Introduction
Organizing pneumonia (OP), a type of chronic
fibrotic disease after lung tissue injury, has several etiologies,
including those related to infection and age (1-3),
and it can accompany lung cancer (4). The histomorphological features of OP
include granulation tissue composed of fibroblasts, myofibroblasts,
collagen and fibrotic exudate within respiratory bronchioles,
alveolar ducts and alveoli (5). OP
has a relatively low incidence, and patients typically present with
atypical symptoms, such as a mild cough or even no symptoms, making
early detection challenging (6).
Due to the lack of specific clinical signs, OP is frequently
misdiagnosed and is often only confirmed through lung biopsy
(7). Notably, OP has been reported
to be associated with lung cancer (8). Squamous lung carcinoma with cystic
air space (LC-CAS), is a rare type of cystic lesion in lung cancer
that has a prevalence of ~3.6% worldwide (9-12).
According to Fintelmann et al (13), the cystic cavity in LC-CAS
represents a pathological expansion of the native airspace within
the lung, often presenting as an isolated, thin-walled cavity
containing air sacs in the lung parenchyma. The pathogenesis of
LC-CAS has been proposed to involve several mechanisms (14): i) Tumor cells proliferate along the
alveolar wall, leading to fusion of the damaged alveolar wall and
formation of a cystic air cavity; ii) liquefaction necrosis causes
the lesion to discharge, creating a cystic air cavity; iii) elastic
retraction of surrounding lung tissue pulls and thins the cavity
wall; and iv) a ‘valve effect’ may occur when tumor cells
originating from the bronchiolar epithelium obstruct the
bronchioles, resulting in distal alveolar dilation and rupture.
This partial bronchiolar obstruction contributes to the formation
of valves, explaining why LC-CAS often arises in peripheral lung
regions. Pneumothorax is a rare complication of LC-CAS, with only a
small number of clinical cases reported in the literature (15). Cases of OP with late LC-CAS
complicated by pneumothorax have been rarely reported. Therefore,
such patients are prone to missed diagnosis, misdiagnosis or
inappropriate treatment. The current report describes the case of a
patient who developed LC-CAS in the early stage after OP resection
and pneumothorax complication in the later stage. To improve the
understanding of the condition and avoid missed or delayed
diagnosis, the literature on the clinical features of the patient,
as well as the pathogenesis of the disease, was reviewed and the
causes of misdiagnosis were analyzed.
Case report
Case presentation
A 66-year-old man was admitted to the Department of
Respiratory and Critical Care Medicine of Daping Hospital, Third
Military Medical University (Chongqing, China) in December 2021 due
to a right lung mass that was discovered 2 years before and right
chest and abdominal pain persisting for 3 months. The patient had a
smoking history of 45 pack-years but quit in December 2019. They
consumed alcohol (20-100 g/day) for 40 years but quit in 2011. The
patient denied a history of lung disease. In March 2019, chest
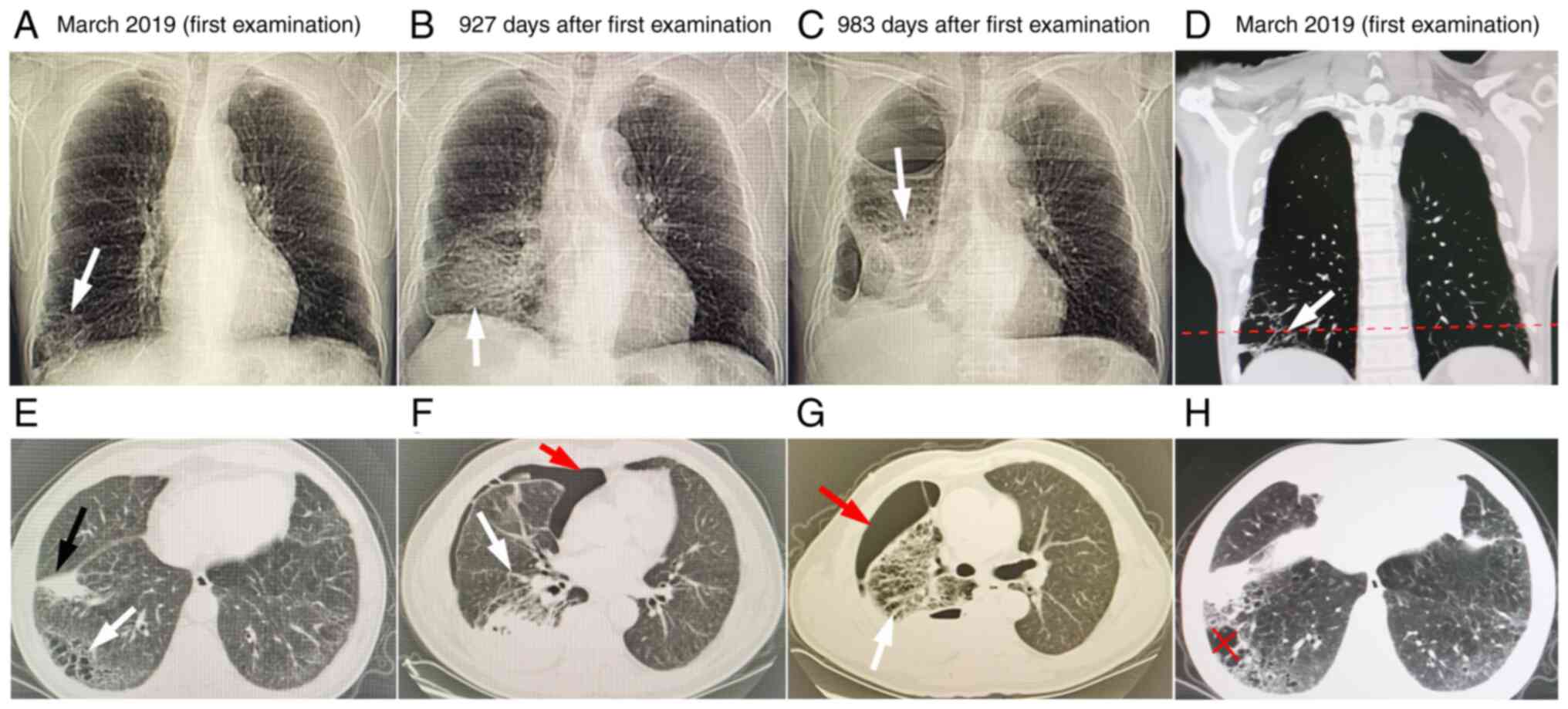
high-resolution computed tomography (HRCT; Fig. 1A, E, D and
H) examined in Chongqing Cancer
Hospital (Chongqing, China) revealed a mass shadow in the basal
segment of the right lower lobe measuring 4.3x2.9x2.6 cm (black
arrow in Fig. 1E). In April 2019,
positron emission tomography-CT (PET-CT) was performed in the
Department of Radiology, Daping Hospital, which revealed a soft
tissue mass shadow in the right lower lobe. The shape of the lesion
was irregular, with lobulation and spiculation signs, and the
boundary between the lesion and the adjacent pleura, oblique
fissure and diaphragm was unclear (Fig. S1). Furthermore,
18F-fluorodeoxyglucose (18F-FDG) PET-CT
revealed a mass with a maximum standardized uptake value
(SUVmax) of 7.25 and average standardized uptake value
(SUVavg) of 3.97. The maximum diameter of the mass was
~4.5 cm; thus, the possibility of peripheral lung cancer was
considered. The peripheral blood carcinoembryonic antigen (CEA) and
carbohydrate antigen 125 levels were both elevated (5.41 ng/ml and
64.68 U/ml, respectively, compared to their normal ranges of
0.00-5.00 ng/ml and 0.00-35.00 U/ml). After 1 day, wedge resection
of the right lower lung lesion was performed in the Department of
Thoracic Surgery, Daping Hospital, and pathology analysis revealed
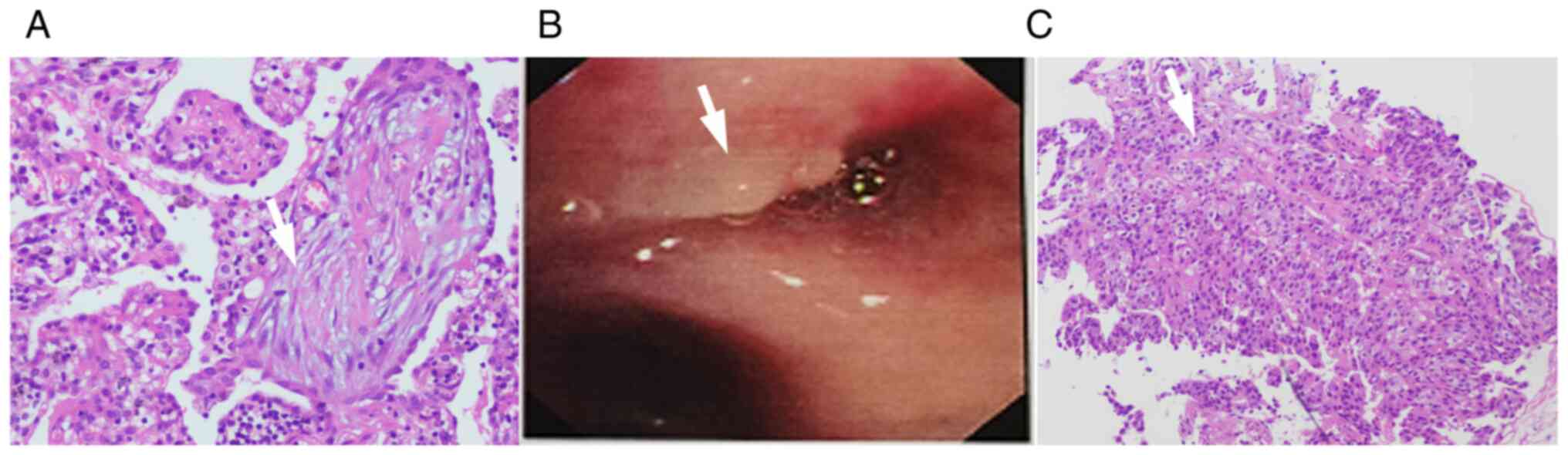
OP of the right lower lung (Fig.
2A). For pathology analysis, the tissue was fixed in 4% neutral
formaldehyde at room temperature for 6 h, followed by paraffin
embedding for 72 h. The tissue was cut into 4-µm sections, which
were stained with hematoxylin and eosin for 10 min at room
temperature. The sections were observed under a light microscope
(BX43; Olympus Corporation). The patient was discharged 5 days
later. During health examination at the same teaching hospital in
November 2020, the patient was found to have pulmonary cysts in the
right lower lung (details unknown), but neither the doctor nor the
patient gave it serious attention due to a lack of any clinical
symptoms. However, the patient gradually experienced pain and
discomfort on the right side of the chest and abdomen, and in
September 2021, the thoracoabdominal pain was accompanied by cough,
expectoration with bloody sputum, and tachypnea and malaise when
walking fast and climbing stairs, which could be relieved after
rest. The patient was hospitalized in a local hospital (Chongqing
Shapingba People's Hospital, Chongqing, China), but the symptoms
were not markedly alleviated by anti-infection (moxifloxacin
hydrochloride sodium chloride injection 400 mg once a day) and
analgesic (oxycodone hydrochloride extended-release tablet 10 mg
twice a day) treatments; thus, the patient was transferred and
admitted to the Department of Radiology of Daping Hospital, Third
Military Medical University in October 2021. Outpatient chest HRCT
revealed a right pneumothorax, with 40-50% right lung compression,
as indicated by the red arrow in Fig.
1B and F. After 3 days, the
patient was admitted again to the current hospital, and laboratory
tests revealed the following: Neuron-specific enolase (NSE), 17.1
ng/ml (normal range <16.3 ng/ml); cytokeratin 19 fragment
(CyFRA21-1), 0.1 ng/ml (normal range <3.3 ng/ml); and squamous
cell carcinoma antigen (SCCA), 21.0 ng/ml (normal range <2.7
ng/ml). The thoracic surgeon diagnosed space-occupying lesions of
the right lower lung and right pneumothorax. Following conservative
treatment (nasal cannula), the patient was discharged after 5 days.
The thoracoabdominal pain gradually aggravated, and the patient was
admitted to the Emergency Department of Daping Hospital ~1.5 months
later. Chest HRCT indicated right liquid pneumothorax (Fig. 1C and G) and 60-70% right lung compression.
Closed thoracic drainage was performed, and the patient was
admitted to the Department of Respiratory and Critical Care
Medicine of the present hospital for further treatment. The patient
was placed in a high semireclining position. Breath sounds were
lower in the right lung than in the left lung, with no obvious
rales observed on either side of the chest. There was right upper
abdominal tenderness, with no signs of rebound pain or muscle
tension. There was no edema observed in either lower limb. The
preliminary differential diagnoses included pneumonia of the right
lung or a tumor of the right lung.
Diagnosis and treatment
Arterial blood gas analysis was completed on the day
of admission (nasal catheter oxygen inhalation; oxygen
concentration, 29%) with the following results: pH, 7.47 (normal
range: 7.35-7.45); partial pressure of CO2, 30 mmHg
(normal range: 35-45 mmHg); arterial oxygen pressure, 76 mmHg
(normal range: 60-100 mmHg); oxygen saturation, 96% (normal range:
95-100%); CEA, 30.7 ng/ml (normal range: 0.0-5.0 ng/ml);
pro-gastrin-releasing-peptide, 53.5 pg/ml (normal range: 28.3-65.7
pg/ml); CyFRA21-1, 30.1 ng/ml (normal range: 0.0-3.3 ng/ml); NSE,
19.5 ng/ml (normal range: 0.0-16.3 ng/ml); and SCCA, 48.5 ng/ml
(normal range: 0.0-2.7 ng/ml). The pleural fluid CEA level was
1,577 ng/ml (normal range <5.0 ng/ml), and cytology examinations
showed suspected cancer cells. For cytology, the samples (from the
dorsal opening of the right lower lobe) were fixed in 10% neutral
formalin at room temperature for 12 h, and were stained with
hematoxylin and eosin for 10 min at room temperature, before being
visualized under an Olympus BX43 light microscope (Olympus
Corporation). The right pleura pathology report revealed squamous
cell carcinoma. Bronchoscopy revealed infiltration of new organisms
in the dorsal opening of the right lower lobe, narrowing of the
lumen and white foam-like secretions in a part of the lumen
(Fig. 2B). The pathology revealed
squamous cell carcinoma (Fig. 2C).
For pathology analysis, the samples (wedge resection of the right
lower lung lesion) were fixed in 10% neutral formalin at room
temperature for 12 h, and 4-µm paraffin-embedded sections were
stained with hematoxylin and eosin for 10 min at room temperature,
and were observed under a light microscope (BX43; Olympus
Corporation). Finally, the patient was diagnosed as having a right
LC-CAS with pleural metastasis and right pulmonary fluid
pneumothorax, and underwent resection for OP (right lower lung;
pathological section diagnosis in April 2019). The findings were
discussed with the patient's family, who then agreed to genetic
testing. The PD-L1 (22C3) test was positive (tumor cells, >15%;
Fig. S2) through
immunohistochemical detection. The samples from the dorsal opening
of the right lower lobe were fixed with 10% neutral formalin at
20-25˚C for 6 h, were embedded in paraffin at room temperature for
72 h, and then cut into 4-µm sections. Subsequently, antigen
retrieval was performed in EDTA (pH 9.0) at 97˚C for 50 min,
followed by blocking with 3% H2O2 at 20-25˚C
for 5 min. The sections were then incubated with a monoclonal mouse
anti-PD-L1 antibody (dilution 1:50; cat. no. SK006; Agilent
Technologies, Inc.) at 4˚C for 175 min, and with a secondary
antibody (dilution 1:20) conjugated to horseradish peroxidase at
4˚C for 40 min (cat. no. SK006; Agilent Technologies, Inc.). DAB
staining was carried out for 5 min, and counterstaining with
hematoxylin was performed for 1 min (using the PD-L1 IHC 22C3
pharmDx detection kit; cat. no. SK006; Agilent Technologies, Inc.),
and then observed under a light microscope (BX43; Olympus
Corporation). Genetic tests of lung cancer (amplification
refractory mutation system-PCR method) indicated PIK3CA gene
mutation in paraffin-embedded tissue samples, and no mutations in
other genes or loci were found. DNA was extracted from tissue
samples using the column extraction method, with DNA polymerase and
a Five Mutation Gene Detection Kit used according to the
manufacturer's protocol (cat. no. CSX1800164; Amoy Diagnostics Co.,
Ltd.) used. The forward and reverse primers were included in the
kit. The amplification procedure used was as follows: First stage
(reverse transcription reaction, inactivation of reverse
transcriptase), one cycle: 42˚C for 5 min and 95˚C for 5 min;
second stage (specific amplification), 10 cycles: 95˚C for 25 sec,
64˚C for 20 sec and 72˚C for 20 sec; stage 3 (specific
amplification, fluorescence acquisition), 36 cycles: 93˚C for 25
sec, 60˚C for 35 sec and 72˚C for 20 sec. The method of detection
of the mutations was fluorescence PCR. As the symptoms of chest and
abdominal pain were relieved, the patient was discharged in
December 2021. After a telephone follow-up (January 2022), the
general condition of the patient was poor, and despite not
receiving immunosuppressive therapy, they were hospitalized in a
local hospital for symptomatic treatment. The patient did not
undergo CT follow-up or other tests, and died at home in June 2022.
The key diagnostic characteristics of the patient are summarized in
Table I.
 | Table IKey diagnostic characteristics of the
patient. |
Table I
Key diagnostic characteristics of the
patient.
| Diagnostic
characteristic | Description |
|---|
| Age, years | 66 |
| Sex | Male |
| Past medical
history | No history of lung
disease |
| Smoking history | Yes |
| CT imaging
features | Chest HRCT (March
2019) showed LC-CAS with irregular shape, uneven wall thickness and
nourishing blood vessels, and subsequent HRCTs (October 2021 and
December 2021) revealed that the lesions gradually expanded and the
cystic cavity became solid. |
| Tumor markers (CEA
and CA-125) | Early elevation
(April 2019) |
| Clinical
presentation | There was no obvious
discomfort in the early stage (March 2019). Late-stage clinical
(October 2021 and December 2021) manifestations included chest and
abdominal pain, cough, sputum with blood, and shortness of breath
after activity. |
Literature review
Literature search
A literature search using the retrieval terms ‘lung
cancer’ OR ‘lung cancer with cystic air space’ AND ‘organizing
pneumonia’ was performed using the PubMed (https://pubmed.ncbi.nlm.nih.gov/), EMBASE (https://www.embase.com), Web of Science (https://www.webofknowledge.com), and China
Biomedical Literature Service System (https://www.sinomed.ac.cn), Wanfang Data (http://www.wanfangdata.com), China Journal Network
full-text database (http://www.cnkie.net) and China HowNet databases
(https://www.cnki.net) to identify the clinical,
pathological and imaging features of LC-CAS based on studies before
February 2022. The screening inclusion and exclusion criteria were
as follows: i) Lung cancer confirmed by histopathological or
cytological results; ii) reporting of CT imaging features of lung
cancer at single or multiple time points; iii) LC-CAS identified on
CT; and iv) co-occurrence with OP. Ultimately, only one study was
identified (8), and along with the
patient in the present case report, there are 2 patients in total.
The clinical data of the 2 patients were analyzed.
General conditions and clinical
manifestations
Both patients were elderly adult men (65 years old
in the literature and 66 years old in the present case) with a
history of heavy smoking. The early clinical manifestations of the
case from the literature included dry cough and fever, and the
later manifestations were chest pain and dyspnea. In the present
case, the patient showed no obvious discomfort at the early stage,
but later clinical manifestations included chest and abdominal
pain, cough, expectoration, bloody sputum, and shortness of breath
after activity.
Imaging features
Chest CT of the patient in the literature showed
irregular consolidation around the right lower lobe with bronchial
air sign, which was connected with the lower lung hilum. In the
present case, early chest HRCT (March 2019, Fig. 1A, D, E and
H) showed that the LC-CAS had an
irregular shape, uneven wall thickness and feeding vessels, whereas
later HRCT (October 2021, Fig. 1B
and F; December 2021, Fig. 1C and G) showed gradual lesion enlargement and
cystic cavity consolidation. The patient in the literature did not
undergo PET-CT examination, whereas the patient in the present case
underwent PET-CT examination, and the results showed that the
18F-FDG value in OP in October, 2021 was increased
compared with that in March, 2019.
Tumor markers
The patient in the literature did not undergo tumor
marker tests. In the present case, early peripheral blood CEA
levels were already elevated, and the level of tumor markers (CEA,
NSE, CyFRA21-1 and SCCA) in both the serum and pleural effusion
gradually increased in the late stage.
Treatment and prognosis
The patient in the literature underwent bronchoscopy
examination and was diagnosed with OP secondary to LC-CAS. They
were in a generally good condition 6 months postoperatively. In the
present case, OP was pathologically confirmed in the right lung
tissue, and wedge resection was performed on the right lung.
Bronchoscopy was performed 32 months postoperatively to monitor the
tumor, and a biopsy was performed to confirm the diagnosis of
LC-CAS. After a telephone follow-up (January 2022), the general
condition of the patient was poor, and despite not receiving
immunosuppressive therapy, they were hospitalized in a local
hospital for symptomatic treatment.
Discussion
LC-CAS, a special type of lung cancer, is a
relatively rare manifestation (16), while OP with LC-CAS is even rarer.
At present, the pathogenesis of LC-CAS is still unclear (17), and the cystic components of LC-CAS
may increase, stabilize or decrease over time (14). Obtaining cystic pathological
tissues of LC-CAS is difficult due to a high risk for injury;
therefore, it is challenging to diagnose this disease (18). The patient in the present case had
no obvious discomfort in the early stage, but they developed major
clinical symptoms, such as chest and abdominal pain, cough,
expectoration, bloody sputum and shortness of breath after activity
in the later stage. Cysts associated with LC-CAS are usually
multilocular, polycystic or thick-walled, while thin-walled cysts
are less common (17). The present
report highlights the presence of uneven cyst walls and irregular,
non-spherical edges, which are characteristics not documented in
previous reports. Early HRCT of LC-CAS revealed an irregular shape,
uneven wall thickness and feeding vessels that gradually expanded;
gradual cystic cavity consolidation occurred later, and finally,
pneumothorax occurred. The chest HRCT imaging features of LC-CAS,
which showed gradual enlargement over time, are consistent with
those reported in previous studies (14,17).
Based on the aforementioned reasons, dynamic
evaluation and comprehensive judgment should be performed for
patients with suspected LC-CAS based on chest HRCT combined with
risk factors, tumor markers and other data (such as bronchoscopy).
The chest HRCT data of the patient in the current case prior to OP
resection in March 2019 showed the aforementioned abnormal changes
in LC-CAS. Given the slightly elevated CEA levels and other lung
cancer markers (CYFRA21-1, NSE and SCCA), as well as a history of
heavy smoking, regular follow-up should include monitoring cystic
lesions in the right lower lung through chest HRCT and cancer
marker assessments. Because the doctor did not consider or inform
the patient about the possibility of LC-CAS, neither the patient
nor the doctor paid attention to the enlargement of the right lower
lung cyst until November 2020, which ultimately delayed the best
treatment opportunity.
It is generally considered that PET-CT can provide
metabolic information on tumors. Furthermore, the
18F-FDG value of lesions is closely related to different
malignant degrees, including the change indices of
SUVavg and SUVmax (19,20).
OP has a high PET-CT metabolism, which is consistent with the
increased 18F-FDG uptake during the early PET-CT
examination of the patient in the present case (21). However, the thin air cavity wall
and irregular shape of LC-CAS lesions reduce the total density of
metabolic cells, resulting in no or poor uptake of
18F-FDG, measurement difficulties and false-negative
imaging results, which affect the judgment of radiologists
(16,22-24).
In the present case, the PET-CT examination performed during an
earlier stage showed that LC-CAS had a low 18F-FDG
uptake value, which was also an important reason for the missed
diagnosis of LC-CAS during the early stage.
A previous study (22) demonstrated that the check-valve
mechanism of LC-CAS (the infiltration of tumor cells into the
bronchiolar lumen leading to elastic retraction) is the cause of
pneumothorax as a complication, particularly in cases of liquid
pneumothorax that may persist despite active treatment. Further
examination, including bronchoscopy, is required if the liquid is
bloody. The liquid amount in the present case was moderate (lung
compression, 30-70%). A pneumothorax complicated by LC-CAS was
revealed in the later stage of the present case, and despite active
treatment and drainage of bloody hydrothorax, achieving
re-expansion of the lung was challenging, with drainage exceeding
moderate levels. Furthermore, bronchoscopy revealed a neoplasm. The
check valve was caused by the tumor in the patient's right lower
lung, which may lead to the formation of refractory pneumothorax
and bronchial obstruction during the early stage, resulting in
OP.
In the present case, the confirmation of LC-CAS was
delayed for 32 months, which warrants reconsideration. There are at
least two factors that can justify the long-term missed diagnosis
and misdiagnosis of LC-CAS by clinicians and radiologists. The
first is the insufficient knowledge about LC-CAS. The chest HRCT in
March 2019 showed a cystic structure (diameter, 4.0x2.5 cm) behind
the right lower lung mass, with neovascular presence and multiple
enlarged feeding vessels on the coronal plane <1 cm away from
the pleura. The characteristic of the HRCT image is not consistent
with the narrowing of pulmonary arterial vascular structures in the
pulmonary bullae and non-vascular structures in lung cyst lesions.
The cystic changes of lymphatic vessels, such as lymphocytic
interstitial pneumonia, are usually in the periphery of the cystic
cavity, while the cystic changes in this patient are also not
characteristic of previously described cases. The aforementioned
abnormal changes were neither mentioned in the radiology report nor
was the LC-CAS in the right lower lung mentioned in the
preoperative discussion and postoperative review by thoracic
surgeons, indicating that there is limited knowledge of this type
of imaging. Second, incomplete judgment existed in clinical and
radiological diagnosis. A previous study (25) demonstrated that 80% of misdiagnoses
stemmed from incomplete thinking and resulting thinking biases and
that one-third of serious medical errors resulted from thinking
biases. When assessing the imaging data, the radiologist
potentially only found the first right lung-occupying lesion by
‘pattern recognition’ or ‘sample recognition’ in order to achieve a
quick diagnosis. However, the relaxed vigilance to the
aforementioned abnormal changes in the right lower lung reduced the
accuracy of diagnosis. Despite the reported increase in peripheral
blood CEA levels, the doctors did not change the primary diagnosis
or monitor changes in tumor markers and the cystic lesion in the
right lower lobe of the patient. They also did not investigate
other potential reasons for the elevated CEA levels. The patient
visited the hospital only when LC-CAS complicated by pneumothorax
had been present for 32 months postoperatively. Furthermore, the
value of tumor markers, such as peripheral blood CEA levels, was
markedly increased during the second admission of the patient to
the Department of Thoracic Surgery, Daping Hospital. However, the
possibility of cancer was still not fully considered. Clinicians
and radiologists should pay close attention to the aforementioned
misjudgments in order to improve clinical data evaluation during
the initial diagnosis. This includes utilizing chest HRCT,
monitoring CEA levels and other tumor markers, performing
fiberoptic bronchoscopy, and integrating these findings closely
with the medical history of the patient. Following the initial
detection of abnormalities, clinicians should systematically review
clinical data, images and reports to identify any potential
counter-evidence. If the clinical manifestations or other evidence
are inconclusive, a peer or superior doctor should be consulted,
and if necessary, a second evaluation for potential lesions should
be conducted to ensure a comprehensive differential diagnosis,
thereby reducing the risk of missed diagnoses and misdiagnosis. The
diagnosis of LC-CAS in the present case took 32 months from the
initial detection on CT to confirmation, which warrants reflection.
This prolonged interval was partly attributed to reviewing previous
imaging studies rather than deliberate long-term imaging follow-up.
Additionally, annual follow-up for a simple parenchymal cyst is not
part of routine lung cancer screening (26). Therefore, cases of pulmonary cystic
disease with wall thickening or nodules should raise suspicion of
lung cancer, necessitating long-term follow-up to ensure stability
and exclusion, thereby preventing misdiagnosis. After discharge,
the patient did not undergo CT follow-up or other tests, and died
at home in June 2022. Therefore, it is not possible to analyze and
discuss the psychological and physiological effects on patients
following misdiagnosis.
In conclusion, OP with LC-CAS is a rare clinical
entity that is prone to missed and delayed diagnosis. Cultivating
good clinical judgment skills and continuous learning are the keys
to reducing misdiagnosis and inappropriate treatment.
Supplementary Material
Representative image of positron
emission tomography-CT performed in April 2019.
Representative PD-L1 (22C3)
image.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JF and HC wrote the original draft and were involved
in analyzing patient data. FS and QM were involved in analyzing
patient data. SZ wrote, reviewed and edited the manuscript, and was
involved in analyzing patient data. WD designed the study and was
involved in analyzing patient data. GC designed the study, wrote,
reviewed and edited the manuscript, and supervised the work. JF and
GC confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for publication was
obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shen L, Yu H and Liu J: Advances in
imaging studies of latent mechanized pneumonia. J Appl Radiol.
34:133–135. 2018.
|
|
2
|
Baha A, Yildirim F, Kokturk N, Galata Z,
Akyürek N, Demirci NY and Türktaş H: Cryptogenic and secondary
organizing pneumonia: Clinical presentation, radiological and
laboratory findings, treatment and prognosis in 56 cases. Turk
Thorac J. 19:201–208. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liu J and Cai X: A case of cave-forming
focal mechanized pneumonia and literature review. J Hainan Med.
30:395–396. 2019.(In Chinese).
|
|
4
|
Arenas-Jiménez JJ, García-Garrigós E,
Ureña Vacas A, Sirera Matilla M and Feliu Rey E: Neumonía
organizada. Radiología (Engl Ed). 64 (Suppl 3):S240–S249.
2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cao L, Huang Y and Hua M: Clinical
diagnosis and treatment of 42 cases of organizing pneumonia. Chin J
Lung Dis. 11:471–473. 2018.(In Chinese).
|
|
6
|
Khan MS, Khateeb F, Akhtar J, Khan Z, Lal
A, Kholodovych V and Hammersley J: Organizing pneumonia related to
electronic cigarette use: A case report and review of literature.
Clin Respir J. 12:1295–1299. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lu J, Yin Q, Zha Y, Deng S, Huang J, Guo Z
and Li Q: Acute fibrinous and organizing pneumonia: Two case
reports and literature review. BMC Pulm Med. 19(141)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Radzikowska E, Nowicka U, Wiatr E,
Jakubowska L, Langfort R, Chabowski M and Roszkowski K: Organising
pneumonia and lung cancer-case report and review of the literature.
Pneumonol Alergol Pol. 75:394–397. 2007.PubMed/NCBI
|
|
9
|
Mascalchi M: Lung cancer associated with
cystic airspaces in the screening perspective. Ann Surg Oncol. 27
(Suppl 3):S960–S961. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shen Y, Xu X, Zhang Y, Li W, Dai J, Jiang
S, Wu T, Cai H, Sihoe A, Shi J and Jiang G: Lung cancers associated
with cystic airspaces: CT features and pathologic correlation. Lung
Cancer. 135:110–115. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tan Y, Gao J, Wu C, Zhao S, Yu J, Zhu R,
Zhang Q, Wu G, Xue X and Wu J: CT characteristics and pathologic
basis of solitary cystic lung cancer. Radiology. 291:495–501.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vlahos I, Stefanidis K, Sheard S, Nair A,
Sayer C and Moser J: Lung cancer screening: Nodule identification
and characterization. Transl Lung Cancer Res. 7:288–303.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fintelmann FJ, Brinkmann JK, Jeck WR,
Troschel FM, Digumarthy SR, Mino-Kenudson M and Shepard JO: Lung
cancers associated with cystic airspaces: Natural history,
pathologic correlation, and mutational analysis. J Thorac Imaging.
32:176–188. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pan X, Wang H, Yu H, Chen Z, Wang Z, Wang
L and Chen J: Lung cancer associated with cystic airspaces: CT and
pathological features. Transl Cancer Res. 9:3960–3964.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang X, Tao YX, Zhang M, Wu WB, Yang DP
and Wang M: Solitary thin-walled cystic lung cancer with extensive
extrapulmonary metastasis: A case report and review of the
literature. Medicine (Baltimore). 97(e12950)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Opoka LM, Szturmowicz M, Oniszh K,
Korzybski D, Podgajny Z, Błasińska-Przerwa K, Szołkowska M and
Bestry I: CT imaging features of thin-walled cavitary squamous cell
lung cancer. Adv Respir Med. 87:114–117. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mendoza D, Heeger A, Mino-Kenudson M,
Lanuti M, Shepard JO, Sequist LV and Digumarthy SR:
Clinicopathologic and longitudinal imaging features of lung cancer
associated with cystic airspaces: A systematic review and
meta-analysis. AJR Am J Roentgenol. 216:318–329. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sheard S, Moser J, Sayer C, Stefanidis K,
Devaraj A and Vlahos I: Lung cancers associated with cystic
airspaces: Underrecognized features of early disease.
Radiographics. 38:704–717. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Han Y, Ma Y, Wu Z, Zhang F, Zheng D, Liu
X, Tao L, Liang Z, Yang Z, Li X, et al: Histologic subtype
classification of non-small cell lung cancer using PET/CT images.
Eur J Nucl Med Mol Imaging. 48:350–360. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Snoeck A, Reyntiens P, Carp L, Spinhoven
MJ, El Addouli H, Van Hoyweghen A, Nicolay S, Van Schil PE, Pauwels
P, van Meerbeeck JP and Parizel PM: Diagnostic and clinical
features of lung cancer associated with cystic airspaces. J Thorac
Dis. 11:987–1004. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Erdogan Y, Ozyurek BA, Ozmen O, Yılmaz
Demirci N, Duyar SŞ, Dadalı Y, Demirağ F and Karakaya J: The
evaluation of FDG PET/CT scan findings in patients with organizing
pneumonia mimicking lung cancer. Mol Imaging Radionucl Ther.
24:60–65. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Aldaghlawi F, Von Holzen U, Li L and Hadid
W: A case of squamous cell lung cancer presented as a cystic lesion
and recurrent pneumothoraces. Respir Med Case Rep.
33(101382)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tang X, Liu G, Tan X, Liu C, Xiang J and
Jiang Y: Solitary multicystic lesion lung cancer: Two case reports
and review of the literature. BMC Pulm Med. 21(368)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ioannis V, Konstantinos S, Sarah S, Nair
A, Sayer C and Moser J: Lung cancer screening: Nodule
identification and characterization. Transl Lung Cancer Res.
7:288–303. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Eichbaum Q, Adkins B, Craig-Owens L,
Ferguson D, Long D, Shaver A and Stratton C: Mortality and
morbidity rounds (MMR) in pathology: Relative contribution of
cognitive bias vs. systems failures to diagnostic error. Diagnosis
(Berl). 6:249–257. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mazzone PJ, Silvestri GA, Patel S, Kanne
JP, Kinsinger LS, Wiener RS, Soo Hoo G and Detterbeck FC: Screening
for lung cancer: CHEST guideline and expert panel report. Chest.
153:954–985. 2018.PubMed/NCBI View Article : Google Scholar
|