Introduction
Ischemic stroke is a one of the worldwide leading
causes of death, with an incidence of ~7 million individuals
annually, and a leading cause of disability, as expressed by
>160 million disability-adjusted life-years lost, representing a
significant socioeconomic burden. The estimated global cost is
>US$890 billion annually (1).
The primary treatment strategy for ischemic stroke is the rapid
restoration of blood flow. However, reperfusion of the ischemic
brain tissue can worsen the injury, which is caused a phenomenon
known as cerebral ischemia/reperfusion (I/R) injury (2). This condition involves multiple
pathological processes, including pyroptosis, apoptosis, oxidative
stress and the inflammatory response (3). Current treatment options for cerebral
I/R injury are limited, as although substances such as antioxidants
and anti-inflammatory agents have demonstrated neuroprotective
effects in models of cerebral I/R injury, consistent results have
not yet been achieved in further clinical trials (4). This highlights the urgent need for
the development of novel therapeutic strategies.
Pyroptosis is a form of inflammatory programmed cell
death that has been documented to serve a key role in cerebral I/R
injury (5,6). It is characterized by activation of
the NLR family pyrin domain containing 3 (NLRP3) inflammasome,
which in turn activates caspase-1 to cleave gasdermin D (GSDMD) and
promotes the release of various inflammatory cytokines, such as
IL-1β and IL-18(7). These
cytokines, through binding to their respective receptors, initiate
inflammatory signaling cascades that ultimately result in
pyroptotic cell death (8).
Adenosine is an endogenous purine nucleoside and can
mediate a variety of physiological functions through interactions
with its receptors (9). There are
four currently known subtypes of adenosine receptors (A1, A2a, A2b
and A3), which are widely distributed throughout the body and are
therefore implicated in a range of pathological conditions,
including ischemic cerebrovascular diseases, immune disorders,
cardiovascular diseases and inflammation, thereby underscoring the
critical importance of selecting adenosine receptors based on their
subtypes (10). Amongst them,
adenosine A1 receptors (A1Rs) exert neuroprotective effects in the
central nervous system (11).
Recent studies have shown that adenosine A1R agonist
[2-chloro-N(6)-cyclopentyladenosine (CCPA)] can
alleviate cerebral I/R injury in the MCAO model (12), although the exact mechanisms remain
unclear.
The Nrf2/NLRP3 pathway is a key pathway in
regulating pyroptosis (13).
Therefore, the present study aimed to investigate the effects of
the adenosine A1R agonist on the Nrf2/NLRP3 signaling pathway and
pyroptosis in rats, to elucidate the possible mechanism of
adenosine A1R agonist in cerebral I/R injury.
Materials and methods
Experimental animals
A total of 36 healthy, male Sprague-Dawley rats
(age, 6-7 weeks; weight, 230-250 g) were provided by Beijing
Weitong Lihua Experimental Animal Technology Co., Ltd. [license no.
SCXK (Beijing) 2021-0011]. The temperature in the animal room was
controlled at 25±1˚C and the air humidity was 60-65%. Animals were
housed under a 12-h light/dark cycle and had free access to food
and water ad libitum. The present study was approved by the
Animal Experiment Ethics Committee of Xinxiang Medical University
(approval no. K2023-64-01; Xinxiang, China).
Main drugs, reagents and
instruments
Adenosine A1R agonist CCPA (cat. no. 119136) was
purchased from MilliporeSigma. The NLRP3 (cat. no. 38679) and IL-1β
antibodies (cat. no. 41059) were obtained from Signalway Antibody
LLC. The GSDMD (cat. no. AF4012), caspase-1 (cat. no. AF5418), Nrf2
(cat. no. AF0639) and GAPDH antibodies (cat. no. AF7021) were
obtained from Affinity Biosciences. The Nrf2 inhibitor ML385 (cat.
no. T4360) was purchased from TargetMol Chemicals, Inc. Western
blotting electrophoresis equipment was obtained from Bio-Rad
Laboratories, Inc. A cryogenic grinder was purchased from Shanghai
Jingxin Industrial Development Co., Ltd.
Animal grouping and model
establishment
A total of 36 Sprague-Dawley rats were randomly
divided into the following four groups: Sham, I/R, adenosine A1
receptor agonist preconditioning (AP) and ML385, with 9 rats in
each group. Amongst these, 3 rats were used for
2,3,5-triphenyltetrazolium chloride (TTC) staining, 3 for western
blot analysis and 3 rats for immunofluorescence. The sham and I/R
groups were given intraperitoneal injections with an equal amount
of physiological saline (0.9% NaCl, 2 ml/kg) (14) 3 days before surgery, once per day.
The AP group was administered the adenosine A1R agonist (200 µg/kg
dissolved in physiological saline) by intraperitoneal injection 3
days before surgery, once per day (15). The ML385 group received
intraperitoneal injections of ML385 + adenosine A1R agonist (30
mg/kg + 200 µg/kg, respectively) 3 days before surgery, once per
day. The dosages used were based on previous studies (16,17).
The middle cerebral artery occlusion (MCAO) model
was prepared using the Longa method (18). After an intraperitoneal injection
of 40 mg/kg pentobarbital sodium for anesthesia, the rats were
fixed in a supine position and a midline longitudinal incision was
made in the neck to separate the left common carotid artery (CCA),
external carotid artery (ECA) and the internal carotid artery
(ICA). A V-shaped oblique incision was made at the bifurcation of
the ECA and ICA using vascular scissors. The arterial clamp was
then reopened and the embolization line was inserted through the
residual end of the ECA into the ICA until slight resistance was
felt, after which the insertion was stopped. The upper end of the
CCA was then ligated to prevent bleeding and movement. Blood flow
was blocked for 2 h, the filament was withdrawn, and after
reperfusion for 24 h, neurological function scoring was conducted.
The sham group did not undergo middle cerebral artery
embolization.
The following criteria were used to determine when
animals should be immediately euthanized during the 24 h of
reperfusion: i) Lack of movement or unresponsiveness to gentle
stimuli; ii) respiratory distress (typical symptoms include
drooling from the mouth or nose and/or cyanosis); iii) diarrhea or
urinary incontinence; iv) weight loss of >20% compared with the
pre-experiment body weight; v) inability to eat or drink; vi)
persistent seizures or stereotyped behavior; and vii) skin lesions
covering >30% of the body or signs of purulent infection. The
surgical procedure was smooth, with no rat fatalities.
All rats were euthanized with an intraperitoneal
injection of sodium pentobarbital (150 mg/kg) 24 h after MCAO.
Death was confirmed based on the absence of pain responses,
complete cessation of cardiac and respiratory activity and pupil
dilation.
Neurobehavioral function score
Neurobehavioral function score was assessed at 24 h
after reperfusion using the Longa method (19). Scores of 1-4 were considered
indicative of a successful model. The scoring scale used was as
follows: 0, no neurological deficits; 1, failure to fully extend
the right forepaw (mild deficits); 2, turning in circles to the
right while crawling (moderate deficits); 3, falling to the right
(moderate deficits); 4, not able to walk independently and
exhibiting a depressed level of consciousness (severe
deficits).
TTC staining
After rat euthanasia, the brain was quickly removed,
placed in a -20˚C freezer for 20 min. The brain was cut into five
2-mm coronal sections. The slices were soaked in a 2% TTC solution
and stained in the dark at 37˚C, then the brain slices were fixed
with 4% paraformaldehyde at 4˚C for 24 h. The non-ischemic area
appeared red, whilst the infarcted area was white. ImageJ software
(version 1.6.0; National Institutes of Health) was used for image
analysis, where the infarct volume ratio (%) was calculated using
the following formula: Infarct volume ratio=(infarct area/total
brain volume) x100% = (∑ infarct area x slice thickness)/(∑ total
brain area x slice thickness) x100%.
Western blotting
The separated cerebral cortex was stored at -80˚C
for subsequent protein extraction. The tissue samples (50 mg each)
were lysed with RIPA lysis buffer (Beyotime Institute of
Biotechnology) at 4˚C, before the tissue homogenate was centrifuged
at 4˚C and at 12,000 x g for 30 min to extract proteins. The
protein concentration was determined using the BCA method (Bio-Rad
Laboratories, Inc.). Proteins samples (50 µg/lane) were separated
on a 10% gel by SDS-PAGE and transferred onto a PVDF membrane.
After blocking the membrane with 5% milk at room temperature for 2
h, diluted primary antibodies (1:1,000; diluted in blocking
solution) against GAPDH, Nrf2, NLRP3, caspase-1, GSDMD and IL-1β
were added and incubated overnight at 4˚C. Subsequently,
anti-rabbit IgG (H+L) secondary antibody (cat. no. 14708; 1:1,000;
Cell Signaling Technology, Inc.) was added and the membrane was
incubated in the dark at room temperature for 1 h. The membrane was
visualized using an enhanced chemiluminescence detection kit (cat.
no. WBULS0100; Merck KGaA). ImageJ software (version 1.6.0;
National Institutes of Health) was used to analyze the target bands
and calculate the band density, before protein expression was
normalized to GAPDH levels.
Immunofluorescence
The brain tissue was fixed in a 4% paraformaldehyde
solution at 4˚C for 24 h, before 20-µm-thick slices were prepared
in coronal position using a cryostat at -20˚C. Slices containing
the hippocampal dentate gyrus region were selected for
immunofluorescence analysis. The slices were permeabilized with
0.2% Triton X-100 and blocked with 10% normal goat serum (cat. no.
16210064; Thermo Fisher Scientific, Inc.) at room temperature for
45 min. Subsequently, 35 µl diluted (1:200) Nrf2 and GSDMD primary
antibodies were added to the sections and incubated overnight at
4˚C. After washing three times with PBS, the slices were incubated
with 35 µl goat anti-rabbit IgG (H+L) Fluor488-conjugated secondary
antibody (cat. no. S0018; 1:1,000; Affinity Biosciences) at room
temperature in the dark for 2 h. The tissue was stained with DAPI
(cat. no. C1005; 1:1; Shanghai Biotium Biological Co., Ltd.) at
25˚C for 5 min, followed by mounting with glycerol. Images were
obtained using a Leica microscope (Leica DM 2000, Leica
Microsystems, Inc.) and Leica microscope software (LAS V3.7). The
number of positive stained cells was counted using ImageJ software
(version 1.6.0; National Institutes of Health).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 9 software (Dotmatics). The neurobehavioral function score is
presented as the median (interquartile range), whilst all other
data are presented as the mean ± standard deviation. The normality
of the data was assessed using the Shapiro-Wilk test, whereas the
homogeneity of variance was examined using the Brown-Forsythe test.
One-way ANOVA followed by Tukey's post hoc test was used for
comparisons among multiple groups, whilst the neurobehavioral
function score was evaluated using Kruskal-Wallis followed by
Dunn's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Neurobehavioral function score and
cerebral infarction volume of rats
Compared with that in the sham group, the
neurobehavioral function score in the I/R group was found to be
significantly increased (P<0.05). Compared with that in the I/R
group, the AP group exhibited a significant decrease in the
neurobehavioral function score (P<0.05). By contrast, compared
with that in the AP group, the neurobehavioral function score of
the ML385 group was significantly higher (P<0.05; Table I).
 | Table INeurobehavioral function score in each
group. |
Table I
Neurobehavioral function score in each
group.
| Group | Neurobehavioral
function score |
|---|
| Sham | 0 (0,0) |
|
Ischemia/reperfusion | 4 (3,4)a |
| AP | 2 (1,2)b |
| ML385 | 3
(3,3.5)c |
| Overall P-value | <0.01 |
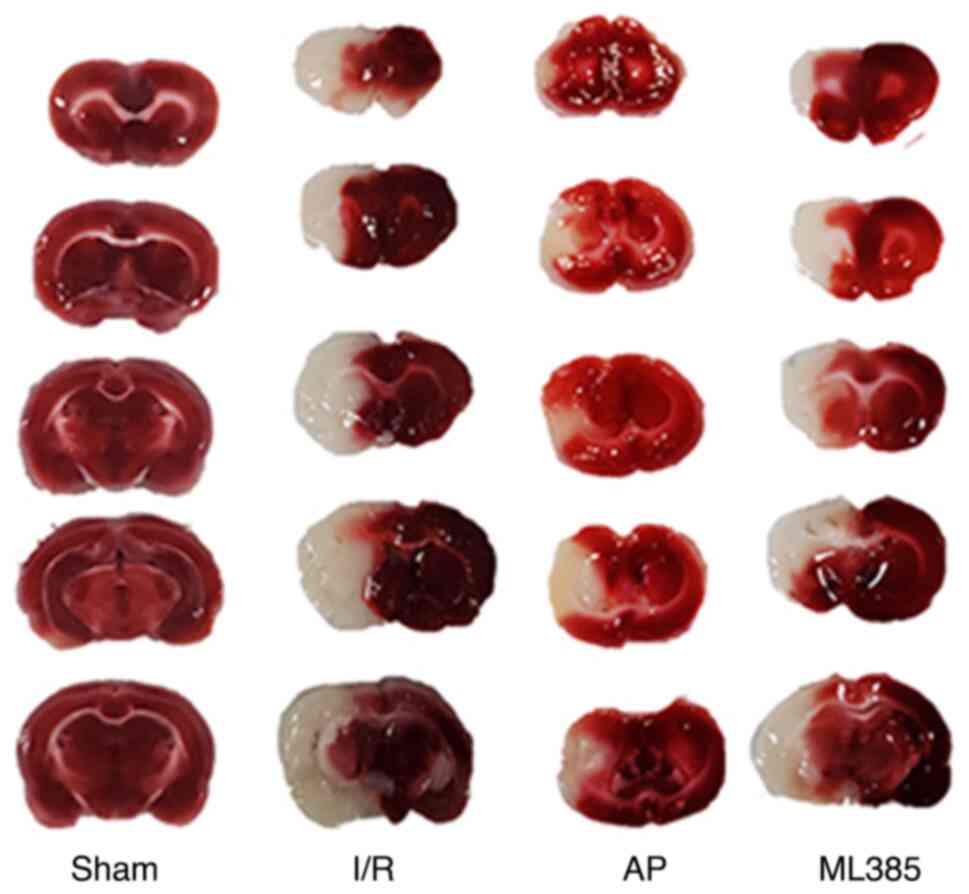
TTC staining of the brain tissue showed minimal
infarcted area in the sham group. However, compared with that in
the sham group, the I/R group showed a significantly larger area of
ischemic infarction (P<0.05). Compared with that in the I/R
group, the cerebral infarction volume of the rats in the AP group
was significantly decreased (P<0.05). Compared with that in the
AP group, the ML385 group exhibited a significant increase in the
cerebral infarction volume (P<0.05; Fig. 1 and Table II).
 | Table IICerebral infarction volume and protein
expression levels of GSDMD, caspase-1 and IL-1β in each group. |
Table II
Cerebral infarction volume and protein
expression levels of GSDMD, caspase-1 and IL-1β in each group.
| | Western blotting | |
|---|
| Group | Cerebral infarct
volume, % | GSDMD | Caspase-1 | IL-1β | Immunofluorescence
GSDMD |
|---|
| Sham | 0.00±0.00 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 | 2.00±1.00 |
| I/R |
0.37±0.02a |
1.43±0.03a |
2.54±0.07a |
2.99±0.16a |
34.33±3.06a |
| AP |
0.14±0.02b |
1.14±0.02b |
1.48±0.05b |
1.67±0.08b |
5.67±2.08b |
| ML385 |
0.30±0.01c |
1.27±0.03c |
2.39±0.06c |
2.78±0.09c |
27.00±2.65c |
| F-value | 455.96 | 184.43 | 620.25 | 269.36 | 127.73 |
| P-value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
Expression of pyroptosis-associated
proteins in the hippocampal tissue of rats
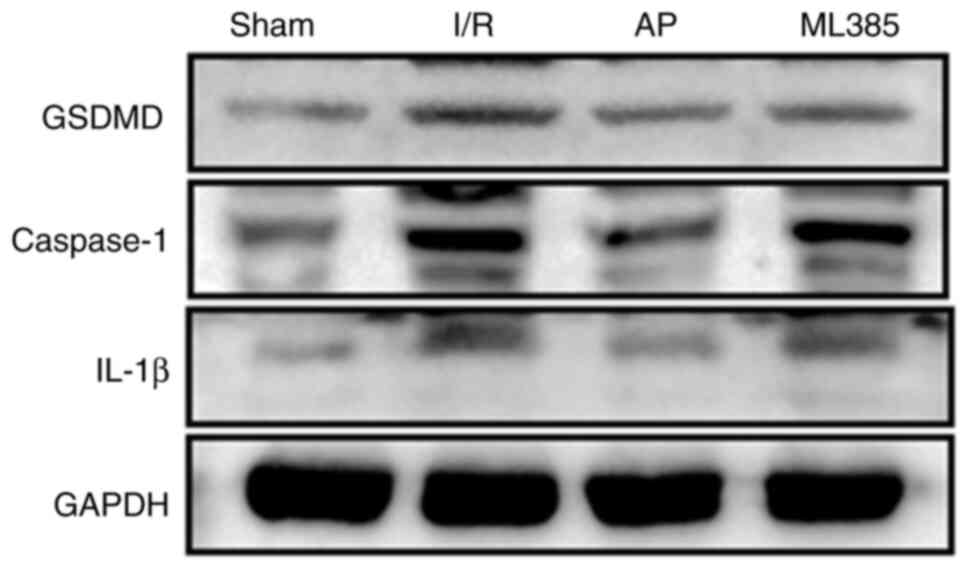
The protein expression levels of caspase-1, GSDMD
and IL-1β in the hippocampus of rats were next detected through
western blotting. The results showed compared with those in the
sham group, the protein expression levels of caspase-1, GSDMD and
IL-1β were all significantly increased in the I/R group
(P<0.05). Compared with those in the I/R group, the expression
levels of caspase-1, GSDMD and IL-1β were significantly reduced in
the AP group (P<0.05). By contrast, compared with those in the
AP group, the ML385 group exhibited significantly increased protein
expression levels of caspase-1, GSDMD and IL-1β (P<0.05;
Fig. 2 and Table II).
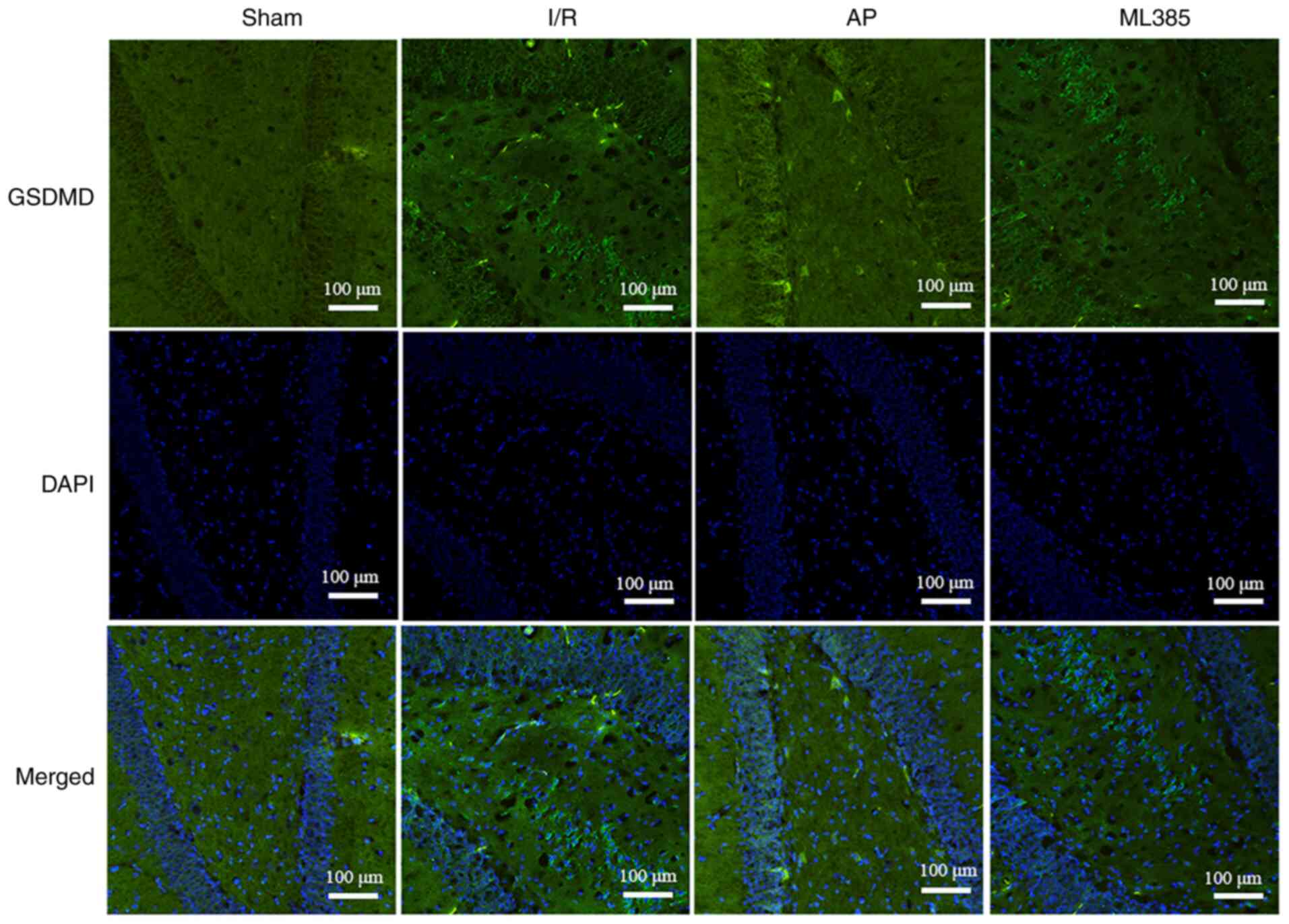
Immunofluorescence assay was then used to detect the
expression of GSDMD in rat hippocampal dentate gyrus. The results
showed that compared with that in the sham group, the I/R group
showed significantly increased expression of GSDMD (P<0.05).
Compared with that in the I/R group, the AP group showed a
significant decrease in GSDMD expression (P<0.05). However,
compared with that in the AP group, the ML385 group showed a
significant increase in GSDMD expression (P<0.05; Fig. 3 and Table II).
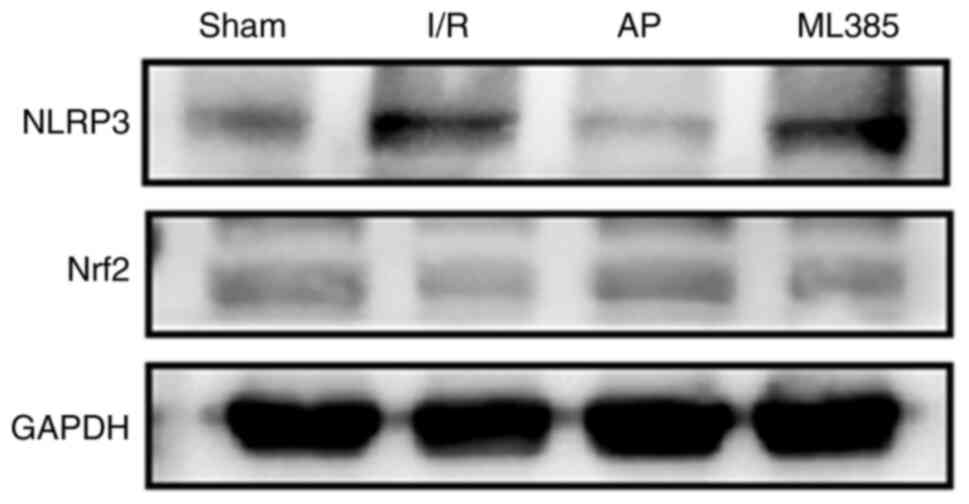
Expression of Nrf2 and NLRP3 proteins
in the hippocampal tissue of rats
The Nrf2/NLRP3 pathway is a key pathway in
regulating pyroptosis (13). The
protein expression levels of Nrf2 and NLRP3 were detected via
western blotting. The results showed that compared with those in
the sham group, the I/R group exhibited a significant decrease in
the Nrf2 protein expression level (P<0.05) and a significant
increase in the NLRP3 protein expression level (P<0.05).
Compared with that in the I/R group, the expression level of Nrf2
was significantly increased (P<0.05) whilst the expression level
of NLRP3 was significantly decreased (P<0.05) in the AP group.
Furthermore, compared with those in the AP group, the ML385 group
showed a significant decrease in the Nrf2 protein expression level
(P<0.05) and a significant increase in the NLRP3 protein
expression level (P<0.05; Fig.
4 and Table III).
 | Table IIIProtein expression levels of Nrf2 and
NLRP3 in each group. |
Table III
Protein expression levels of Nrf2 and
NLRP3 in each group.
| | Western
blotting | |
|---|
| Group | Nrf2 | NLRP3 | Immunofluorescence
Nrf2 |
|---|
| Sham | 1.00±0.00 | 1.00±0.00 | 17.33±2.52 |
| I/R |
0.67±0.02a |
2.34±0.16a |
2.00±1.00a |
| AP |
0.92±0.01b |
1.46±0.08b |
8.67±2.08b |
| ML385 |
0.81±0.01c |
1.90±0.06c |
2.67±1.53c |
| F-value | 324.64 | 106.20 | 43.30 |
| P-value | <0.01 | <0.01 | <0.01 |
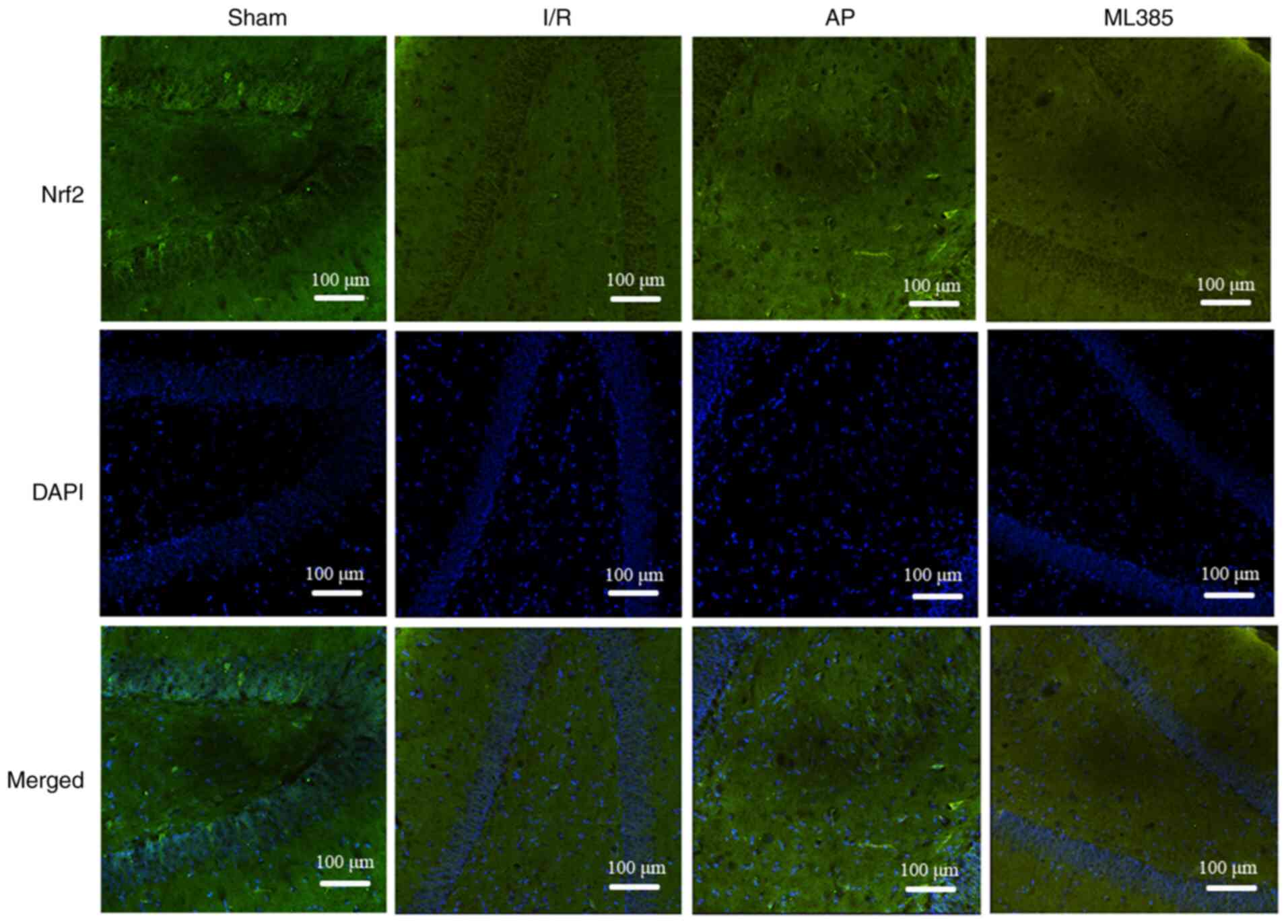
Immunofluorescence assay was then used to detect
Nrf2 protein expression in rat hippocampal dentate gyrus. The
results showed that compared with that in the sham group, the I/R
group showed a significant decrease in Nrf2 protein expression
(P<0.05). Compared with that in the I/R group, the expression of
Nrf2 was significantly increased in the AP group (P<0.05). By
contrast, compared with that in the AP group, the ML385 group
exhibited a significant decrease in Nrf2 expression (P<0.05;
Fig. 5 and Table III).
Discussion
Under ischemic, hypoxic, inflammatory and/or
stressful conditions, CD39 and CD73 catalyze the conversion of
extracellular ATP into adenosine, resulting in a notable increase
in adenosine concentrations (20).
It has been previously demonstrated that the elevation of
extracellular adenosine levels is associated with an endogenous
neuroprotective response against neuronal damage caused by ischemic
or inflammatory stress (21,22).
In particular, adenosine A1R serves an important regulatory role in
the central nervous system, participating in various physiological
and pathological processes, such as the regulation of
neurotransmitter release, modulation of neuronal excitability,
neuroprotection in ischemic injury and the inhibition of
inflammation (23). A previous
study has shown that intraperitoneal injection of CCPA could reduce
the infarct size in an MCAO rat model (24). Another previous study has also
suggested that activation of adenosine A1R is beneficial for
individuals with acute ischemic hypoxic injury, particularly in the
brain (25). Consistent with the
aforementioned previous studies, the present study demonstrated
neuroprotective effects of adenosine A1R agonist in alleviating
cerebral I/R injury. Unlike a previous study (12), the present study revealed that the
adenosine A1R agonist not only significantly reduced the cerebral
infarct volume and ameliorated neurological deficits in rats, but
also inhibited pyroptosis downstream of the Nrf2/NLRP3 signaling
pathway (Fig. 6). To the best of
our knowledge, the present study was the first to reveal that
adenosine A1R agonist can alleviate cerebral I/R injury,
potentially by activating the Nrf2/NLRP3 pathway to inhibit
pyroptosis, thereby providing novel insights into the mechanism by
which adenosine A1R agonist can ameliorate cerebral I/R injury.
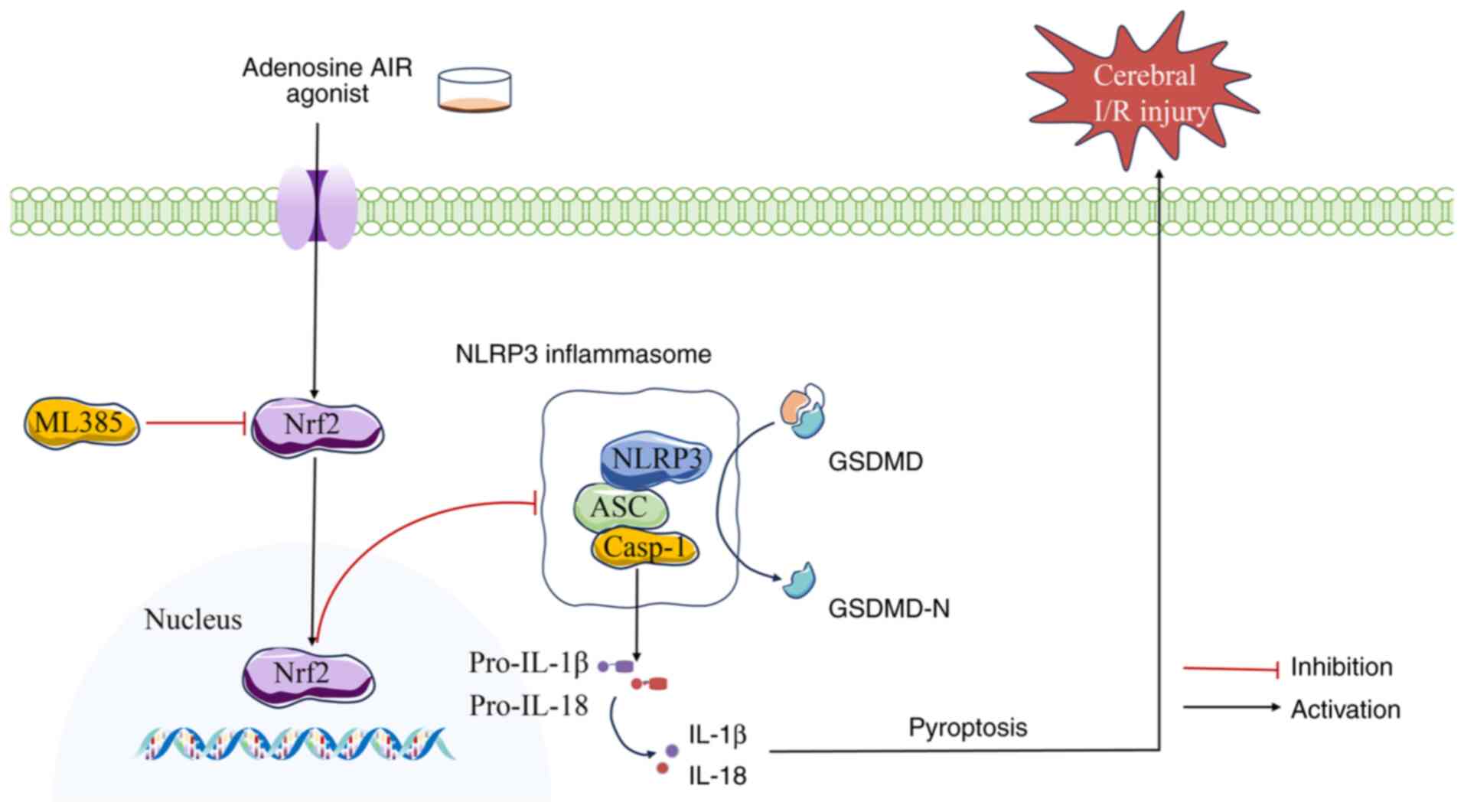 | Figure 6Adenosine A1R agonist inhibits
pyroptosis mediated by the Nrf2/NLRP3 signaling pathway, thereby
ameliorating cerebral I/R injury. The adenosine A1R agonist
improves cerebral I/R injury by activating Nrf2, inhibiting the
NLRP3 inflammasome and downregulating pyroptosis-related proteins
such as caspase-1, GSDMD and IL-1β, thereby suppressing pyroptosis.
The effects of the A1R agonist are reversed upon the use of the
Nrf2 inhibitor ML385. A1R, A1 receptor; Nrf2, nuclear factor
erythroid 2-related factor 2; NLRP3, NLR family pyrin domain
containing 3; GSDMD, gasdermin D; I/R, ischemia/reperfusion; AP,
adenosine A1 receptor agonist preconditioning. Casp-1, caspase-1;
GSDMD-N, N-terminal domain of GSDMD; ASC, apoptosis-associated
speck-like protein containing a caspase recruitment domain. |
Pyroptosis is a form of cell death that is typically
associated with inflammatory responses. During pyroptosis, rupture
of the cell membrane leads to the release of intracellular contents
and proinflammatory factors into the extracellular environment.
Pro-caspase-1 is cleaved into its active form, caspase-1, by
inflammasomes, which then cleaves GSDMD into two N-terminal
fragments and mediates the formation of membrane pores, allowing
water to enter the cell, causing swelling, cell lysis and the
release of IL-1β and IL-18(26).
It has been previously demonstrated that pyroptosis is associated
with the increased expression of caspase-1, GSDMD, IL-1β and
IL-18(27). A previous study
demonstrated that the expression of pyroptosis-associated proteins
increases after cerebral I/R injury, thereby indicating that
pyroptosis may be involved in this process (28). Consistent with the aforementioned
studies, the present study observed an increase in the expression
of caspase-1, GSDMD and IL-1β after cerebral I/R injury. Notably,
administration of adenosine A1R agonist resulted in the decreased
expression of these proteins, suggesting that the neuroprotective
effect of the adenosine A1R agonist in cerebral I/R injury is
closely associated with pyroptosis.
To investigate the specific mechanism by which
adenosine A1R agonist alleviates pyroptosis after cerebral I/R
injury, the signaling pathways involved were further examined. Nrf2
is an important regulatory factor in cerebral I/R injury. Upon
cellular injury, Nrf2 is activated and transferred to the nucleus,
where it binds to antioxidant response elements in the promoter
regions of various target genes. These target genes include those
involved in oxidative stress regulation, cellular repair and
inflammation (29). Notably,
activation of the Nrf2/NLRP3 pathway has been demonstrated to
mitigate cerebral I/R injury in the rat MCAO model (30). The present study also supports this
finding, revealing that the amelioration of cerebral I/R injury
after the administration of adenosine A1R agonist was accompanied
by the upregulation of Nrf2 and the downregulation of NLRP3. The
Nrf2/NLRP3 pathway is a key signaling pathway regulator of
pyroptosis (31). A previous study
has indicated that activation of the Nrf2/NLRP3 signaling pathway
can suppress the expression of pyroptosis-associated proteins in
neuronal cells of the brain in the cerebral I/R injury model
(32). The present study
demonstrated that adenosine A1R agonist not only led to the
activation of the Nrf2/NLRP3 pathway but also the inhibition of
pyroptosis. However, in contrast with a previous study (33), after the co-administration of
adenosine A1R agonist and the Nrf2-specific inhibitor ML385, the
effect of adenosine A1R agonist in inhibiting pyroptosis was
reversed, thereby demonstrating that the inhibition of pyroptosis
by the adenosine A1R agonist following cerebral I/R injury is
dependent on the Nrf2/NLRP3 signaling pathway. To the best of our
knowledge, the present study demonstrates for the first time that
adenosine A1R agonist can alleviate cerebral ischemia/reperfusion
injury by inhibiting Nrf2/NLRP3 signaling-mediated pyroptosis.
In conclusion, the present study demonstrated that
adenosine A1R agonist can improve cerebral I/R injury, potentially
by inhibiting pyroptosis mediated by the Nrf2/NLRP3 signaling
pathway. However, there are certain limitations. Future studies
should examine the active/cleaved forms of certain proteins, such
as GSDMD, caspase-1 and IL-1β, to confirm the effect of A1R agonist
on pyroptosis. The association between adenosine A1R and the
Nrf2/NLRP3 signaling pathway is complex, where other signaling
pathways, such as PI3K, may also be involved. Previous studies have
shown that adenosine A1R agonist can activate the PI3K/Akt pathway
in neurons (34,35). Nrf2 is a key molecule in inhibiting
pyroptosis to ameliorate cerebral I/R injury, but it is also a
downstream molecule of the PI3K/Akt pathway (36). Therefore, future research will
further explore the association between adenosine A1R and the
Nrf2/NLRP3 signaling pathway and validate these findings in
clinical settings.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Henan Medical
Science and Technology Research and Development Program (grant no.
LHGJ20200533).
Availability of data of materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
QM and ZL designed the study protocol, performed
data analysis and wrote the final manuscript. JT and YL contributed
to the study design, data analysis and revised the manuscript. QM
and ZL confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by Xinxiang Medical
University (approval no. K2023-64-01; Xinxiang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Feigin VL, Brainin M, Norrving B, Martins
SO, Pandian J, Lindsay P, F Grupper M and Rautalin I: World stroke
organization: Global stroke fact sheet 2025. Int J Stroke.
20:132–144. 2025.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liu D, Ji Q, Cheng Y, Liu M, Zhang B, Mei
Q, Huan M and Zhou S: Cyclosporine A loaded brain targeting
nanoparticle to treat cerebral ischemia/reperfusion injury in mice.
J Nanobiotechnology. 20(256)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Guo XB, Deng X, Wang J, Qi Y, Zhao W and
Guan S: HAX-1 interferes in assembly of NLRP3-ASC to block
microglial pyroptosis in cerebral I/R injury. Cell Death Discov.
10:1–13. 2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang C, Ma Y, Zhao Y, Guo N, Han C, Wu Q,
Mu C, Zhang Y, Tan S, Zhang J and Liu X: Systematic review of
melatonin in cerebral ischemia-reperfusion injury: Critical role
and therapeutic opportunities. Front Pharmacol.
15(1356112)2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang K, Zhao H, Chen J, Yan LL, Zhao B,
Chen Y, Dong YY, Li ZC and He Z: Toona sinensis fruit polyphenols
alleviate cerebral ischemia-reperfusion injury in rats by
inhibiting MAPK signaling pathways and NLRP3
inflammasome/pyroptosis. J Ethnopharmacol.
342(119375)2025.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gao P, Shi H, Jin X, Guo S, Zhou X and Gao
W: Mechanism of astragaloside IV regulating NLRP3 through
LOC102555978 to attenuate cerebral ischemia reperfusion induced
microglia pyroptosis. Int Immunopharmacol.
131(111862)2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
He C, Zhao Y, Jiang X, Liang X, Yin L, Yin
Z, Geng Y, Zhong Z, Song X, Zou Y, et al: Protective effect of
Ketone musk on LPS/ATP-induced pyroptosis in J774A.1 cells through
suppressing NLRP3/GSDMD pathway. Int Immunopharmacol. 71:328–335.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu Y, Lei H, Zhang W, Xing Q, Liu R, Wu
S, Liu Z, Yan Q, Li W, Liu X and Hu Y: Pyroptosis in renal
inflammation and fibrosis: Current knowledge and clinical
significance. Cell Death Dis. 14(472)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li J, Teng D, Jia W, Gong L, Dong H, Wang
C, Zhang L, Xu B, Wang W, Zhong L, et al: PLD2 deletion ameliorates
sepsis-induced cardiomyopathy by suppressing cardiomyocyte
pyroptosis via the NLRP3/caspase 1/GSDMD pathway. Inflamm Res.
73:1033–1046. 2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pinna A, Parekh P and Morelli M: Serotonin
5-HT1A receptors and their interactions with adenosine A2A
receptors in Parkinson's disease and dyskinesia. Neuropharmacology.
226(109411)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Khan H, Kaur P, Singh TG, Grewal AK and
Sood S: Adenosine as a key mediator of neuronal survival in
cerebral ischemic injury. Neurochem Res. 47:3543–3555.
2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shi Y, Dai Q, Ji B, Huang L, Zhuang X, Mo
Y and Wang J: Electroacupuncture pretreatment prevents cognitive
impairment induced by cerebral ischemia-reperfusion via adenosine
A1 receptors in rats. Front Aging Neurosci.
13(680706)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li MZ, Zhao Y, Dai XY, Talukder M and Li
JL: Lycopene ameliorates DEHP exposure-induced renal pyroptosis
through the Nrf2/Keap-1/NLRP3/caspase-1 axis. J Nutr Biochem.
113(109266)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fabera P, Parizkova M, Uttl L, Vondrakova
K, Kubova H, Tsenov G and Mares P: Adenosine A1 receptor agonist
2-chloro-N6-cyclopentyladenosine and hippocampal excitability
during brain development in rats. Front Pharmacol.
10(656)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Geng W, Cai L, Han K, Li D, Mo Y, Dai Q,
Tang H, Zhang M, Akuetteh PDP, Balelang MF and Wang J:
Electroacupuncture pretreatment alleviates cerebral
ischemia-reperfusion injury by increasing GSK-3β phosphorylation
level via adenosine a1 receptor. BioMed Res Int.
2020(6848450)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fan GB, Li Y, Xu GS, Zhao AY, Jin HJ, Sun
SQ and Qi SH: Propofol inhibits ferroptotic cell death through the
Nrf2/Gpx4 signaling pathway in the mouse model of cerebral
ischemia-reperfusion injury. Neurochem Res. 48:956–966.
2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen L, Huang J, Yao ZM, Sun XR, Tong XH,
Hu M, Zhang Y and Dong SY: Procyanidins alleviated cerebral
ischemia/reperfusion injury by inhibiting ferroptosis via the
Nrf2/HO-1 signaling pathway. Molecules. 28(3582)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu H, Li J, Jiang L, He J, Zhang H and
Wang K: Dexmedetomidine pretreatment alleviates cerebral
ischemia/reperfusion injury by inhibiting neuroinflammation through
the JAK2/STAT3 pathway. Braz J Med Biol Res.
55(e12145)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Han W, Zhang E, Tian Y, Wang S and Chen Y:
Reversible middle cerebral artery occlusion without craniectomy in
rats. Exp Brain Res. 241:1471–1488. 2023.
|
|
21
|
Ramos-Zepeda G and Herrero JF: Interaction
of the adenosine A1 receptor agonist N6-cyclopentyladenosine (CPA)
and opioid receptors in spinal cord nociceptive reflexes. Life Sci.
93:233–239. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu H, Sun Q, Ding Z, Shi W, Wang WH and
Zhang C: Adenosine stimulates the basolateral 50 pS K+ channel in
renal proximal tubule via adenosine-A1 receptor. Front Physiol.
14(1242975)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schädlich IS, Winzer R, Stabernack J,
Tolosa E, Magnus T and Rissiek B: The role of the ATP-adenosine
axis in ischemic stroke. Semin Immunopathol. 45:347–365.
2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Martire A, Lambertucci C, Pepponi R,
Ferrante A, Benati N, Buccioni M, Dal Ben D, Marucci G, Klotz KN,
Volpini R and Popoli P: Neuroprotective potential of adenosine A1
receptor partial agonists in experimental models of cerebral
ischemia. J Neurochem. 149:211–230. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Martí Navia A, Dal Ben D, Lambertucci C,
Spinaci A, Volpini R, Marques-Morgado I, Coelho JE, Lopes LV,
Marucci G and Buccioni M: Adenosine receptors as neuroinflammation
modulators: Role of A1 Agonists and A2A Antagonists. Cells.
9(1739)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu J, Wang X, Wang X, Wang J, Ma Y, Cao Y
and Zhang W: Chicken gasdermins mediate pyroptosis after the
cleavage by caspases. Int J Biol Macromol.
270(132476)2024.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Luo E, Li Z, Zhang S, Wen Y, Yang Z, Zeng
H and Ding H: Hyperglycemia induces microglial pyroptosis by
increasing oxygen extraction rate: Implication in neurological
impairment during ischemic stroke. Mol Med Rep.
30(146)2024.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tuo QZ, Zhang ST and Lei P: Mechanisms of
neuronal cell death in ischemic stroke and their therapeutic
implications. Med Res Rev. 42:259–305. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tastan B, Arioz BI and Genc S: Targeting
NLRP3 inflammasome with Nrf2 inducers in central nervous system
disorders. Front Immunol. 13(865772)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
She Y, Shao L, Jiao K, Sun R, Lang T, Long
H, Tang Y, Zhang W, Ding C and Deng C: Glycosides of Buyang Huanwu
decoction inhibits pyroptosis associated with cerebral
ischemia-reperfusion through Nrf2-mediated antioxidant signaling
pathway both in vivo and in vitro. Phytomedicine.
120(155001)2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cheng Y, Cheng L, Gao X, Chen S, Wu P,
Wang C and Liu Z: Covalent modification of Keap1 at Cys77 and
Cys434 by pubescenoside a suppresses oxidative stress-induced NLRP3
inflammasome activation in myocardial ischemia-reperfusion injury.
Theranostics. 11:861–877. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shi M, Wang J, Bi F and Bai Z: Diosmetin
alleviates cerebral ischemia-reperfusion injury through
Keap1-mediated Nrf2/ARE signaling pathway activation and NLRP3
inflammasome inhibition. Environ Toxicol. 37:1529–1542.
2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liston TE, Hama A, Boltze J, Poe RB,
Natsume T, Hayashi I, Takamatsu H, Korinek WS and Lechleiter JD:
Adenosine A1R/A3R (adenosine A1 and A3 receptor) agonist AST-004
reduces brain infarction in a nonhuman primate model of stroke.
Stroke. 53:238–248. 2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhong M, Song WL, Xu YC, Ye Y and Feng LY:
Paeoniflorin ameliorates ischemic neuronal damage in vitro via
adenosine A1 receptor-mediated transactivation of epidermal growth
factor receptor. Acta Pharmacol Sin. 36:298–310. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Rabie MA, Ibrahim HI, Nassar NN and Atef
RM: Adenosine A1 receptor agonist, N6-cyclohexyladenosine,
attenuates Huntington's disease via stimulation of
TrKB/PI3K/Akt/CREB/BDNF pathway in 3-nitropropionic acid rat model.
Chem Biol Interact. 369(110288)2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Guo Y, Mao M, Li Q, Yu X and Zhou L:
Extracts of Ginkgo flavonoids and ginkgolides improve cerebral
ischaemia-reperfusion injury through the PI3K/Akt/Nrf2 signalling
pathway and multicomponent in vivo processes. Phytomedicine.
99(154028)2022.PubMed/NCBI View Article : Google Scholar
|