1. Introduction
One of the most common complications associated with
the peritoneum is the formation of abdominal adhesions after
surgery. Adhesions are bands of connective tissue that form between
organs or between organs and the abdominal wall, typically in
response to inflammation or (surgical) trauma. Such adhesions can
remain symptom-free for life, but they can lead to organ
dysfunction. These include restricted movement, pain, intestinal
obstruction or infertility. The formation of adhesions is a
significant problem after abdominal operations such as caesarean
sections, appendectomies or hysterectomies.
As aforementioned, adhesions are significant
regarding frequent issues faced in many surgical disciplines. They
are associated with chronic pelvic pain, infertility, and
intestinal obstruction, which lead to significant clinical
challenges, as noted across multiple studies (1,2). In
2004, one in 20 gastrointestinal procedures was primarily indicated
for adhesion lysis according to US Health System analyses. This has
resulted in 900,000 days of hospitalization as well as annual
adhesion-related costs of $2.3 billion (3).
The subsequent problems caused by adhesions have not
diminished (4,5). Capmas et al (6) describe that adhesions are widespread
in abdomino-pelvic surgery, particularly in gynecological and
colorectal procedures. The study was conducted in France and
demonstrated costs for hospitalization and surgery due to adhesions
of approximately €4 million in 2019. In this time period, more than
25.000 surgeries were performed in France with either the primary
or secondary goal of adhesiolysis. The adhesion-dependent economic
burden on the healthcare system arises primarily from the direct
costs of readmissions and repeat operations (6). The costs associated with loss of
productivity and long-term patient morbidity are also considerable.
However, these indirect costs are usually not even considered, but
would significantly increase the impact (5). Moreover, the process of adhesiolysis
itself was a part of one in three laparoscopic surgeries, as
detailed per diagnosis-related group codings. A 2023 systematic
review concerning the health care costs of adhesion-related small
bowel obstruction in different countries found national costs
reaching up to $1.77 billion (7).
The literature review included 7 clinical trials conducted
worldwide between 1999 and 2016. The mean total cost of the 39,573
patients was $1,814-$2,568 with medical treatment vs.
$4,914-$25,321 in the surgical treatment group. The mean length of
stay was 3.0-7.0 days for conservative treatment and 8.0-16.3 days
for surgical treatment. On average, the length of stay for patients
who underwent surgery was about three times longer than for
patients who received conservative treatment, which explains the
significantly higher treatment costs.
2. Adhesion types and diagnostics
Adhesions are attachments of tissue areas, which are
not physiologically connected to each other. They consist of
fibrous bands of scar tissue and thus connect areas of tissue or
organs that were originally not connected to each other. Adhesions
often arise as a result of surgery, inflammation or trauma. As
such, they are part of the body's natural healing process, but can
form abnormal connections that lead to medical complications
(8,9). In surgical context, one usually
refers to adhesions involving abdominal and pelvic organs as well
as the peritoneum.
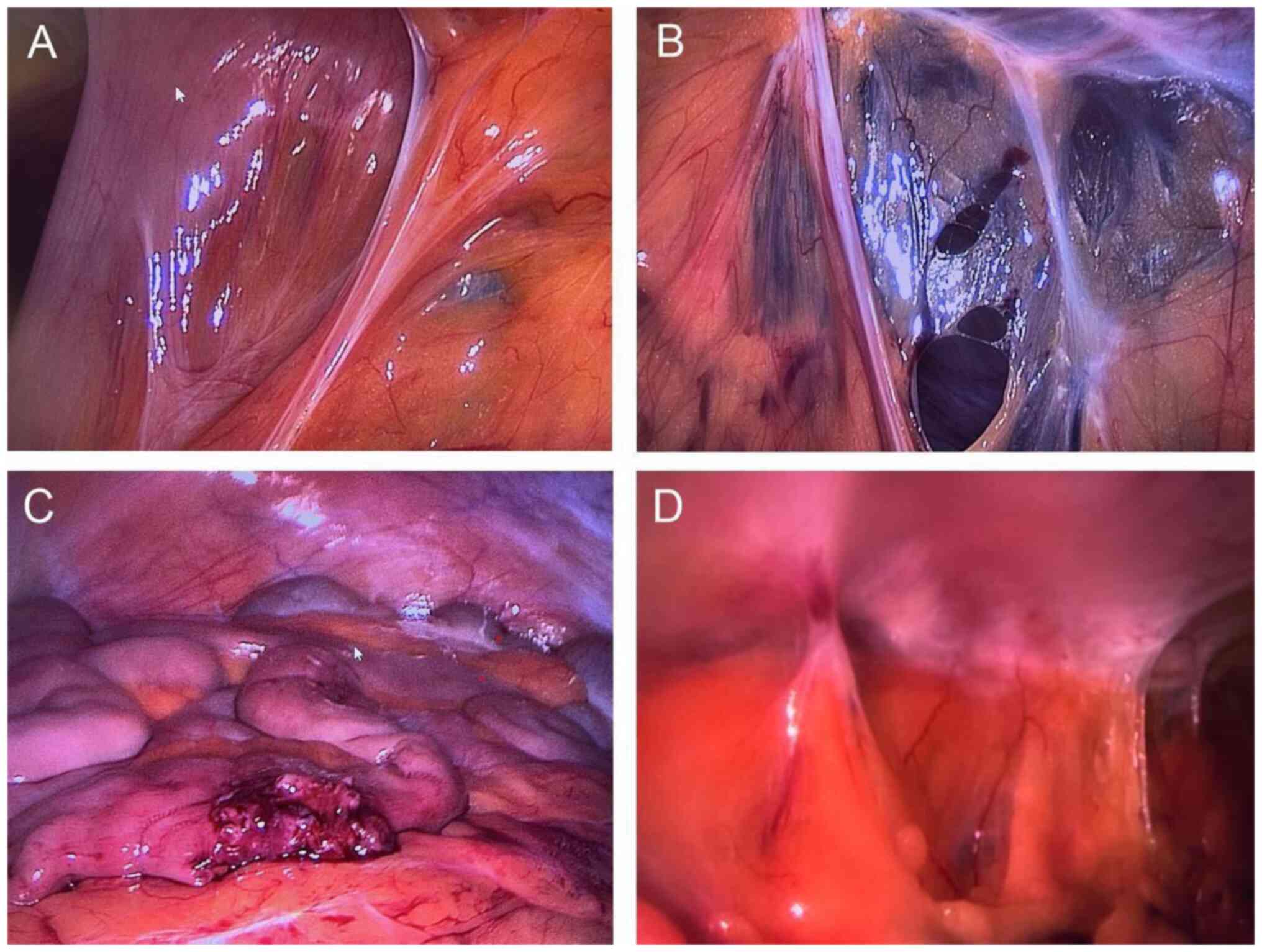
Fig. 1 shows that
adhesions can form between almost any intra-abdominal surface, such
as the intestines or the greater omentum. These surfaces are then
connected to either each other or the abdominal wall via such
adhesions. Adhesions are generally classified into three main types
according to their location, structure and functional effect.
One type of adhesion may occur during embryonic
organogenesis. This form of adhesion is congenital and is the
result of abnormal embryonic development. These adhesions usually
involve the gastrointestinal tract, e.g. the persistence of a
congenital band causing small bowel obstruction. However, they tend
to be more isolated and less extensive than acquired adhesions.
This happens comparatively rarely and is usually only incidentally
diagnosed. In most cases, such adhesions remain asymptomatic and do
not require therapy (8).
Adhesions can also be formed post-inflammatorily as
a consequence of acute or chronic underlying diseases. These
adhesions arise as a reaction to pathological processes such as
infections, endometriosis or chronic inflammation. Such
pathological adhesions tend to form dense and highly vascularized
fibrous bands. The clinical presentation is often asymptomatic or
mild in these cases. However, they can also occur with certain
diseases such as pelvic inflammatory disease or intra-abdominal
sepsis. In the clinical presentation, this can lead to chronic
pelvic pain, infertility and complications during surgical
interventions (9,10). A post-mortem study revealed
adhesions in 28% of all cases in probands who had never undergone
surgery (9). Etiologically, such
adhesions can be attributed to intra-abdominal inflammatory
processes (such as peritonitis, Crohn's disease, endometriosis, and
radiotherapy-induced inflammation) (2,10).
The largest adhesion group consists of clinically
significant postoperative adhesions (Fig. 1). Here, the peritoneum has been
damaged, which is estimated to occur in 70-100% of patients
(11,12). These adhesions are the result of
surgical trauma and are by a wide margin the most common adhesions.
The formation of such acquired adhesions is a response to injury
and subsequent inflammation of the peritoneum. The adhesions can
vary greatly in density and extent and range from very fine to
extremely strong and dense fibrous bands (10,12).
Although adhesions can also be completely
asymptomatic, all three main types can also lead to a variety of
complications. These range from the mildest symptoms to very
serious complications. For one thing, unclear symptoms such as
chronic abdominal or pelvic pain can occur. In many cases, however,
there are also defined clinical manifestations such as inflammatory
diseases or functional limitations of the affected organs, e.g.
peritonitis, intestinal obstruction or infertility (13). As a diagnosis of adhesions is
nearly impossible without directly seeing them, adhesions are
mostly detected incidentally during surgical procedures. The
patient's medical history may provide indications. When the
displaceability of organs and organ areas in relation to each other
is restricted, further evidence can sometimes be found with the use
of imaging techniques.
Modern imaging techniques enable very accurate
clarification of anatomical abnormalities. The use of a cine
magnetic resonance imaging (MRI) device makes it possible to
analyze the abdominal cavity with a high image sequence. It was
seen that adhesions could be detected with a sensitivity of 86.0%
and a specificity of 80.0% (14).
In addition, the non-invasive procedure also made it possible to
assess the localization and extent of adhesions as well as the
mobility of affected organs. Another advantage of MRI is that
patients are not exposed to ionizing radiation. However, the method
has its limitations with extremely thin adhesion bands and in areas
where only very low organ dynamics are naturally present. However,
MRI-based mapping of adhesions facilitates better planning of a
surgical procedure and reduces potential surgical risks and thus
also postoperative complications. Future advances in suitable
imaging techniques could significantly improve the diagnosis and
subsequent treatment of adhesions. However, routine adhesion
diagnostics using such devices is hampered by the high technical
and financial requirements.
3. Peritoneum
The peritoneum plays a crucial pathophysiological
role in the development of adhesions. Intra-abdominal adhesions are
mostly adhesions of the peritoneum itself, resulting from damage
during the surgical procedure. The larger the surface of an organ
covered by peritoneum and the denser the intra-abdominal position
of each organ in relation to each other, the higher the risk of
adhesions. This risk is particularly high during ovarian
interventions, which achieve adhesion rates of 90% in gynecologic
adnexal surgery (15,16).
The peritoneal membrane has two sheets that line the
peritoneal cavity in humans. The parietal sheet covers the
abdominal wall from the inside, while the visceral sheet envelops
the abdominal organs located intraperitoneally (17). The peritoneum is derived from the
mesoderm and develops from the lateral squamous mesoderm during
early embryogenesis. During embryogenesis, the peritoneum forms a
continuous membrane that lines the abdominal cavity and covers most
of the visceral organs. It later develops into the abdominal
cavity, which allows the abdominal organs to move. Structurally,
the peritoneum can be divided into two layers (18). Histologically, the peritoneum
possesses an outer single-layered mesothelial cell layer that
carries microvilli. In humans, this brush border increases the
secretory surface area by approximately 1.8 m² (19). Under the mesothelium, there is a
basement membrane and further below there is a connective tissue
layer with nerves, blood, and lymph vessels (20).
The primary functions of the peritoneum include
regulation of the fluid balance within the abdominal cavity,
maintaining organ mobility within the abdominal cavity and
recruitment and modulation of immune cell actions and.
Intra-abdominal homeostasis is sustained by secreted or resorbed
peritoneal fluid (19,20). This allows the mesothelial cells to
provide 50-75 ml of peritoneal fluid on the surface, which enables
low-friction displacement of the intraperitoneal organs (21). Finally, the peritoneum functions as
an immunologically active tissue. The peritoneal mesothelial cells
respond to infections or injuries and can release cytokines and
other inflammatory mediators. Cell-free areas of the stroma contain
fat-associated lymphoid clusters (macula lactea) with lymph
node-like functions (22,23). Neutrophilic granulocytes and
various lymphocyte types are located here for local immune defense
against infection and inflammation (24,25).
The adhesion formation is one of the most common
complications associated with the peritoneum, but there are also
other diseases of the peritoneum that can significantly restrict
its function. Peritonitis is an inflammation of the peritoneum and
can occur after infections, trauma or surgical interventions in the
abdominal cavity. The increased secretion of immunomodulators leads
to an increase in vascular permeability. This intensifies local
inflammatory effects and disrupts peritoneal fluid homeostasis
(18,20). Furthermore, fibrotic and sclerotic
processes can harden the peritoneum and thus impair its
functionality (19). Finally, the
peritoneum is also frequently affected by abdominal cancers.
Peritoneal carcinomas can occur in particular in stomach, colon and
ovarian cancer. Malignant cells penetrate the peritoneal mucosa,
causing inflammation and worsening the oncological prognosis
(15,19).
4. Cell biology of adhesion tissue
formation
As in wound healing, the pathophysiological
processes involved in adhesion formation include exudation,
resorption, and repair. At the cell biological level, complex
interactions between mesothelial cells and the extracellular matrix
(ECM) are involved, which are orchestrated by different signaling
pathways (26). Following
abdominal surgery, these biological events also take place leading
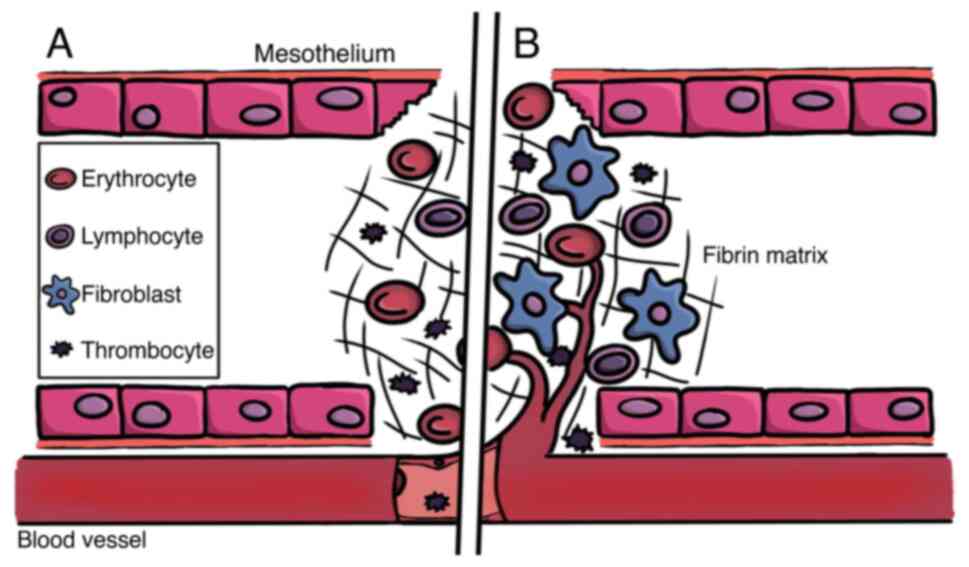
to the repair of the damaged tissue (Fig. 2).
The reparative processes begin with activation of
the fibrin system, accompanied by local inflammation. As tissue
repair progresses, hypoxia occurs and redox-regulated signaling and
effector cascades are stimulated in the affected tissue.
Revascularization occurs in the late phase of repair. Dysregulation
in these complex and finely orchestrated mechanisms, e.g.
imbalances of fibrin synthesis and degradation, leads to
abnormalities in tissue formation and ultimately to tissue
adhesions (27,28).
The formation of tissue adhesions begins with the
activation of the coagulation cascade. Injury to the mesothelial
layer leads to the release of permeability-increasing substances,
such as histamine or different cytokines and increases vascular
permeability (29). Moreover,
activation of the coagulation cascade occurs in areas of trauma. In
the extrinsic pathway, tissue factor (tissue thromboplastin, factor
III) localized in the subendothelial tissue activates factor VII in
the blood. In parallel, the intrinsic pathway is stimulated.
Collagen of the endothelium, exposed due to trauma, leads to
contact activation of additional coagulation factors (factor XIII,
factor IX, factor XI, factor XII). Both pathways lead to the
activation of factor V and factor X, and subsequently to
proteolytic cleavage of fibrinogen to fibrin by the enzyme
thrombin. Polymerization and cross-linking of the fibrin monomers
results in building a fibrin matrix (30,31).
Inflammatory reactions also play an important role
in adhesion formation. Following injury, immune cells infiltrate
the site and release cytokines and growth factors such as tumor
necrosis factor-alpha (TNF-α) and transforming growth factor-beta
(TGF-β), which promote fibrosis and activate mesothelial cells.
Activation of inflammatory pathways also induces the expression of
adhesion molecules on the surface of mesothelial cells, which
facilitates interaction with ECM components and neighboring cells
(30,32).
Peritoneal mesothelial cells undergo a so-called
mesothelial-to-mesenchymal transition (MMT). During this process,
these cells largely lose their epithelial properties and take on a
fibroblastic or myofibroblastic phenotype (33). MMT leads to an increased release of
ECM components such as collagen and fibronectin. The pivotal
regulator of MMT is TGFβ1 (34,35).
As a result, fibrous components form, which can be regarded as the
initial adhesion tissue.
During the process, the ECM continues to be
extensively modified. Most commonly, collagen, fibronectin and
hyaluronic acid are upregulated and integrated into the ECM in
excess at injury sites. This remodeling is primarily mediated by
matrix metalloproteinases (MMP) and their regulators Tissue
Inhibitors of MMP (TIMP). After MMT, the fibroblasts and
myofibroblasts secrete alpha-smooth muscle actin (α-SMA), a marker
for myofibroblastic differentiation. Furthermore, the cells produce
additional ECM proteins, which increases the mechanical stiffness
of the adherent tissue. (32).
The formation of new blood vessels (angiogenesis) in
the adherent tissue is another important characteristic of adhesion
formation. Reactive oxygen species (ROS) and signaling molecules
such as vascular endothelial growth factor (VEGF) are essential
factors in angiogenic initiation and progression (34,35).
The adhesion becomes irreversible when the adhesion tissue is
vascularized (36).
As in wound healing, redox processes are seemingly
involved in adhesion formation. ROS, such as hydroxyl radical,
hydrogen peroxide (H2O2), superoxide anion,
and singlet oxygen, serve as cofactors for cellular proteins and as
second messengers. Nitric oxide controls ECM production and is
released from adhesion-associated fibroblasts (37). H2O2 is an
important signaling molecule for the activation of VEGF-dependent
vascularization processes (38).
Furthermore, it controls various processes regarding
pro-inflammatory and anti-inflammatory cell responses including
immunomodulation. Immune cells, such as monocytes and macrophages,
migrate into the tissue and further contribute to an adhesive
microenvironment through immune modulatory protein factor secretion
(18,20,39).
Thus, a strategy of using specific therapeutics in
order to suppress ROS levels seems a promising way to intervene in
the process of adhesion formation. Several recent publications
regarding chronic wound healing discuss the use of
anti-oxidant-incorporating hydrogels for wound management (40,41).
Some of the incorporated anti-oxidant substances include gallic
acid-conjugated gelatin, Lignin, and Pseurotin A (42-44).
5. Cellular misregulations leading to
adhesion formation
There are two auto-regulated processes in which
dysregulation may primarily occur during the formation of adhesive
tissue. These are fibrinogenesis/fibrinolysis and ECM
synthesis/degradation (30). A
disturbed balance of synthesis and degradation of the fibrin matrix
during coagulation is primarily responsible for adhesion formation.
The extent of the coagulation pathway activation correlates with
the degree of inflammation in the area of trauma.
The mesothelial cells making up the outermost layer
of the peritoneum express cell adhesion-molecules (CAM), e.g.
intercellular adhesion molecule 1 or vascular cell adhesion
molecule 1. CAM, cytokines and growth factors (TGF-β, VEGF)
stimulate the invasion of granulocytes and lymphocytes as part of
the pathogenic process (45).
Important cytokines involved in adhesion formation include
interleukin 1 (IL-1), IL-6 and TNF-α (46). Further insight may be provided by
modulating these cytokines using molecular strategies such as small
molecules in future experiments may. Cytokine levels also
correspond to the local inflammation status and reflect the risk of
adhesion formation. Cytokine monitoring after surgical
interventions would therefore potentially also be a biomarker for
adhesion formation.
VEGF, which induces angiogenesis, is important in
adhesion formation as vascularization precludes reversal of
adhesions. VEGF inhibitors (i.e. humanized monoclonal antibodies
against VEGF such as Bevacizumab) are especially used in the
treatment of oncologic diseases. VEGF inhibitors could also inhibit
the proliferation of adhesive tissue and, in particular, reduce the
formation of adhesion-associated blood vessels. Their use could
therefore also be promising in adhesion prevention. (47).
On the other hand, complications have also been
described following the use of VEGF inhibitors. In a study of
cancer patients receiving bevacizumab, wound healing complications
were observed (48). The more
closely a surgical incision was made after bevacizumab
administration, the greater the wound healing complications. Since
VEGF contributes significantly to growth and vascularization, it is
an important factor in wound healing. This is in contrast to its
use as an adhesion inhibitor, as this application is intended to
suppress the adhesion-associated growth and wound healing
processes. These side effects significantly limit VEGF blockade for
adhesion prophylaxis and must be investigated further.
Fibrinogenesis is regulated by fibrinolysis, in
which fibrin is enzymatically digested. Sufficient degradation of
fibrin polymers usually leads to healing of the traumatized
peritoneal area. Plasmin, a serine protease formed from the
enzymatically inactive precursor plasminogen, is responsible for
this degradation (49). During
surgery, the fibrinolytic capacity of the peritoneum decreases
significantly. The enzymatic activation of plasmin occurs locally
in the peritoneum mainly induced by tissue-type plasminogen
activator (tPA) and by urokinase-type plasminogen activator (uPA)
(36). Fibrin levels induce the
activity of tPA/uPA, while plasminogen activator inhibitor (PAI)
inhibits it (31,49,50).
PAI is secreted by both adhesive tissue cells and migratory immune
cells (36). A study of tissue
expression of PAI and tPA in the peritoneum showed that in patients
with severe adhesions, synthesis of tPA was significantly decreased
while synthesis of PAI significantly increased. The resulting
reduced fibrinolytic capacity represented a risk factor for the
development of adhesions (50,51).
Another critical step in adhesion formation is the
synthesis of ECM components. Here, too, there is a balance between
ECM synthesis by adhesive myofibroblasts and ECM degradation by
extracellular MMP. MMP are in turn inhibited by the MMP inhibitors
TIMP in order to prevent an excessive cell response. As with the
fibrin-plasmin system, a pathological imbalance of the ECM-MMP-TIMP
system leads to impaired wound healing and subsequently to an
increased risk of adhesion tissue formation (52,53).
Elevated PAI-1 levels in the peritoneum can impair fibrinolysis and
promote adhesion formation (50).
Accordingly, increased tPA levels have been shown to correlate with
a lower risk of adhesion, whereas increased PAI-1 levels correlate
with an increased risk of adhesion (51). Another regulator of MMP enzyme
activity is the neurokinin receptor-1 (NKR-1). By antagonizing
NKR-1, MMP activity could be increased and thus adhesion formation
was reduced (53).
6. Adhesion prophylaxis
One has to differentiate between surgical methods
and the use of so-called barrier methods when discussing strategies
for minimizing adhesion formation.
As far as suitable surgical procedures are
concerned, the goal is an option with minimal intraoperative trauma
and thorough hemostasis (54).
Thus, minimally invasive surgery, such as laparoscopy, seems to
have several advantages over laparotomy (55,56).
There are several factors that determine the risk of
adhesion formation during laparoscopy. One of these factors is the
intraoperative hypoxia caused by carbon dioxide (CO2)
insufflation. In a mouse model, hypoxia was correlated with an
upregulation of the hypoxia-induced factor 1a (HIF-1a) and HIF-1b
(57). Best results with the
intention to minimize this risk could be achieved with a
CO2 insufflation with a concentration of 3% added oxygen
(58).
Another factor is the peritoneal insufflation
pressure during laparoscopic surgery. Animal studies demonstrated
more severe adhesions when higher pressures (6, 9, 12 mmHg) were
applied (59). In postoperative
analyses, more, larger and more severe adhesions were observed with
increasing pressures. The results suggest that higher insufflation
pressures may increase both ischemia and trauma to the peritoneum,
thus raising the risk of adhesion formation.
Nevertheless, laparoscopic techniques can in
principle be used to treat adhesions. The minimally invasive nature
causes less tissue trauma and thus reduces the risk of new adhesive
tissue formation (60). However,
in very complex situations with dense or extensive adhesions, it
can be very challenging. It may then be necessary to switch to open
surgery for technical reasons. Based on the literature data, the
authors assume that laparoscopic procedures offer a small but
significant advantage in terms of adhesion prevention.
Several studies have investigated the molecular
mechanisms of adhesion formation and gained insights into the
possible prophylactic application of fibrinolytics (61). Thus, pharmacological activation of
plasmin appears to be a potentially effective application to reduce
adhesion. The data were confirmed with recombinant tPA,
streptokinase-streptodornase and pepsin/trypsin in clinical
studies. Despite promising adhesion-reducing results, however,
there are only few clinical data available (61-64).
One reason may be that protein/enzyme preparations are expensive to
produce and sensitive in clinical use.
Barrier methods are currently the most promising
methods for preventing adhesions. They act as spacers between
peritoneal wound surfaces and thus reduce the formation of fibrin
bridges (65). There is currently
a lack of sufficient and systematic clinical evidence on adhesion
prophylaxis to adequately assess the effectiveness of the various
procedures. Therefore, some medical societies do not yet provide a
clear recommendation for the use of adhesion prophylaxis. A
consensus article from 2022 explicitly emphasizes that more and
coordinated research activities on adhesion prophylaxis should be
encouraged (65). Standardized
examination procedures (study design) and evaluation criteria (e.g.
second-look laparoscopy) are also important instruments.
Three barrier methods have been approved by the US
Food and Drug Administration: ‘Interceed’, ‘Seprafilm’ and ‘Adept’.
The medical product ‘Adept’ consists of a 4% icodextrin-solution.
The resorption of this solution after surgery takes more than 96 h,
as the fluid cannot be resorbed directly, but must be removed from
the intraperitoneal cavity via the lymphatic system (54,66,67).
In comparison, Ringer's solution is resorbed in less than 24 h and
thus serves as a poor spacer between individual peritoneal layers
(68). The barrier methods called
‘Interceed’ and ‘Seprafilm’ are bioresorbable membranes, which are
applied intraoperatively, and have a similar purpose and way of
functioning as ‘Adept.’
There are also gelatinous barrier methods, e.g.,
‘SprayGel,’ which is made of polyethylene glycol (54). These can be applied during
laparotomy and endoscopically. Finally, the relatively novel
barrier method called ‘4DryField’ is applied in the form of a
powder and effects both adhesion formation and hemostasis (69,70).
Some studies have also failed to demonstrate sufficient efficacy of
anti-adhesion candidates. For example, ibuprofen, dexamethasone and
heparin exhibited no prophylactic effects with regard to the
formation of adhesions.
Another innovative method for adhesion prophylaxis
is the use of non-invasive physical plasma (NIPP), also known as
cold atmospheric plasma. NIPP treatment is on the threshold between
preclinical investigations including clinical trials and routine
use in everyday clinical practice, e.g. in tissue repair and wound
management (71,72). The main biomedical effect factors
are ROS including free oxygen radicals. These locally modulate the
immunological environment of the tissue area and can therefore
reduce postoperative adhesions (73-76).
NIPP inhibits both cell growth and cell-cell contacts of
proadhesive peritoneal fibroblasts (77). Such anti-adhesive effects have
already been described in connection with the treatment of
keloid-forming fibroblasts (78).
This treatment does not necessarily require the direct
intraoperative use of a NIPP device, but it is also possible to
apply physiologic saline solution that has itself been treated with
NIPP. Such indirect treatment with NIPP has been reported to be
successful in various in vitro cell models (79,80).
Therefore, postoperative irrigation of the abdominal cavity with
NIPP-treated physiologic saline could be a promising concept for
adhesion prophylaxis.
7. Adhesion therapy
The primary treatment for adhesions is adhesiolysis,
a surgical procedure to remove or separate the adhesions. This
procedure, like any surgical intervention, carries risks, including
the possibility of recurrence of adhesions. However, clinical
studies on non-invasive, pharmacological and physiotherapeutic
procedures have not yet revealed any significant effects in
adhesion patients (81-83).
The results of clinical studies show that
laparoscopic adhesiolysis significantly reduces the risk of
morbidity, mortality and postoperative infections. Furthermore,
serious complications and incisional complications are
significantly reduced. These clinical data suggest that
laparoscopic adhesiolysis, when feasible, may offer better
short-term outcomes compared to open surgery (84,85).
Due to their minimally invasive nature, laparoscopic procedures are
considered the adhesiolysis method of choice (60).
However, surgical adhesiolysis does not always lead
to a reduction in adhesion-related symptoms. In the case of
adhesion-associated chronic abdominal pain, adhesiolysis did not
lead to any pain relief in 33% of patients (10). In another clinical study, no
improvement in pain was observed at all (86). A meta-analysis showed initial pain
relief in about two-thirds of laparoscopically treated adhesion
patients. However, the results did not allow any conclusions to be
drawn about long-term effects (87). Abdominal pain in particular is more
difficult to correlate with specific clinical pathophysiology.
In reproductive medicine, surgical adhesiolysis is
evaluated much more positively. In the case of diagnosed
adhesion-related infertility, surgical adhesiolysis leads to a
postoperative one-year pregnancy rate of 61%. In patients with
severe adhesions, however, this rate drops to In the case of
diagnosed adhesion-related infertility, surgical adhesiolysis leads
to a postoperative one-year pregnancy rate of 61%. In patients with
severe adhesions, however, this rate drops to 20% (88).
The use of robot-assisted surgery, e.g. with the da
Vinci robotic surgery system, to combat adhesions is particularly
interesting with regard to innovative new procedures in surgery
(89). In gynecological patients
with extensive adhesions, robotic surgery showed more favorable
results compared to laparoscopy, both in terms of operative time
and intraoperative blood loss (90). In addition, literature analysis
showed that the rate of conversion from laparoscopic surgery to
open surgery due to complicated adhesions is lower when a robotic
approach is used (91).
However, it should be borne in mind that curative
surgical adhesion therapy carries the risk of causing new
adhesions. As with adhesion prophylaxis, this underlines the
unsatisfactory situation with regard to therapeutic options for
tissue adhesions.
8. Conclusion
The formation of tissue adhesions is a major medical
and health economic problem. Adhesions can remain asymptomatic, but
they can also cause unpleasant to severe symptoms such as abdominal
pain, obstruction and sterility. Furthermore, the anatomical
consequences of adhesions often lead to longer and additional
surgical interventions as well as longer postoperative hospital
stays. This ultimately increases the healthcare costs of the
treatment (92-94).
Adhesions are often diagnosed by chance during the
originally planned operation. It would be possible to identify
areas of adhesion prior to surgery using imaging techniques such as
MRI. This can even be done with relatively good accuracy, so that
such findings could be incorporated into surgical planning.
However, imaging is complex and expensive and has not yet been able
to establish itself in routine practice. Adhesion-minimizing
prophylactic procedures are only available to a limited extent. The
only effective procedures are barrier methods. These involve
introducing agents into the operated area that delay contact
between traumatized tissue areas in the abdominal cavity for as
long as possible and thus reduce the adhesion rate. Innovative
molecular approaches relate to fibrin turnover. This can be
regulated by means of fibrin-associated factors, which reduces
fibrin synthesis and subsequent adhesion events. There is also
initial data on using physical plasma to affect the ROS biology of
the wound area, which can also inhibit adhesive processes. However,
both approaches have not yet been integrated into everyday clinical
practice. The treatment of identified adhesions is currently only
possible surgically. Attempts with pharmacological agents have
failed as ineffective. However, surgical interventions carry the
risk of renewed adhesions. This can be countered to a certain
extent by using minimally invasive procedures. Minimizing
traumatological influences also reduces the formation of new
adhesions. It has also been shown that robotic-assisted surgery can
have positive and anti-adhesive effects.
As the clinical data situation is still very
heterogeneous overall and there is a lack of comprehensive
systematic clinical studies, there are only a few clear and
guideline-based recommendations for diagnosis, prophylaxis and
treatment of adhesions. The medical associations and health and
science policy are called upon to take action here. In terms of the
medical treatment of such tissue defects, a clear awareness of the
dangers but also of the medical care options must be created. In
addition, it is essential in the current situation that the
political framework conditions are created that place the problem
of adhesions more at the center of structured research approaches
in accordance with its enormous medical and health economic
significance. There are some promising approaches for the
prophylaxis and treatment of adhesions, but there is still a long
way to go before they can be used clinically.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
MCKS, AA and MBS were responsible for
conceptualization. AA and MCKS provided the resources for the
figures included. MCKS prepared the original draft. MCKS, AA and
MBS reviewed and edited the manuscript. MBS supervised the project.
All authors have read and approved the final version of the
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patients for the use of the images in Fig. 1.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
diZerega GS: Contemporary adhesion
prevention. Fertil Steril. 61:219–235. 1994.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Brüggmann D, Tchartchian G, Wallwiener M,
Münstedt K, Tinneberg HR and Hackethal A: Intra-abdominal
adhesions: Definition, origin, significance in surgical practice,
and treatment options. Dtsch Arztebl Int. 107:769–775.
2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sikirica V, Bapat B, Candrilli SD, Davis
KL, Wilson M and Johns A: The inpatient burden of abdominal and
gynecological adhesiolysis in the US. BMC Surg.
11(13)2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cheong Y, Sadek K, Watson A, Metwally M
and Li TC: Adhesion reduction agents in gynaecological procedures:
can NHS aff ord it? An economic cost efficiency analysis. J Obstet
Gynaecol. 31:631–635. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Davey AK and Maher PJ: Surgical adhesions:
A timely update, a great challenge for the future. J Minim Invasive
Gynecol. 14:15–22. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Capmas P, Payen F, Lemaire A and Fernandez
H: Adhesions in abdomino-pelvic surgeries: A real economic impact?
PLoS One. 17(e0276810)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Garoufalia Z, Gefen R, Emile SH, Zhou P,
Silva-Alvarenga E and Wexner SD: Financial and inpatient burden of
adhesion-related small bowel obstruction: A systematic review of
the literature. Am Surg. 89:2693–2700. 2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yang KH, Lee TB, Lee SH, Kim SH, Cho YH
and Kim HY: Congenital adhesion band causing small bowel
obstruction: What's the difference in various age groups, pediatric
and adult patients? BMC Surg. 16(79)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Weibel MA and Majno G: Peritoneal
adhesions and their relation to abdominal surgery. A postmortem
study. Am J Surg. 126:345–353. 1973.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tabibian N, Swehli E, Boyd A, Umbreen A
and Tabibian JH: Abdominal adhesions: A practical review of an
often overlooked entity. Ann Med Surg (Lond). 15:9–13.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Leung TT, Dixon E, Gill M, Mador BD,
Moulton KM, Kaplan GG and MacLean AR: Bowel obstruction following
appendectomy: What is the true incidence? Ann Surg. 250:51–53.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nunobe S, Hiki N, Fukunaga T, Tokunaga M,
Ohyama S, Seto Y and Yamaguchi T: Previous laparotomy is not a
contraindication to laparoscopy-assisted gastrectomy for early
gastric cancer. World J Surg. 32:1466–1472. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Robb WB and Mariette C: Strategies in the
prevention of the formation of postoperative adhesions in digestive
surgery; a systematic review of the literature. Dis Colon Rectum.
57:1228–1240. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Buhmann-Kirchhoff S, Lang R, Kirchhoff C,
Steitz HO, Jauch KW, Reiser M and Lienemann A: Functional cine MR
imaging for the detection and mapping of intraabdominal adhesions:
Method and surgical correlation. Eur Radiol. 18:1215–1223.
2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pittaway DE, Daniell JF and Maxson WS:
Ovarian surgery in an infertility patient as an indication for a
short-interval second-look laparoscopy: A preliminary study. Fertil
Steril. 44:611–614. 1985.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Diamond MP, Pellicer A, Boyers SP and
DeCherney AH: The effect of periovarian adhesions on follicular
development in patients undergoing ovarian stimulation for in vitro
fertilization-embryo transfer. Fertil Steril. 49:100–103.
1988.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Blackburn SC and Stanton MP: Anatomy and
physiology of the peritoneum. Semin Pediatr Surg. 23:326–330.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sompayrac SW, Mindelzun RE, Silverman PM
and Sze R: The greater omentum. AJR Am J Roentgenol. 168:683–687.
1997.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kastelein AW, Vos LMC, de Jong KH, van
Baal JOAM, Nieuwland R, van Noorden CJF, Roovers JWR and Lok CAR:
Embryology, anatomy, physiology and pathophysiology of the
peritoneum and the peritoneal vasculature. Semin Cell Dev Biol.
92:27–36. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pfister F, Büttner-Herold M, Kitsche B,
Bulian DR, Kielstein JT, Wanninger R, Eden G, Alscher D, Nebel M,
Schwenger V and Amann K: Standardized histomorphological processing
of peritoneal biopsies as part of the German Peritoneal Biopsy
Registry (GRIP, German registry in PD). Pathologe. 41:634–642.
2020.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
21
|
Rudralingam V, Footitt C and Layton B:
Ascites matters. Ultrasound. 25:69–79. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Doherty NS, Griffiths RJ, Hakkinen JP,
Scampoli DN and Milici AJ: Post-capillary venules in the ‘milky
spots’ of the greater omentum are the major site of plasma protein
and leukocyte extravasation in rodent models of peritonitis.
Inflamm Res. 44:169–177. 1995.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jackson-Jones LH and Bénézech C: FALC
stromal cells define a unique immunological niche for the
surveillance of serous cavities. Curr Opin Immunol. 64:42–49.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jackson-Jones LH, Smith P, Portman JR,
Magalhaes MS, Mylonas KJ, Vermeren MM, Nixon M, Henderson BEP,
Dobie R, Vermeren S, et al: Stromal cells covering omental
fat-associated lymphoid clusters trigger formation of neutrophil
aggregates to capture peritoneal contaminants. Immunity.
52:700–715.e6. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cruz-Migoni S and Caamaño J:
Fat-associated lymphoid clusters in inflammation and immunity.
Front Immunol. 7(612)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Brochhausen C, Schmitt VH, Planck CN,
Rajab TK, Hollemann D, Tapprich C, Krämer B, Wallwiener C,
Hierlemann H, Zehbe R, et al: Current strategies and future
perspectives for intraperitoneal adhesion prevention. J
Gastrointest Surg. 16:1256–1274. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sen CK and Roy S: Redox signals in wound
healing. Biochim Biophys Acta. 1780:1348–1361. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Koninckx PR, Gomel V, Ussia A and Adamyan
L: Role of the peritoneal cavity in the prevention of postoperative
adhesions, pain, and fatigue. Fertil Steril. 106:998–1010.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Buţureanu SA and Buţureanu TA:
Pathophysiology of adhesions. Chirurgia (Bucur). 109:293–298.
2014.
|
|
30
|
Capella-Monsonís H, Kearns S, Kelly J and
Zeugolis DI: Battling adhesions: From understanding to prevention.
BMC Biomed Eng. 1(5)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Maciver AH, McCall M and James Shapiro AM:
Intra-abdominal adhesions: Cellular mechanisms and strategies for
prevention. Int J Surg. 9:589–594. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Smith SA, Travers RJ and Morrissey JH: How
it all starts: Initiation of the clotting cascade. Crit Rev Biochem
Mol Biol. 50:326–336. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mutsaers SE, Prêle CMA, Pengelly S and
Herrick SE: Mesothelial cells and peritoneal homeostasis. Fertil
Steril. 106:1018–1024. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li Y, Wang J and Asahina K: Mesothelial
cells give rise to hepatic stellate cells and myofibroblasts via
mesothelial-mesenchymal transition in liver injury. Proc Natl Acad
Sci USA. 110:2324–2329. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yang AH, Chen JY and Lin JK:
Myofibroblastic conversion of mesothelial cells. Kidney Int.
63:1530–1539. 2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Arung W, Meurisse M and Detry O:
Pathophysiology and prevention of postoperative peritoneal
adhesions. World J Gastroenterol. 17:4545–4553. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Amadeu TP and Costa AM: Nitric oxide
synthesis inhibition alters rat cutaneous wound healing. J Cutan
Pathol. 33:465–473. 2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhou Q, Liu LZ, Fu B, Hu X, Shi X, Fang J
and Jiang BH: Reactive oxygen species regulate insulin-induced VEGF
and HIF-1alpha expression through the activation of p70S6K1 in
human prostate cancer cells. Carcinogenesis. 28:28–37.
2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
De Oliveira-Marques V, Cyrne L, Marinho HS
and Antunes F: A quantitative study of NF-kappaB activation by
H2O2: Relevance in inflammation and synergy with TNF-alpha. J
Immunol. 178:3893–3902. 2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kang JI and Park KM: Advances in
gelatin-based hydrogels for wound management. J Mater Chem B.
9:1503–1520. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ou Q, Zhang S, Fu C, Yu L, Xin P, Gu Z,
Cao Z, Wu J and Wang Y: More natural more better: triple natural
anti-oxidant puerarin/ferulic acid/polydopamine incorporated
hydrogel for wound healing. J Nanobiotechnology.
19(237)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Thi PL, Lee Y, Tran DL, Thi TTH, Kang JI,
Park KM and Park KD: In situ forming and reactive oxygen
species-scavenging gelatin hydrogels for enhancing wound healing
efficacy. Acta Biomater. 103:142–152. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kim B, Kim Y, Lee Y, Oh J, Jung Y, Koh WG
and Chung JJ: Reactive oxygen species suppressive kraft
lignin-gelatin antioxidant hydrogels for chronic wound repair.
Macromol Biosci. 22(e2200234)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chen K, Qiu P, Yuan Y, Zheng L, He J, Wang
C, Guo Q, Kenny J, Liu Q, Zhao J, et al: Pseurotin A inhibits
osteoclastogenesis and prevents ovariectomized-induced bone loss by
suppressing reactive oxygen species. Theranostics. 9:1634–1650.
2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Brochhausen C, Schmitt VH, Rajab TK,
Planck CN, Krämer B, Wallwiener M, Hierlemann H and Kirkpatrick CJ:
Intraperitoneal adhesions-an ongoing challenge between biomedical
engineering and the life sciences. J Biomed Mater Res A.
98:143–156. 2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Cheong YC, Laird SM, Shelton JB, Ledger
WL, Li TC and Cooke ID: The correlation of adhesions and peritoneal
fluid cytokine concentrations: A pilot study. Hum Reprod.
17:1039–1045. 2002.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Cahill RA and Redmond HP: Cytokine
orchestration in post-operative peritoneal adhesion formation.
World J Gastroenterol. 14:4861–4866. 2008.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Scappaticci FA, Fehrenbacher L, Cartwright
T, Hainsworth JD, Heim W, Berlin J, Kabbinavar F, Novotny W, Sarkar
S and Hurwitz H: Surgical wound healing complications in metastatic
colorectal cancer patients treated with bevacizumab. J Surg Oncol.
91:173–180. 2005.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Holmdahl L, Eriksson E, Al-Jabreen M and
Risberg B: Fibrinolysis in human peritoneum during operation.
Surgery. 119:701–705. 1996.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Andreasen PA, Georg B, Lund LR, Riccio A
and Stacey SN: Plasminogen activator inhibitors: Hormonally
regulated serpins. Mol Cell Endocrinol. 68:1–19. 1990.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ivarsson ML, Bergström M, Eriksson E,
Risberg B and Holmdahl L: Tissue markers as predictors of
postoperative adhesions. Br J Surg. 85:1549–1554. 1998.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Bourboulia D and Stetler-Stevenson WG:
Matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs): Positive and negative regulators in
tumor cell adhesion. Semin Cancer Biol. 20:161–168. 2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Cohen PA, Gower AC, Stucchi AF, Leeman SE,
Becker JM and Reed KL: A neurokinin-1 receptor antagonist that
reduces intraabdominal adhesion formation increases peritoneal
matrix metalloproteinase activity. Wound Repair Regen. 15:800–808.
2007.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Diamond MP, Wexner SD, diZereg GS, Korell
M, Zmora O, Van Goor H and Kamar M: Adhesion prevention and
reduction: current status and future recommendations of a
multinational interdisciplinary consensus conference. Surg Innov.
17:183–188. 2010.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Buunen M, Gholghesaei M, Veldkamp R,
Meijer DW, Bonjer HJ and Bouvy ND: Stress response to laparoscopic
surgery: A review. Surg Endosc. 18:1022–1028. 2004.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Tazeoğlu D, Benli S, Tikici D, Esmer AC
and Dirlik MM: Can minimally invasive surgical techniques reduce
the incidence of postoperative adhesions? Pol Przegl Chir.
94:23–30. 2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Molinas CR, Campo R, Elkelani OA, Binda
MM, Carmeliet P and Koninckx PR: Role of hypoxia inducible factors
1alpha and 2alpha in basal adhesion formation and in carbon dioxide
pneumoperitoneum-enhanced adhesion formation after laparoscopic
surgery in transgenic mice. Fertil Steril. 80 (Suppl 2):S795–S802.
2003.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Elkelani OA, Binda MM, Molinas CR and
Koninckx PR: Effect of adding more than 3% oxygen to carbon dioxide
pneumoperitoneum on adhesion formation in a laparoscopic mouse
model. Fertil Steril. 82:1616–1622. 2004.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Yesildaglar N and Koninckx PR: Adhesion
formation in intubated rabbits increases with high insufflation
pressure during endoscopic surgery. Hum Reprod. 15:687–691.
2000.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Otani K, Ishihara S, Nozawa H, Kawai K,
Hata K, Kiyomatsu T, Tanaka T, Nishikawa T, Yasuda K, Sasaki K, et
al: A retrospective study of laparoscopic surgery for small bowel
obstruction. Ann Med Surg (Lond). 16:34–39. 2017.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Hellebrekers BW, Trimbos-Kemper TC,
Trimbos JB, Emeis JJ and Kooistra T: Use of fibrinolytic agents in
the prevention of postoperative adhesion formation. Fertil Steril.
74:203–212. 2000.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Sulaiman H, Dawson L, Laurent GJ,
Bellingan GJ and Herrick SE: Role of plasminogen activators in
peritoneal adhesion formation. Biochem Soc Trans. 30:126–131.
2002.PubMed/NCBI
|
|
63
|
Menzies D and Ellis H: Intra-abdominal
adhesions and their prevention by topical tissue plasminogen
activator. J R Soc Med. 82:534–535. 1989.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Meier H, Dietl KH and Willital GH: First
clinical results of intraoperative application of
streptokinase-streptodornase in children. Langenbecks Arch Chiv.
366:191–193. 1985.
|
|
65
|
De Wilde RL, Devassy R, Ten Broek RPGT,
Miller CE, Adlan A, Aquino P, Becker S, Darmawan F, Gergolet M,
Habana MAE, et al: The future of adhesion prophylaxis trials in
abdominal surgery: An expert global consensus. J Clin Med.
11(1476)2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Brown CB, Luciano AA, Martin D, Peers E,
Scrimgeour A and diZerega GS: Adept Adhesion Reduction Study Group.
Adept (icodextrin 4% solution) reduces adhesions after laparoscopic
surgery for adhesiolysis: A double-blind, randomized, controlled
study. Fertil Steril. 88:1413–1426. 2007.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Hosie K, Gilbert JA, Kerr D, Brown CB and
Peers EM: Fluid dynamics in man of an intraperitoneal drug delivery
solution: 4% icodextrin. Drug Deliv. 8:9–12. 2001.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Wiseman DM, Trout JR and Diamond MP: The
rates of adhesion development and the effects of crystalloid
solutions on adhesion development in pelvic surgery. Fertil Steril.
70:702–711. 1998.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Ahmad M and Crescenti F: Significant
adhesion reduction with 4DryField PH after release of adhesive
small bowel obstruction. Surg J (N Y). 5:e28–e34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Blumhardt G, Haas M and Polte S: Effect of
4DryField® PH, a novel adhesion barrier, on recurrence of
intestinal adhesions after extensive visceral adhesiolysis. Case
Rep Surg. 2018(9628742)2018.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Khalaf AT, Abdalla AN, Ren K and Liu X:
Cold atmospheric plasma (CAP): A revolutionary approach in
dermatology and skincare. Eur J Med Res. 29(487)2024.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Abazid A, Babak A, Huschitt N, Badendieck
S and Stope MB: Application of non-invasive physical plasma in an
in vitro tissue model of fibroblasts and epithelial cells: enhanced
cell adhesion in mesh implants. Wehrmed Monatsschr. 69:6–12.
2025.
|
|
73
|
Nakagawa H, Matsumoto Y, Matsumoto Y, Miwa
Y and Nagasaki Y: Design of high-performance anti-adhesion agent
using injectable gel with an anti-oxidative stress function.
Biomaterials. 69:165–173. 2015.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Förster S, Niu Y, Eggers B, Nokhbehsaim M,
Kramer FJ, Bekeschus S, Mustea A and Stope MB: Modulation of the
tumor-associated immuno-environment by non-invasive physical
plasma. Cancers (Basel). 15(1073)2023.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Freund E, Moritz J, Stope M, Seebauer C,
Schmidt A and Bekeschus S: Plasma-derived reactive species shape a
differentiation profile in human monocytes. Appl Sci.
9(2530)2019.
|
|
76
|
Niu Y, Förster S, Badendieck S,
Nokhbehsaim M, Abazid A and Stope MB: Non-invasive physical plasma
modulates macrophage polarization: A potential strategy for tumor
microenvironment remodeling. Anticancer Res. 44:2437–2444.
2024.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Holl M, Rasch ML, Becker L, Keller AL,
Schultze-Rhonhof L, Ruoff F, Templin M, Keller S, Neis F, Keßler F,
et al: Cell type-specific anti-adhesion properties of peritoneal
cell treatment with plasma-activated media (PAM). Biomedicines.
10(927)2022.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Kang SU, Kim YS, Kim YE, Park JK, Lee YS,
Kang HY, Jang JW, Ryeo JB, Lee Y, Shin YS and Kim CH: Opposite
effects of non-thermal plasma on cell migration and collagen
production in keloid and normal fibroblasts. PLoS One.
12(e0187978)2017.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Koensgen D, Besic I, Gümbel D, Kaul A,
Weiss M, Diesing K, Kramer A, Bekeschus S, Mustea A and Stope MB:
Cold atmospheric plasma (CAP) and CAP-stimulated cell culture media
suppress ovarian cancer cell growth-a putative treatment option in
ovarian cancer therapy. Anticancer Res. 37:6739–6744.
2017.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Nitsch A, Sander C, Eggers B, Weiss M,
Egger E, Kramer FJ, Erb HHH, Mustea A and Stope MB: Pleiotropic
devitalization of renal cancer cells by non-invasive physical
plasma: Characterization of molecular and cellular efficacy.
Cancers (Basel). 15(481)2023.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Bateman BG, Nunley WC Jr and Kitchin JD
III: Prevention of postoperative peritoneal adhesions with
ibuprofen. Fertil Steril. 38:107–108. 1982.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Puchalski A: The influence of cumulative
dexamethasone, promethazine and dextran 70 used as protection
against intraperitoneal adhesions on selected parameters of humoral
immunity in women operated on for infertility. Ann Acad Med Stetin.
44:115–136. 1998.PubMed/NCBI(In Polish).
|
|
83
|
Jansen RP: Failure of peritoneal
irrigation with heparin during pelvic operations upon young women
to reduce adhesions. Surg Gynecol Obstet. 166:154–160.
1988.PubMed/NCBI
|
|
84
|
Kelly KN, Iannuzzi JC, Rickles AS,
Garimella V, Monson JRT and Fleming FJ: Laparotomy for small-bowel
obstruction: First choice or last resort for adhesiolysis? A
laparoscopic approach for small-bowel obstruction reduces 30-day
complications. Surg Endosc. 28:65–73. 2014.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Sajid MS, Khawaja AH, Sains P, Singh KK
and Baig MK: A systematic review comparing laparoscopic vs open
adhesiolysis in patients with adhesional small bowel obstruction.
Am J Surg. 212:138–150. 2016.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Swank DJ, Swank-Bordewijk SC, Hop WC, Van
Erp WF, Janssen IM, Bonjer HJ and Jeekel J: Laparoscopic
adhesiolysis in patients with chronic abdominal pain: A blinded
randomised controlled multi-centre trial. Lancet. 361:1247–1251.
2003.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Van den Beukel BA, de Ree R, van Leuven S,
Bakkum EA, Strik C, van Goor H and ten Broek RPG: Surgical
treatment of adhesion-related chronic abdominal and pelvic pain
after gynaecological and general surgery: A systematic review and
meta-analysis. Hum Reprod Update. 23:276–288. 2017.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Elgergawi A, Dawood AS, Salem HA and
Alhalwagy A: Outcome of laparoscopic adhesiolysis in infertile
patients with pelvic adhesions following cesarean delivery: A
randomized clinical trial. Fertil Steril. 112(e36)2019.
|
|
89
|
Wang PY, Lee YC, Liu WM and Chen CH:
Surgical outcome of benign cases with pelvic adhesions undergoing
robotic total hysterectomy. J Chin Med Assoc. 85:853–858.
2022.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Chiu LH, Chen CH, Tu PC, Chang CW, Yen YK
and Liu WM: Comparison of robotic surgery and laparoscopy to
perform total hysterectomy with pelvic adhesions or large uterus. J
Minim Access Surg. 11:87–93. 2015.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Milone M, Manigrasso M, Anoldo P, D'Amore
A, Elmore U, Giglio MC, Rompianesi G, Vertaldi S, Troisi RI,
Francis NK and De Palma GD: The role of robotic visceral surgery in
patients with adhesions: a systematic review and meta-analysis. J
Pers Med. 12(307)2022.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Van Goor H: Consequences and complications
of peritoneal adhesions. Colorectal Dis. 9 (Suppl 2):S25–S34.
2007.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Aguayo P, Fraser JD, Ilyas S, St Peter SD,
Holcomb GW III and Ostlie DJ: Laparoscopic management of small
bowel obstruction in children. J Laparoendosc Adv Surg Tech A.
21:85–88. 2011.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Poves I, Sebastián Valverde E, Puig
Companyó S, Dorcaratto D, Membrilla E, Pons MJ and Grande L:
Results of a laparoscopic approach for the treatment of acute small
bowel obstruction due to adhesions and internal hernias. Cir Esp.
92:336–340. 2014.PubMed/NCBI View Article : Google Scholar
|