80% dose)
according to the National Comprehensive Cancer Network guidelines
for CML. The total sunitinib trough serum concentration on day 32
was 33.3 ng/ml (sunitinib: 28.6 ng/ml, N-desethyl sunitinib:
4.7 ng/ml, Fig. 3). Although the
total sunitinib trough serum concentration was below the target
serum concentration (Fig. 3), with
the risk of AEs due to the combination of sunitinib and dasatinib,
we decided to continue 37.5 mg/day. On day 57, CTCAE grade 3
neutrophil count decreased [absolute neutrophil count (ANC):
0.9x103/µl] and CTCAE grade 1 PLT decreased (PLT:
78x103/µl) were observed, so dasatinib was again paused.
On day 70, both the ANC and PLT levels returned to the normal
range, and dasatinib treatment was resumed at 50 mg/day. The total
sunitinib trough serum concentration on day 70.0 was 36.6 ng/ml
(sunitinib: 27.5 ng/ml, N-desethyl sunitinib: 9.1 ng/ml,
Fig. 3). As no worsening of AEs
was subsequently observed, the sunitinib dose was changed to 50.0
and 37.5 mg/day alternately (14 days on/7 days off) on day 81. The
total sunitinib trough serum concentration on day 84 was 46.1 ng/ml
(sunitinib: 38.7 ng/ml, N-desethyl sunitinib: 7.4 ng/ml,
Fig. 3), and no new AEs occurred.
The patient achieved a complete cytogenetic response
(BCR::ABL1IS≤1%) for CML on day 154. In contrast, the
treatment of the patient's renal cancer was changed from sunitinib
to axitinib on day 186 because the pancreatic and left adrenal
metastases were enlarged. Thereafter, a combination of axitinib and
dasatinib was continued. Thereafter, dasatinib treatment was
continued and multiple drugs (nivolumab, pazopanib, and
cabozantinib) were administered for mRCC, but the patient died of
progression of mRCC 4.4 years after starting dasatinib combination
therapy.
To elucidate the reasons for the AEs, dasatinib
serum concentration was measured using liquid chromatography-tandem
mass spectrometry with the residue sample for sunitinib serum
concentration analysis. The dasatinib serum concentrations on days
7, 32, and 84 (8 h after administration) were 1.16 ng/ml, less than
the lower limit of quantification (LLOQ: 0.5 ng/ml), and 2.75
ng/ml, respectively. In Japan, sunitinib is subject to TDM, while
dasatinib is not. No clear target serum concentration for dasatinib
has been specified thus far, which is why it is not routinely
measured. Therefore, at the time at which this case was treated,
our hospital did not have a system for measuring dasatinib.
Therefore, the blood concentration of dasatinib could not be
monitored in real time, and analysis was performed at a later
date.
Discussion
This is the first case report of the effect of the
dasatinib combination on the serum concentrations of sunitinib and
the occurrence of AEs in a patient receiving sunitinib. During the
treatment period, no significant differences were observed in the
trough levels of total sunitinib, sunitinib, and N-desethyl
sunitinib between the monotherapy and combination therapy with
dasatinib. Dasatinib may have moderate CYP3A4 inhibitory activity
(10,11), but this study showed that the
administration of dasatinib did not affect the pharmacokinetics of
sunitinib, at least under clinical conditions.
Grade 1 HFSR and diarrhea progressed to grade 2
shortly after initiation of sunitinib and dasatinib combination
therapy. Although dasatinib achieved sufficient efficacy against
CML, its concentration (3 ng/ml, 8 h post-dose) was lower than the
typical range (12) and the total
sunitinib concentration did not change with the concomitant use of
dasatinib. Therefore, the increase of AEs was attributed not to
abnormalities of either drug concentrations but to a
pharmacodynamic interaction between sunitinib and dasatinib. The
lower level of dasatinib maybe due to reduced absorption resulting
from the effect of the concomitant medication esomeprazole
magnesium. Dasatinib has pH-dependent solubility, and
co-administration of proton pump inhibitors, such as omeprazole,
can decrease its absorption from the gastrointestinal tract,
leading to a reduced area under the curve (13-15).
Inhibition of the vascular endothelial growth factor
(VEGF) and platelet-derived growth factor receptor (PDGFR) is
necessary to cause HFSR (16).
Imatinib, a PDGFR inhibitor, and bevacizumab, a VEGF inhibitor,
which target the inhibition of either one of these receptors alone
rarely cause HFSR (17). However,
the incidence increased when bevacizumab was combined with
sorafenib, which inhibits VEGF and PDGFR (18). Therefore, enhanced inhibition of
these related pathways may lead to an increased risk of HFSR.
Sunitinib inhibits both PDGFR and VEGFR, whereas dasatinib inhibits
PDGFR, suggesting that their combination may have intensified the
inhibition of related pathways, potentially leading to the
development of HFSR. Diarrhea caused by multi-TKIs is caused by
c-Kit, which is expressed in the intestinal cells of Cajal
(19). As both sunitinib and
dasatinib inhibit c-kit, their combined effects may have
contributed to the occurrence of diarrhea. Thus, the
pharmacodynamic interaction between sunitinib and dasatinib may
play a significant role in the development of HFSR and
diarrhea.
Al-Najjar and Jarkowski (20) similarly reported that treatment of
CML and mRCC with a combination of everolimus and dasatinib at the
same time caused severe pneumonia at a relatively early stage. They
pointed out that the combination of multiple molecular-targeted
drugs with similar target molecules may cause serious AEs. The case
of Al-Najjar and Jarkowski (20)
was similar to our patient in that the patient was a man in his 60s
and had comorbidities such as diabetes mellitus. When different
molecular-targeted drugs were used in combination, their
synergistic effects may have contributed to the occurrence of AEs.
Therefore, as in the case of our patient, when using
molecular-targeted drugs in combination, caution is required
regarding the occurrence of AEs caused by inhibition of those
target molecules. These AEs are likely to occur relatively
early.
Although the AEs observed after starting the
combination therapy of sunitinib and dasatinib were within the
tolerable range under supportive care, they were more severe than
those observed during sunitinib monotherapy. Following the dose
reduction or pause of both drugs, sunitinib and dasatinib were
finally maintained at 87.5 and 83.3% of their initial doses,
respectively, and no severe AEs were observed.
At the time treatment for this case was started,
pembrolizumab had not yet been approved, and there were fewer
treatment options available than there are today. However, even now
that pembrolizumab is available, molecular-targeted drugs such as
sunitinib are key drugs for patients who cannot use immune
checkpoint inhibitors due to AEs or who do not achieve sufficient
efficacy. The combination of molecular-targeted drugs can be
considered an option for cases with multiple tumors. This report
shows that in cases in which molecular-targeted drugs with the same
target molecule are used in combination, the drug dose should be
reduced and the occurrence of AEs should be carefully monitored,
especially in the early stages of combination treatment. In
addition, our case suggests that it may be possible to maximize
therapeutic efficacy by increasing the dose of each drug to the
target dose while controlling AEs.
This study has two limitations. First, although the
presence of specific gene mutations has been confirmed in multiple
tumors, searching for gene mutations using methods such as genome
profiling has, to date, not been performed in general clinical
practice in urological cancers, including renal cancer, in Japan.
Therefore, the causative gene was not searched for this case.
However, we cannot rule out the possibility that a specific gene
mutation existed and created a situation different from the general
case. Second, sunitinib may affect dasatinib plasma levels given
the lower dasatinib concentrations observed. More research is
needed to assess the effect of sunitinib on the plasma levels of
dasatinib when used in combination, and the safety of higher
dasatinib concentrations.
In conclusion, although dasatinib concentrations
were lower than usual in this case, they were sufficient for CML
treatment, and dasatinib did not affect the serum concentration of
sunitinib. While HFSR and diarrhea increased after initiating the
combination of sunitinib and dasatinib, temporary dose reductions
or pauses, and finally maintaining at 87.5 and 83.3% of the initial
doses for sunitinib and dasatinib, respectively, allowed for the
continuation of therapy without severe AEs. Combination therapy at
a reduced dose and escalating it based on AE monitoring may enable
safe and effective treatment.
Acknowledgements
Not applicable.
Funding
Funding: This research was supported by a Grant-in-Aid for
Scientific Research (grant no. 20K16454) from the Japan Society for
the Promotion of Science.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MTS, HY, TA, MN, KS and KY contributed to the
conception and design of this study. MTS, HY, TA, and MN collected
the data. MN and KS diagnosed and treated the patient. MTS, HY, and
TA performed the data analysis. MTS, HY, and TA confirm the
authenticity of all the raw data. MTS, HY, TA, MN, KS and KY
interpreted the data. MTS wrote the first draft of the manuscript,
and all authors commented on previous versions of the manuscript.
All the authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Informed written consent was obtained from the
patient for publication of this report.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Dr Miki Takenaka Sato, ORCID 0000-0002-0474-2007; Dr
Hideaki Yashima, ORCID 0000-0002-8982-2312; Dr Takuya Araki, ORCID
0009-0005-9162-5405; Dr Kazuhiro Suzuki, ORCID
0000-0002-1448-7273.
References
|
1
|
Verheijen RB, Yu H, Schellens JHM, Beijnen
JH, Steeghs N and Huitema ADR: Practical recommendations for
therapeutic drug monitoring of kinase inhibitors in oncology. Clin
Pharmacol Ther. 102:765–776. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Mueller-Schoell A, Groenland SL,
Scherf-Clavel O, van Dyk M, Huisinga W, Michelet R, Jaehde U,
Steeghs N, Huitema ADR and Kloft C: Therapeutic drug monitoring of
oral targeted antineoplastic drugs. Eur J Clin Pharmacol.
77:441–464. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Noda S, Otsuji T, Baba M, Yoshida T,
Kageyama S, Okamoto K, Okada Y, Kawauchi A, Onishi H, Hira D, et
al: Assessment of sunitinib-induced toxicities and clinical
outcomes based on therapeutic drug monitoring of sunitinib for
patients with renal cell carcinoma. Clin Genitourin Cancer.
13:350–358. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Numakura K, Fujiyama N, Takahashi M,
Igarashi R, Tsuruta H, Maeno A, Huang M, Saito M, Narita S, Inoue
T, et al: Clinical implications of pharmacokinetics of sunitinib
malate and N-desethyl-sunitinib plasma concentrations for treatment
outcome in metastatic renal cell carcinoma patients. Oncotarget.
9:25277–25284. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Takasaki S, Kawasaki Y, Kikuchi M, Tanaka
M, Suzuka M, Noda A, Sato Y, Yamashita S, Mitsuzuka K, Saito H, et
al: Relationships between sunitinib plasma concentration and
clinical outcomes in Japanese patients with metastatic renal cell
carcinoma. Int J Clin Oncol. 23:936–943. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Houk BE, Bello CL, Poland B, Rosen LS,
Demetri GD and Motzer RJ: Relationship between exposure to
sunitinib and efficacy and tolerability endpoints in patients with
cancer: Results of a pharmacokinetic/pharmacodynamic meta-analysis.
Cancer Chemother Pharmacol. 66:357–371. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mendel DB, Laird AD, Xin X, Louie SG,
Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, et
al: In vivo antitumor activity of SU11248, a novel tyrosine kinase
inhibitor targeting vascular endothelial growth factor and
platelet-derived growth factor receptors: Determination of a
pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res.
9:327–337. 2003.PubMed/NCBI
|
|
8
|
Faivre S, Delbaldo C, Vera K, Robert C,
Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, et
al: Safety, pharmacokinetic, and antitumor activity of SU11248, a
novel oral multitarget tyrosine kinase inhibitor, in patients with
cancer. J Clin Oncol. 24:25–35. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang J, Cui X, Cheng C, Wang Y, Sun W,
Huang CK, Chen RJ and Wang Z: Effects of CYP3A inhibitors
ketoconazole, voriconazole, and itraconazole on the
pharmacokinetics of sunitinib and its main metabolite in rats. Chem
Biol Interact. 338(109426)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Filppula AM, Neuvonen PJ and Backman JT:
In vitro assessment of time-dependent inhibitory effects on CYP2C8
and CYP3A activity by fourteen protein kinase inhibitors. Drug
Metab Dispos. 42:1202–1209. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li X, He Y, Ruiz CH, Koenig M, Cameron MD
and Vojkovsky T: Characterization of dasatinib and its structural
analogs as CYP3A4 mechanism-based inactivators and the proposed
bioactivation pathways. Drug Metab Dispos. 37:1242–1250.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Eley T, Luo FR, Agrawal S, Sanil A,
Manning J, Li T, Blackwood-Chirchir A and Bertz R: Phase I study of
the effect of gastric acid pH modulators on the bioavailability of
oral dasatinib in healthy subjects. J Clin Pharmacol. 49:700–709.
2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yago MR, Frymoyer A, Benet LZ, Smelick GS,
Frassetto LA, Ding X, Dean B, Salphati L, Budha N, Jin JY, et al:
The use of betaine HCl to enhance dasatinib absorption in healthy
volunteers with rabeprazole-induced hypochlorhydria. AAPS J.
16:1358–1365. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Takahashi N, Miura M, Niioka T and Sawada
K: Influence of H2-receptor antagonists and proton pump inhibitors
on dasatinib pharmacokinetics in Japanese leukemia patients. Cancer
Chemother Pharmacol. 69:999–1004. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hofmann J, Bartůněk A, Hauser T, Sedmak G,
Beránek J, Ryšánek P, Šíma M and Slanař O: Dasatinib anhydrate
containing oral formulation improves variability and
bioavailability in humans. Leukemia. 37:2486–2492. 2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lacouture ME, Reilly LM, Gerami P and
Guitart J: Hand foot skin reaction in cancer patients treated with
the multikinase inhibitors sorafenib and sunitinib. Ann Oncol.
19:1955–1961. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chu D, Lacouture ME, Fillos T and Wu S:
Risk of hand-foot skin reaction with sorafenib: A systematic review
and meta-analysis. Acta Oncol. 47:176–186. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Azad NS, Aragon-Ching JB, Dahut WL,
Gutierrez M, Figg WD, Jain L, Steinberg SM, Turner ML, Kohn EC and
Kong HH: Hand-foot skin reaction increases with cumulative
sorafenib dose and with combination anti-vascular endothelial
growth factor therapy. Clin Cancer Res. 15:1411–1416.
2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chai Y, Huang Y, Tang H, Tu X, He J, Wang
T, Zhang Q, Xiong F, Li D and Qiu Z: Role of stem cell growth
factor/c-Kit in the pathogenesis of irritable bowel syndrome. Exp
Ther Med. 13:1187–1193. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Al-Najjar F and Jarkowski A III: Treatment
of concurrent metastatic renal cell carcinoma and chronic
myelogenous leukemia-easier said than done? A case report. J Oncol
Pharm Pract. 17:436–439. 2011.PubMed/NCBI View Article : Google Scholar
|