Introduction
Cell-in-cell (CIC) structures result from the
internalization of one or more living cells by a neighboring cell,
where the internalized cells are surrounded by a large vacuole
(1,2). The inner and outer cells can be of
the same type (homotypic CIC) or different types (heterotypic CIC)
(3). CIC structures have been
observed in various cancers, including breast (4,5),
lung (6,7), tongue (8), gastric (9), and pancreatic cancers (10) and melanoma (11) as well as in cytologically malignant
samples from urine and effusion fluids (12). CIC structures are associated with
several cellular processes, including cannibalism, emperipolesis,
and entosis (2,13). In tumor cells, cannibalism refers
to the phagocytic engulfment of other tumor or immune cells by a
tumor cell to acquire nutrients or escape from the immune system
(11,14). Emperipolesis, derived from the
Greek meaning ‘inside around wandering about,’ describes the
penetration and residence of inflammatory cells within host cells,
such as tumor cells and megakaryocytes, without being destroyed
(15,16). Entosis, derived from the Greek word
‘entos,’ meaning inside/into/within, was first described by
Overholtzer et al (1) as
the internalization of one cell by a neighboring cell, triggered by
the detachment of the cell from the extracellular matrix.
Although the recent recognition of entosis as a
concept, studies utilizing the human mammary epithelial cell line
MCF-10A and the breast cancer cell line MCF-7 cells (1,17)
reveal that epithelial cadherin (E-cadherin), placental cadherin
(P-cadherin) (17) and α-catenin
(3) as well as the
Rho/Rho-associated coiled-coil containing protein kinase
(ROCK)/actomyosin signaling in internalized cells play crucial
roles in entosis. During this process, neighboring cells compete
with each other and one cell becomes the host cell, the outer cell
whereas the other cell becomes the internalized cell (18), Sun et al (18) demonstrated that cells expressing
E-cadherin and higher levels of phospho-myosin light chain 2 were
more likely to become internalized cells both in MCF-10A and MCF-7
cells and that MCF-10A cells harboring mutant KRAS exhibited
a deformable cell membrane mediated via Rac1 signaling, rendering
them to become host cells. The internalized cells may undergo
lysosomal cell death in MCF-7 cells (1) and may proceed to mitosis, escape, or
survival within the host cell in the breast cancer cell line
MDA-MB-453(18). The role of
entosis in cancer remains unclear, with conflicting findings and
different perspectives (19).
Entosis can lead to the generation of aneuploid cells, as the
presence of the inner cell physically inhibits the cytokinesis of
the host cell, resulting in binucleated host cells in models
utilizing MCF10A and MCF-7 cells (20). Conversely, entosis related to p53
can contribute to the elimination of aneuploid cells resulting from
prolonged mitosis in MCF10A and MCF-7 cells (21).
Clinical studies have also reported the association
of entosis with cancer outcomes. Dziuba et al (5) found that the frequency of entosis was
associated with a high Ki-67 index and lymph node metastasis in
patients with HER2-positive mammary breast cancer. Additionally,
Wen et al (22) reported
that the rate of entotic structures was higher in patients with
castration-resistant human prostate cancer than in those with
benign prostate hyperplasia or androgen-dependent prostate cancer.
The same study also revealed that androgen receptor increased the
frequency of entosis by inhibiting phosphoinositide 3-kinase and
the RhoA/ROCK signaling in LNCaP cells, a prostate cancer cell line
(22). Furthermore, entosis has
been linked to both favorable and poor prognoses in certain
cancers. For example, homotypic CIC in cancer cells was an
indicator of favorable outcomes in patients with breast cancer
(23) whereas entosis was more
prevalent in patients with unfavorable prognosis in pancreatic
ductal adenocarcinoma (10).
Despite considerable advances made in understanding
the underlying entosis (17), it
is still primarily considered a phenomenon observed in epithelial
cells (1), with evidence coming
from a variety of sources, including mammary epithelial (24), breast cancer (25), bronchial epithelial (26), lung cancer (27), pancreatic carcinoma (10), colon cancer (26,28),
and epidermoid carcinoma (27,29)
cell lines. To date, no studies have explored whether entosis might
also occur in nonepithelial cells. Therefore, the aim of this study
was to investigate whether entosis can occur in nonepithelial cells
under nonadherent conditions which typically support entosis in
epithelial cells.
Materials and methods
Samples
The following cell lines were purchased from the
JCRB Cell Bank managed by the National Institutes of Biomedical
Innovation, Health, and Nutrition (Osaka, Japan): MCF-7 breast
cancer cells (JCRB0134), RD rhabdomyosarcoma cells (JCRB9072),
HT1080 fibrosarcoma cells (IFO50354), and ICH-ERMS-1
rhabdomyosarcoma cells (JCRB1648). All cell lines were confirmed to
be negative for mycoplasma by the JCRB Cell Bank.
Cell culture
All cell lines were cultured in Dulbecco's modified
Eagle's medium (DMEM; 043-30085; FujiFilm Wako Pure Chemical,
Osaka, Japan) supplemented with 10% fetal bovine serum (S1600-500,
Biowest, Nuaillé, France) and 1% penicillin-streptomycin
(168-23191; FujiFilm Wako Pure Chemical) at 37˚C in a humidified 5%
CO2 atmosphere.
Cell internalization assay
Cell internalization was evaluated in cultures
maintained in 24-well cell plates for adherent (3473; Corning, NY,
USA) and nonadherent (174930; Thermo Fisher Scientific Inc,
Massachusetts, USA) cultures. First, the cells were cultured in
75-cm² cell culture flasks (130190; Thermo Fisher Scientific Inc.)
to a density of 1.0-2.0x105 cells/ml, detached using
0.25% Trypsin-ethylenediaminetetraacetic acid (EDTA) (Trypsin;
27250018; FUJIFILM Wako Pure Chemical Corporation, EDTA; E5134;
SIGMA, Massachusetts, USA), and adjusted to a density of
0.8-1.0x105 cells/ml. Next, 1 ml of the prepared cell
suspensions was added to each well of the adherent and nonadherent
plates, which were then incubated for 6 h. In adherent plates,
following medium removal from six wells, the wells were rinsed with
1 ml phosphate-buffered saline (PBS; FUJIFILM Wako Pure Chemical
Corporation), 120 µl of 0.25% Trypsin-EDTA was added to each well
of the plate, which was incubated for 5 min at 37˚C. To terminate
the reaction, 1 ml fresh DMEM was added to each well, and all cells
were collected into one tube and centrifuged at 180 x g for 5 min
at room temperature. The supernatant was discarded, and the cell
pellet was transferred to a 1.5-ml microtube for cell block
preparation. In nonadherent plates, trypsinization was performed
after the collection of cells from the plates, as they were not
adherent to the plate. Briefly, for each treatment condition, cells
from six wells were collected in a centrifuge tube, which was
centrifuged at 180 x g for 5 min at room temperature. The
supernatants were discarded, and the cells were resuspended in 10
ml PBS. After centrifugation at 180 x g for 5 min at room
temperature, the supernatant was discarded, 1 ml of 0.25%
Trypsin-EDTA was added to each tube, and the resuspended cells were
incubated for 5 min at 37˚C. Next, 4 ml fresh DMEM was added to
each tube to terminate the reaction, followed by centrifugation and
the transfer of the cell pellets to 1.5-ml microtubes for cell
block preparation.
Cell internalization assay with the
ROCK inhibitor treatment in nonadherent cultures
A 20-mM stock solution of the ROCK inhibitor Y27632
(CS-0131; ChemScene, NJ, USA) was prepared by dissolving 5 mg of
Y27632 in 1 ml of dimethyl sulfoxide (DMSO; D2650; Sigma Aldrich,
Milwaukee, WI, USA). The working solutions for the ROCK inhibitor
and DMSO were prepared by adding 25 µl of the stock solution to 25
ml of DMEM, followed by sterilization with filtration. For
treatment with the ROCK inhibitor, 0.5 ml of the cell suspension at
a density of 1.6-2.0x105 cells/ml was added to each
well, followed by the addition of 0.5 ml of DMEM containing 20 µM
Y27632 or DMSO. Next, the cell internalization assay for
nonadherent cultures was performed as described above.
Cell block preparation
Approximately 30 µl of a 2% fibrinogen solution
(F3879; Sigma Aldrich) in PBS (FujiFilm Wako Pure Chemical) was
added to the cell pellets in microtubes, and finger tapping was
used for gentle mixing. Immediately after adding 20 µl of the
thrombin solution (224092751; Mochida Pharmaceutical, Tokyo,
Japan), the microtubes were gently tapped to mix the solution and
incubated for 5 min for clotting. The fibrin clot was collected
using forceps, placed into an embedding cassette (USM-1900-W;
Youken Science, Tokyo, Japan), and fixed overnight in 10% neutral
buffered formalin solution (062-01661; FujiFilm Wako Pure
Chemical).
Embedding and sectioning of cell
blocks
After fixation, the specimens were processed using a
vacuum infiltration processor (Tissue-Tek VIP; Sakura Finetek
Japan, Tokyo, Japan) and embedded in paraffin. Four-µm-thick
sections were prepared using a rotary microtome (RX-860; Yamato
Kohki Industrial, Saitama, Japan) attached to a continuous cooling
device (PC-110; Yamato Kohki Industrial) to ensure cooling during
slicing.
Hematoxylin and hematoxylin/eosin
staining
The slides prepared from cell blocks were soaked in
xylene (242-00087; FujiFilm Wako Pure Chemical) three times, 5 min
each, followed by 100, 95, and 70% ethanol, for 1 min each. The
slides were rinsed under running water for 1 min, followed by
staining with Mayer's hematoxylin (30141; New Hematoxylin Type M;
Muto Pure Chemicals, Tokyo, Japan) for 10 min. Following rinsing
under running water for 10 min, the slides were stained with eosin
(32081; New Eosin Type M; Muto Pure Chemicals) for 3 min. After a
brief rinse under running water, the slides were dipped in 70 and
95% ethanol, five times each. The slides were dipped in 100%
ethanol twice, for 30 sec each, and in xylene three times, for 5
min each. Finally, the slides were sealed using a mounting medium
(20093; Malinol; Muto Pure Chemicals) and observed under a
microscope (Olympus BX51; Olympus Corporation, Tokyo, Japan). For
hematoxylin-only staining, the slides were processed as described
above, except for immersion in eosin.
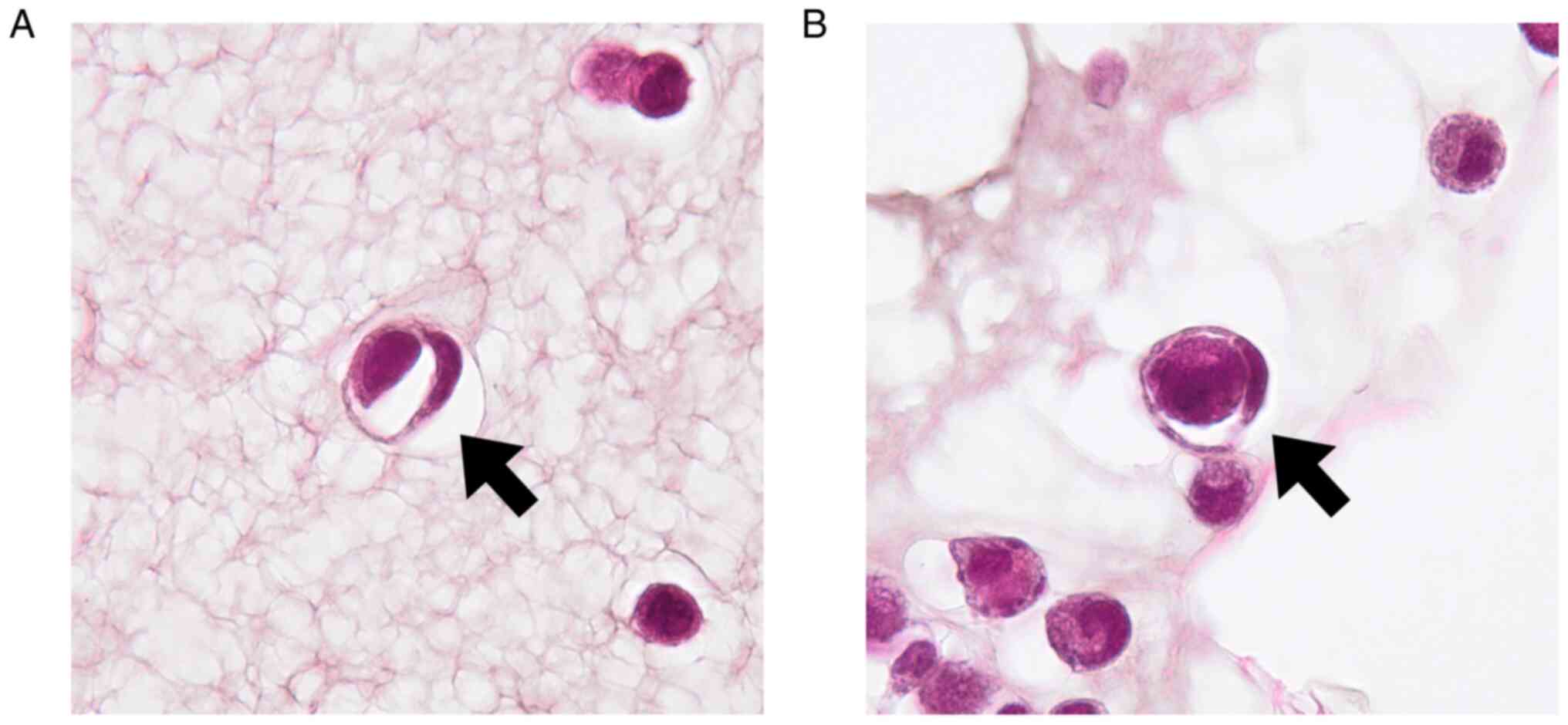
Evaluation of the CIC structures
The hematoxylin/eosin-stained slides were evaluated
to observe the CIC structures, and images of the CIC structures in
the entire viewing area were captured using a digital microscope
with an affixed camera (DP23; Olympus Corporation) using a 100x
oil-immersion objective lens. The capture settings were as follows:
exposure, auto; gain, 2x; exposure compensation, 2/3; and
resolution, 2072x2072 pixels. Each cell exhibiting the
morphological characteristics of engulfing more than two-thirds of
another cell was counted as one CIC structure. CIC structures were
assessed by one cytotechnologist and one pathologist, and consensus
between the two observers was recorded.
Whole-slide imaging
Whole-slide imaging of the slides stained with
hematoxylin/eosin or hematoxylin alone were captured using a
digital slide scanner (Nano Zoomer-SQ C13140-01; Hamamatsu
Photonics, Shizuoka, Japan). The scanning settings were as follows:
objective lens, 20x; numerical aperture, 0.75; scanning speed, 40x;
maximum capture size, 26x76 mm2; pixel size, 0.23
µm/pixel; diode source, light-emitting; image storage format, JPEG;
and focus, automatic.
Cell counting by computer-assisted
image analysis
Whole-slide images of hematoxylin-stained slides
were converted to MRXS files using an image converter software
(version 1.14) from 3DHISTECH (Budapest, Hungary). The files were
analyzed to count cell nuclei using the HistoQuant module in the
Panoramic Viewer software (3DHISTECH). The HistoQuant settings were
as follows: hue, 252-310; saturation: 25-53; separation, 7; noise
reduction (Gauss), 3; minimum size, 23; and maximum size, 211. Of
note, these settings were slightly adjusted depending on the
staining condition for precise identification of the cellular
nuclei in each sample (Table
SI).
Quantitative polymerase chain reaction
(qPCR)
Total RNA extraction was performed using TRIzol™
reagent (15596-026; Thermo Fisher Scientific) according to the
manufacturer's instructions. The synthesis of cDNA was performed
using SuperScript™ III Reverse Transcriptase (18080-044; Thermo
Fisher Scientific). according to the manufacturer's instructions.
Candidate genes were measured using qPCR system according to the
manufacture's protocol of PowerUp™ SYBR™ Green master mix (A25742;
Thermo Fisher Scientific) described. The reaction conditions were
as follows for primer pairs with a melting temperature (Tm) at or
above 60˚C: 1 cycle at 50˚C for 2 min and 95˚C for 2 min, followed
by 40 cycles at 95˚C for 15 sec and 60˚C for 1 min. For primer
pairs with a Tm below 60˚C, the reaction conditions were as
follows: 1 cycle at 50˚C for 2 min and 95˚C for 2 min, followed by
40 cycles at 95˚C for 15 sec, Tm of the primer-pair (Table I) for 15 sec, and 72˚C for 1 min.
In all reactions, melting curve analysis was performed for reaction
specificity, and the single melting curve gained reaction was
considered specific. The primers used for qPCR were synthesized at
FASMAC (Kanagawa, Japan) and purchased from Greiner Bio-One (Tokyo,
Japan) (Table I). Delta Ct (ΔCt)
value was calculated based on the following formula: ΔCt=Ct (a
target gene) - Ct (a reference gene: GAPDH) (30).
 | Table IPrimers used for PCR in the present
study. |
Table I
Primers used for PCR in the present
study.
| Primer | Forward
(5'-3') | Reverse
(5'-3') | Tm (˚C) |
|---|
| E-cadherin |
agggaggattttgagcacgt |
ttgggttgggtcgttgtact | 59.8 |
| M-cadherin |
gtcatctacagcatccaggg |
aggaaggctggccggttgtc | 60.0 |
| N-cadherin |
ggcagaagagagactgggtc |
gaggctggtcagctcctggc | 60.0 |
| P-cadherin |
aacctccacagccaccatag |
aaactgctcatcctcacggt | 60.0 |
| R-cadherin |
ccgaccagccccccatggag | cctggcttg
gagccctcgtcc | 60.0 |
| GAPDH |
aggtgaaggtcggagtcaac |
gcatcgccccacttgatttt | 60.0 |
Statistical analysis
All data were analyzed using JMP Pro version 15 (SAS
Institute, Tokyo, Japan). For the analysis of the cell
internalization assay, Welch's t-test was used to compare means and
the Wilcoxon rank-sum test was used for nonparametric comparisons
between two groups. A P value of <0.05 was considered to
indicate statistical significance.
Results
CIC structures are observed in
rhabdomyosarcoma cell lines under nonadherent conditions
Although widely documented in epithelial cells under
nonadherent conditions (1),
whether entosis can occur in nonepithelial cells remains unknown.
Therefore, we determined whether rhabdomyosarcoma cells exhibited
entosis under nonadherent culture conditions. To this end, we
compared the proportion of cells with CIC structures in a range of
cell lines grown in adherent and nonadherent tissue culture plates.
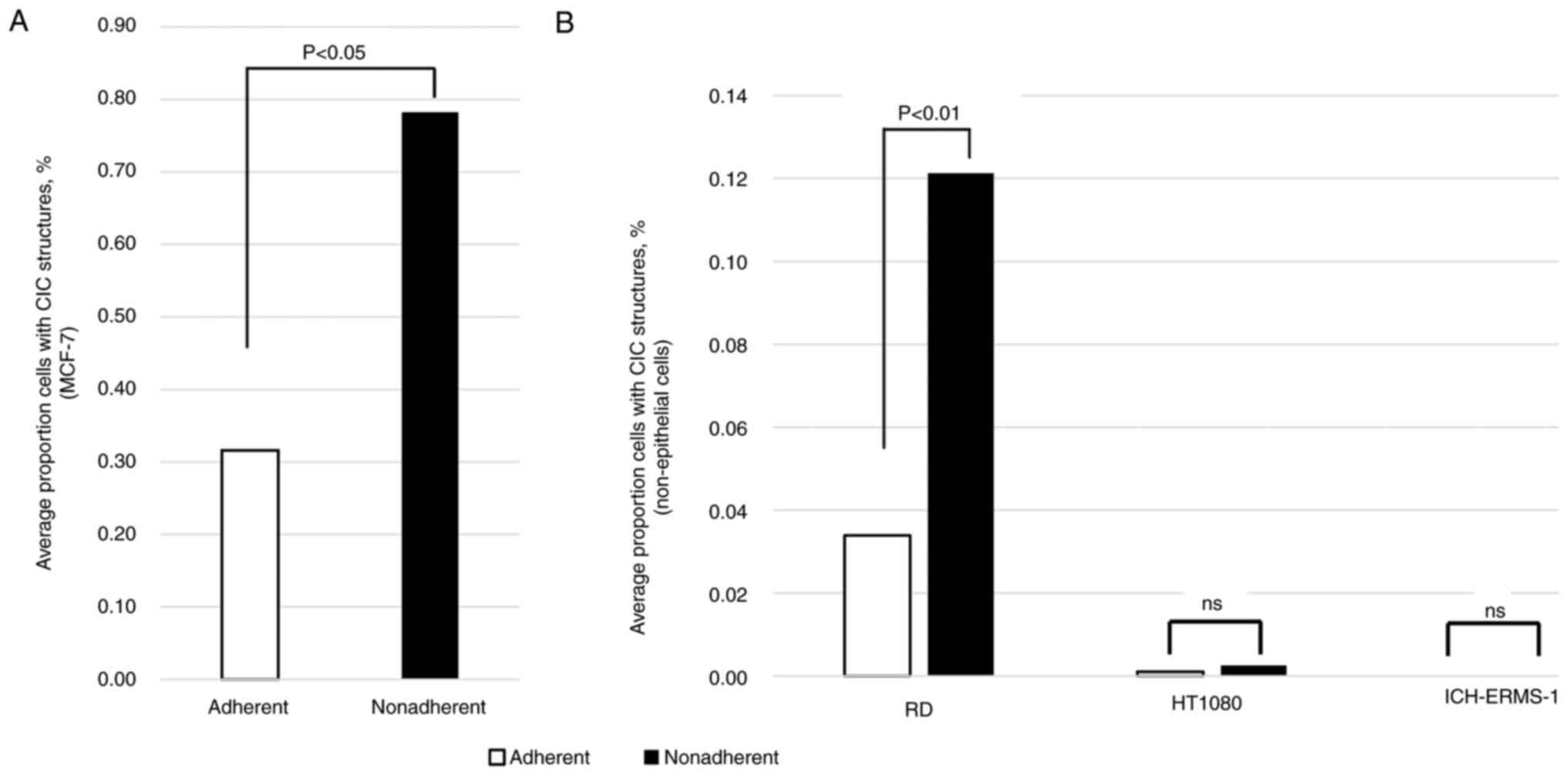
A representative CIC structure is shown in Fig. 1. As shown in Fig. 2A and B, the proportion of cells with CIC
structures was significantly higher in nonadherent culture
conditions than in adherent culture conditions in both the MCF-7
and RD cell lines (P=0.0297 and P=0.0098 respectively). However,
the proportion of cells with CIC structures was low in adherent
conditions and did not significantly change in nonadherent
conditions in either the HT1080 or ICH-ERMS-1 cell lines. These
results suggested that CIC structures could emerge not only in
epithelial but also in nonepithelial cells.
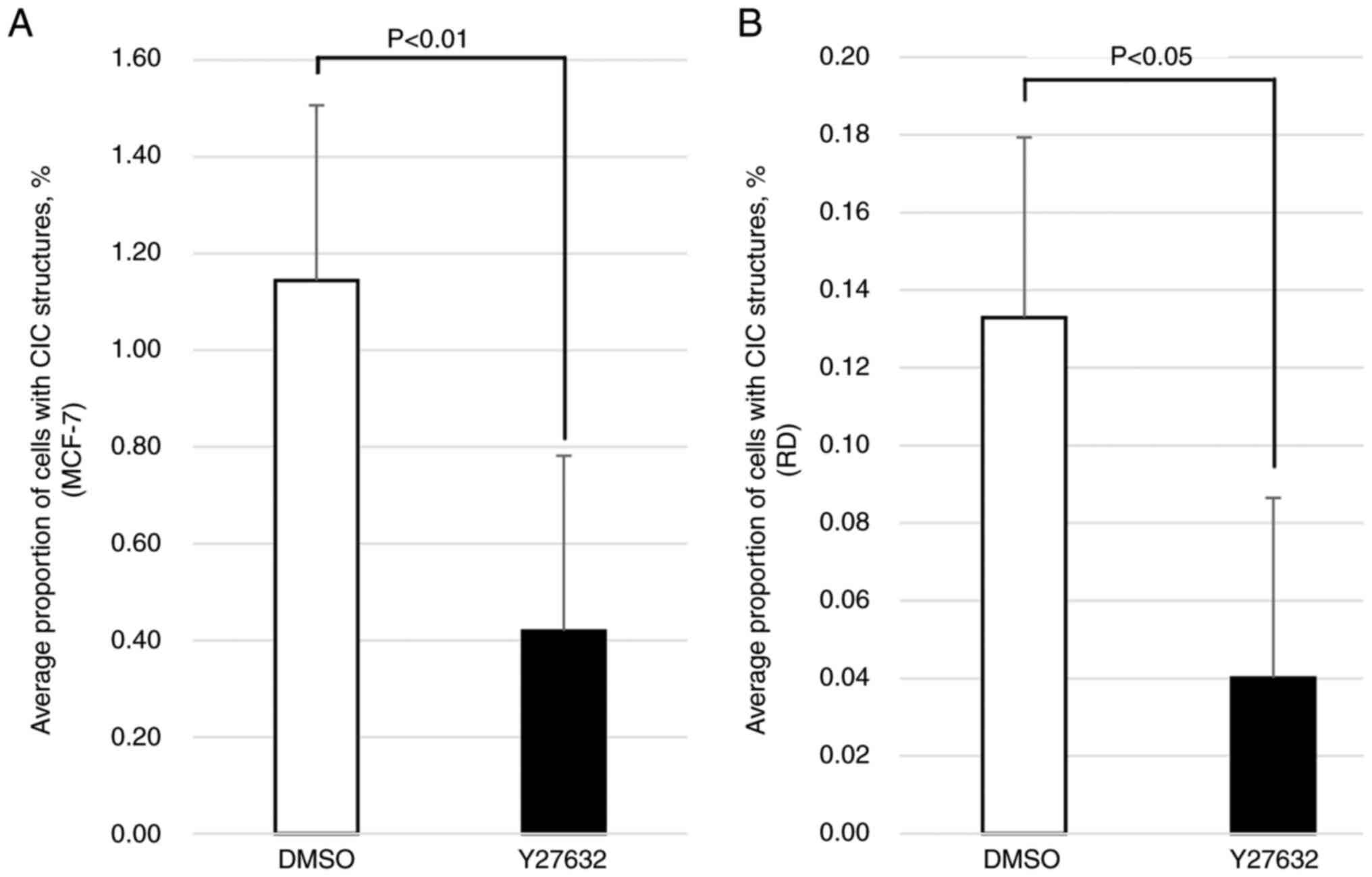
Inhibition of ROCK signaling blocks
the emergence of CIC structures in rhabdomyosarcoma cells
Previous studies (17,18)
demonstrated that entosis could be blocked by the inhibition of
ROCK with Y27632(31). To
determine whether the CIC structures observed in the RD cell line
represented entosis, we evaluated the proportion of cells with CIC
structures in RD and MCF-7 cells cultured in nonadherent conditions
and treated with Y27632. As shown in Fig. 3, the proportion of cells with CIC
structures was significantly decreased in both the MCF-7 (P=0.0021)
and RD (P=0.0407) cells treated with Y27632 compared with the
cultures treated with the DMSO vehicle, suggesting that the
emergence of CIC structures observed in the RD cells cultured in
nonadherent conditions was due to entosis, which was also observed
in the MCF-7 cells, the positive control.
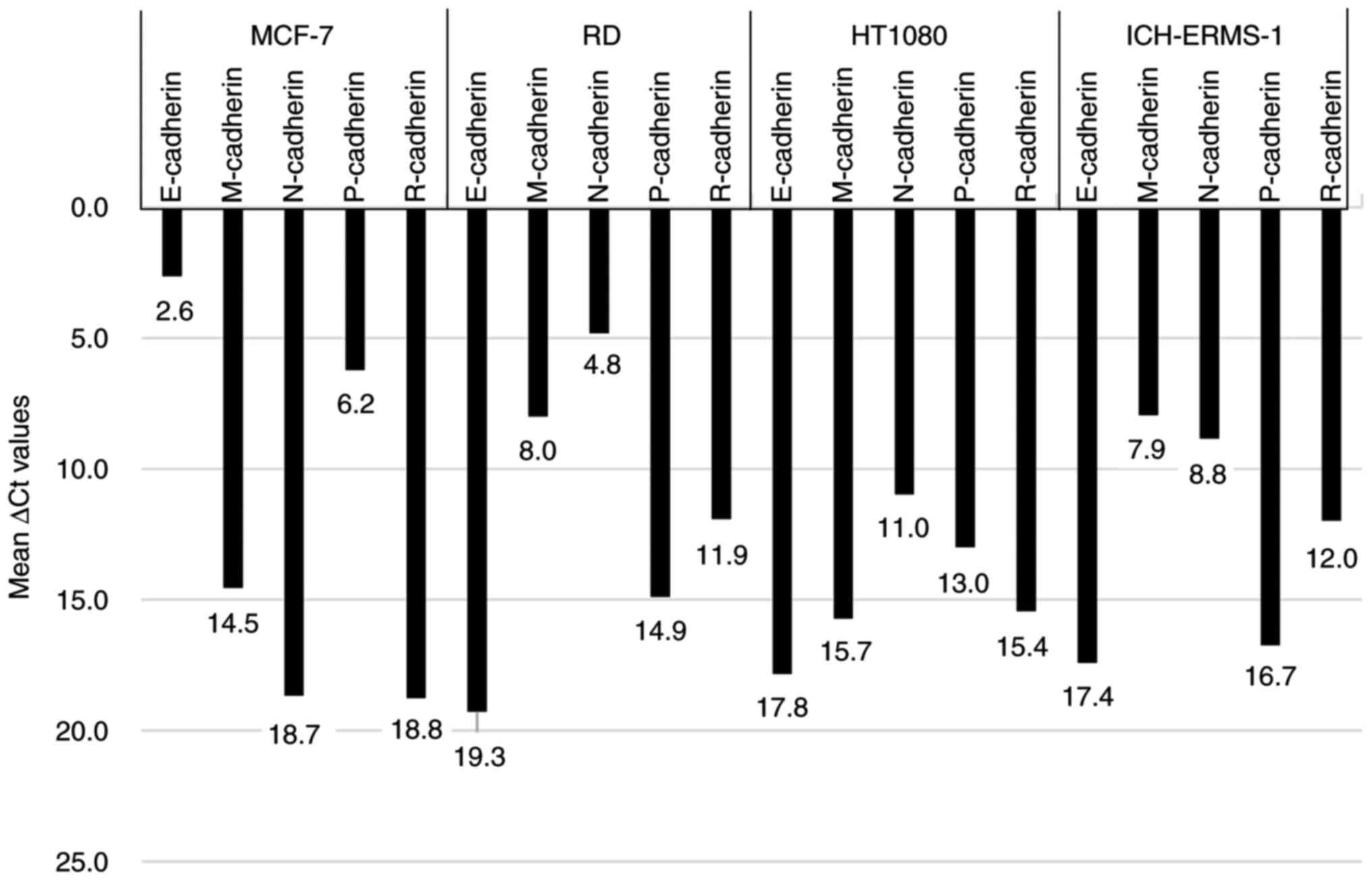
N-cadherin is involved in entosis in
rhabdomyosarcoma cells
To investigate the molecular mechanisms underlying
entosis in rhabdomyosarcoma cells, we compared the expression
levels of the members of the cadherin family of genes using
quantitative PCR between the entotic MCF-7 and RD cell lines and
the nonentotic HT1080 and ICH-ERMS-1 cell lines. As shown in
Fig. 4, the E-cadherin expression
level was the lowest among the five cadherin genes in the MCF-7
cell line, with a ΔCt value of 2.6. Conversely, the neural cadherin
(N-cadherin) expression level was lowest among the five cadherin
genes in the RD cell line, with a ΔCt value of 4.8. However, the
N-cadherin expression levels were relatively higher in the HT1080
(ΔCt value of 11.0) and ICH-ERMS-1 (ΔCt value of 8.8) cell lines
compared with the RD cell line (Fig.
4). Overall, these results suggested that E-cadherin and
N-cadherin were involved in entosis in the MCF-7 and RD cell lines,
respectively.
Discussion
The sequential activation of RhoA, ROCK, and
phospho-myosin light chain 2 leads to the contraction of
actomyosin, resulting in entosis (32); indeed, ROCK inhibition was shown to
block entosis (17,18). In the present study, we found that
the CIC structures observed in the nonepithelial rhabdomyosarcoma
cell line RD were triggered by the detachment of cells from the
matrix in nonadherent culture conditions and that these structures
were blocked by ROCK inhibition, thereby demonstrating that entosis
could occur not only in epithelial but also in nonepithelial cell
lines. In addition, we found that the core adhesion molecule
involved in the entosis of nonepithelial cells was N-cadherin and
not E-cadherin, which was previously shown to be involved in
epithelial cell entosis (1). Sun
et al (17) reported that
the introduction of E- or P-cadherin in cadherin-negative breast
cancer cell lines induced entosis, demonstrating the role of
cadherin proteins in entosis. During entosis, a multimolecular
complex termed the mechanical ring is formed in the interface
between the cellular surfaces of the invading and host cells; this
complex consists of E-cadherin; α-, β-, and γ-catenin; F-actin;
vinculin; and other components (2). In the present study, we found that
N-cadherin, and not E- or P-cadherin, was involved in the entosis
of nonepithelial cells. The cadherin family of proteins includes
E-cadherin (33,34), N-cadherin (33), and P-cadherin (35), and N- and E-cadherin fulfill
similar roles in cell adhesion (35). Notably, increased N-cadherin
expression is observed in some rhabdomyosarcoma cell lines,
including the RD cell line (36),
although it is not expressed in striated muscle (37). Thus, N-cadherin might function as
an adhesion molecule in the entosis of nonepithelial cells.
RD, an embryonal rhabdomyosarcoma cell line, is
originally less invasive than alveolar rhabdomyosarcoma cell lines
(38). Therefore, the entosis of
RD cells cannot be explained as inherently invasive behavior.
Instead, the matrix detachment condition might alter their
behavior. Li et al (39)
demonstrated that RD cells cultured under spheroid conditions
exhibited enhanced migration compared with those cultured under
adherent conditions. In addition, during entosis, the mobility of
inner cells increases, and they themselves invade outer cells
(17). Our experiments under
nonadherent conditions created a situation where cells showed
three-dimensional overlapping, which might have altered the
mobility of RD cells, creating conditions favorable for entosis.
Thus, in nonepithelial cells, entosis may occur when the
environment surrounding tumor cells changes. Because there are no
articles specifically mentioning entosis in non-epithelial tumor
cells, we would like to speculate on the meanings and significance
of entosis in non-epithelial tumor cells by reviewing the
phenomenon as reported in epithelial tumor cells. What should be
understood from reports on the incidence of CIC and the prognosis
of cancer patients is that the relationship between CIC incidence
and prognosis varies depending on the type of cancer. For example,
Schwegler et al (40) found
that lower incidences of CIC in head and neck cancer and colorectal
cancer were associated with a better patient prognosis, while a
higher incidence of CIC in anal cancer was associated with a better
patient prognosis. Song et al (10) reported that the prognosis of
pancreatic cancer patients was worse in cases where CIC was
observed. In addition, Druzhkova et al (41) showed that the incidence of CIC in
colon cancer cell lines increased depending on the chemotherapy
drug concentration. In other words, considering the above reports,
regarding whether the appearance of CIC has a pro-tumor or
anti-tumor effect, it appears that the majority of reports suggest
that CIC formation has a pro-tumor effect. Therefore, even in
non-epithelial tumors, the significance of CIC formation may be
understood more in terms of its possible role as a poor prognostic
factor or as a means of escaping from anticancer drugs, rather than
its anti-tumor effect. Further studies are needed to confirm this
implication.
Homotypic CIC structures can also occur in
cannibalism, which, however, usually arises under starvation
conditions (14). Additionally,
other molecules, such as ezrin, actin, and caveolin-1, play roles
in cannibalism but not in entosis (11,42).
Therefore, the accumulating data suggests that the CIC observed in
the present study is not an indication of cannibalism.
We acknowledge the limitations of the present study.
Among the three nonepithelial cell lines analyzed in the present
study, entosis was observed only in RD cells. Thus, experiments
utilizing additional nonepithelial cell lines are necessary to
confirm our study findings. Additionally, we examined only the role
of N-cadherin in nonepithelial cell-related entosis and did not
expand our evaluations to other potential molecules. Furthermore,
we did not evaluate the histologic aspects of nonepithelial cell
entosis due to the lack of clinical samples containing
rhabdomyosarcoma cells collected from body cavities.
In conclusion, this is the first study to
demonstrate that entosis, a phenomenon previously considered to be
limited to epithelial cells, could occur in nonepithelial cells. We
also show that nonepithelial cell entosis involves N-cadherin, but
not E-cadherin, an entotic finding not previously reported.
Supplementary Material
Settings used in the image
analysis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MO performed cell-in-cell (CIC) structure-related
culture experiments, developed the cell block preparation technique
and prepared cell blocks, manually counted CIC structures and
counted total cells by computer-assisted image analysis, performed
RNA extraction, cDNA preparation and quantitative PCR analysis,
prepared figures and tables, and wrote all parts of the draft of
this manuscript. MS conducted the research as the principal
investigator, cultured and maintained the cell lines, supervised
cell culture, RNA extraction and cDNA preparation, evaluated the
CIC structures, reviewed the data, figures and tables, reviewed all
contents of the manuscript written by MO, and revised all of the
descriptions and references as the corresponding author. RK and YK
developed the cell block preparation technique and prepared the
cell blocks. SK performed RNA extraction, cDNA preparation and
quantitative PCR analysis. YN participated in the preparation of
cell blocks. MO and MS confirmed the authenticity of all the raw
data. All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Overholtzer M, Mailleux AA, Mouneimne G,
Normand G, Schnitt SJ, King RW, Cibas ES and Brugge JS: A
nonapoptotic cell death process, entosis, that occurs by
cell-in-cell invasion. Cell. 131:966–979. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang M, Niu Z, Qin H, Ruan B, Zheng Y,
Ning X, Gu S, Gao L, Chen Z, Wang X, et al: Mechanical ring
interfaces between adherens junction and contractile actomyosin to
coordinate entotic cell-in-cell formation. Cell Rep.
32(108071)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang M, Ning X, Chen A, Huang H, Ni C,
Zhou C, Yu K, Lan S, Wang Q, Li S, et al: Impaired formation of
homotypic cell-in-cell structures in human tumor cells lacking
alpha-catenin expression. Sci Rep. 5(12223)2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bauer MF, Hildebrand LS, Rosahl MC, Erber
R, Schnellhardt S, Büttner-Herold M, Putz F, Ott OJ, Hack CC,
Fietkau R and Distel L: Cell-in-cell structures in early breast
cancer are prognostically valuable. Cells. 12(81)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dziuba I, Gawel AM, Tyrna P, Machtyl J,
Olszanecka M, Pawlik A, Wójcik C, Bialy LP and Mlynarczuk-Bialy I:
Homotypic entosis as a potential novel diagnostic marker in breast
cancer. Int J Mol Sci. 24(6819)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wei Y, Niu Z, Hou X, Liu M, Wang Y, Zhou
Y, Wang C, Ma Q, Zhu Y, Gao X, et al: Subtype-based analysis of
cell-in-cell structures in non-small cell lung cancer. Am J Cancer
Res. 13:1091–1102. 2023.PubMed/NCBI
|
|
7
|
Liu X, Guo R, Li D, Wang Y, Ning J, Yang S
and Yang J: Homotypic cell-in-cell structure as a novel prognostic
predictor in non-small cell lung cancer and frequently localized at
the invasive front. Sci Rep. 14(18952)2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Almangush A, Mäkitie AA, Hagström J,
Haglund C, Kowalski LP, Nieminen P, Coletta RD, Salo T and Leivo I:
Cell-in-cell phenomenon associates with aggressive characteristics
and cancer-related mortality in early oral tongue cancer. BMC
Cancer. 20(843)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kim Y, Choi JW, Lee JH and Kim YS: Spindle
assembly checkpoint MAD2 and CDC20 overexpressions and cell-in-cell
formation in gastric cancer and its precursor lesions. Hum Pathol.
85:174–183. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Song J, Xu R, Zhang H, Xue X, Ruze R, Chen
Y, Yin X, Wang C and Zhao Y: Cell-in-cell-mediated entosis reveals
a progressive mechanism in pancreatic cancer. Gastroenterology.
165:1505–1521.e20. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lugini L, Matarrese P, Tinari A, Lozupone
F, Federici C, Iessi E, Gentile M, Luciani F, Parmiani G, Rivoltini
L, et al: Cannibalism of live lymphocytes by human metastatic but
not primary melanoma cells. Cancer Res. 66:3629–3638.
2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gupta K and Dey P: Cell cannibalism:
Diagnostic marker of malignancy. Diagn Cytopathol. 28:86–87.
2003.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Wang S, He M, Li L, Liang Z, Zou Z and Tao
A: Cell-in-cell death is not restricted by caspase-3 deficiency in
MCF-7 cells. J Breast Cancer. 19:231–241. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fais S: Cannibalism: A way to feed on
metastatic tumors. Cancer Lett. 258:155–164. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Humble JG, Jayne WH and Pulvertaft RJ:
Biological interaction between lymphocytes and other cells. Br J
Haematol. 2:283–294. 1956.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Larsen TE: Emperipolesis of granular
leukocytes within megakaryocytes in human hemopoietic bone marrow.
Am J Clin Pathol. 53:485–489. 1970.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sun Q, Cibas ES, Huang H, Hodgson L and
Overholtzer M: Induction of entosis by epithelial cadherin
expression. Cell Res. 24:1288–1298. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sun Q, Luo T, Ren Y, Florey O, Shirasawa
S, Sasazuki T, Robinson DN and Overholtzer M: Competition between
human cells by entosis. Cell Res. 24:1299–1310. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kianfar M, Balcerak A, Chmielarczyk M,
Tarnowski L and Grzybowska EA: Cell death by entosis: Triggers,
molecular mechanisms and clinical significance. Int J Mol Sci.
23(4985)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Krajcovic M, Johnson NB, Sun Q, Normand G,
Hoover N, Yao E, Richardson AL, King RW, Cibas ES, Schnitt SJ, et
al: A non-genetic route to aneuploidy in human cancers. Nat Cell
Biol. 13:324–330. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Liang J, Niu Z, Zhang B, Yu X, Zheng Y,
Wang C, Ren H, Wang M, Ruan B, Qin H, et al: p53-dependent
elimination of aneuploid mitotic offspring by entosis. Cell Death
Differ. 28:799–813. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wen S, Shang Z, Zhu S, Chang C and Niu Y:
Androgen receptor enhances entosis, a non-apoptotic cell death,
through modulation of Rho/ROCK pathway in prostate cancer cells.
Prostate. 73:1306–1315. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang X, Niu Z, Qin H, Fan J, Wang M,
Zhang B, Zheng Y, Gao L, Chen Z, Tai Y, et al: Subtype-based
prognostic analysis of cell-in-cell structures in early breast
cancer. Front Oncol. 9(895)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ishikawa F, Ushida K, Mori K and Shibanuma
M: Loss of anchorage primarily induces non-apoptotic cell death in
a human mammary epithelial cell line under atypical focal adhesion
kinase signaling. Cell Death Dis. 6(e1619)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang C, Chen A, Ruan B, Niu Z, Su Y, Qin
H, Zheng Y, Zhang B, Gao L, Chen Z, et al: PCDH7 inhibits the
formation of homotypic cell-in-cell structure. Front Cell Dev Biol.
8(329)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Durgan J, Tseng YY, Hamann JC, Domart MC,
Collinson L, Hall A, Overholtzer M and Florey O: Mitosis can drive
cell cannibalism through entosis. Elife. 6(e27134)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mackay HL, Moore D, Hall C, Birkbak NJ,
Jamal-Hanjani M, Karim SA, Phatak VM, Piñon L, Morton JP, Swanton
C, et al: Genomic instability in mutant p53 cancer cells upon
entotic engulfment. Nat Commun. 9(3070)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bozkurt E, Dussmann H, Salvucci M,
Cavanagh BL, Van Schaeybroeck S, Longley DB, Martin SJ and Prehn
JHM: TRAIL signaling promotes entosis in colorectal cancer. J Cell
Biol. 220(e202010030)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Garanina AS, Kisurina-Evgenieva OP,
Erokhina MV, Smirnova EA, Factor VM and Onishchenko GE: Consecutive
entosis stages in human substrate-dependent cultured cells. Sci
Rep. 7(12555)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rao X, Huang X, Zhou Z and Lin X: An
improvement of the 2^(-delta delta CT) method for quantitative
real-time polymerase chain reaction data analysis. Biostat
Bioinforma Biomath. 3:71–85. 2013.PubMed/NCBI
|
|
31
|
Uehata M, Ishizaki T, Satoh H, Ono T,
Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M
and Narumiya S: Calcium sensitization of smooth muscle mediated by
a Rho-associated protein kinase in hypertension. Nature.
389:990–994. 1997.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Gutjahr MC, Rossy J and Niggli V: Role of
Rho, Rac, and Rho-kinase in phosphorylation of myosin light chain,
development of polarity, and spontaneous migration of Walker 256
carcinosarcoma cells. Exp Cell Res. 308:422–438. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hatta K, Okada TS and Takeichi M: A
monoclonal-antibody disrupting calcium-dependent cell cell-adhesion
of brain-tissues-possible role of its target antigen in animal
pattern-formation. Proc Natl Acad Sci USA. 82:2789–2793.
1985.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yoshida-Noro C, Suzuki N and Takeichi M:
Molecular nature of the calcium-dependent cell cell-adhesion system
in mouse teratocarcinoma and embryonic-cells studied with a
monoclonal-antibody. Dev Biol. 101:19–27. 1984.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nose A and Takeichi M: A novel cadherin
cell-adhesion molecule: Its expression patterns associated with
implantation and organogenesis of mouse embryos. J Cell Biol. 103
(6 Pt 2):2649–2658. 1986.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Soler AP, Johnson KR, Wheelock MJ and
Knudsen KA: Rhabdomyosarcoma-derived cell-lines exhibit aberrant
expression of the cell-cell adhesion molecules N-Cam, N-Cadherin,
and cadherin-associated proteins. Exp Cell Res. 208:84–93.
1993.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Derycke LD and Bracke ME: N-cadherin in
the spotlight of cell-cell adhesion, differentiation,
embryogenesis, invasion and signalling. Int J Dev Biol. 48:463–476.
2004.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Rapa E, Hill SK, Morten KJ, Potter M and
Mitchell C: The over-expression of cell migratory genes in alveolar
rhabdomyosarcoma could contribute to metastatic spread. Clin Exp
Metastasis. 29:419–429. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Li M, Nagamori E and Kino-Oka M:
Disruption of myoblast alignment by highly motile rhabdomyosarcoma
cell in tissue structure. J Biosci Bioeng. 123:259–264.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Schwegler M, Wirsing AM, Schenker HM, Ott
L, Ries JM, Büttner-Herold M, Fietkau R, Putz F and Distel LV:
Prognostic value of homotypic cell internalization by
nonprofessional phagocytic cancer cells. Biomed Res Int.
2015(359392)2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Druzhkova I, Potapov A, Ignatova N,
Bugrova M, Shchechkin I, Lukina M, Shimolina L, Kolesnikova E,
Shirmanova M and Zagaynova E: Cell hiding in colorectal cancer:
Correlation with response to chemotherapy in vitro and in vivo. Sci
Rep. 14(28762)2024.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lugini L, Lozupone F, Matarrese P, Funaro
C, Luciani F, Malorni W, Rivoltini L, Castelli C, Tinari A, Piris
A, et al: Potent phagocytic activity discriminates metastatic and
primary human malignant melanomas: A key role of ezrin. Lab Invest.
83:1555–1567. 2003.PubMed/NCBI View Article : Google Scholar
|