Introduction
The S100 protein family is associated with cell
differentiation, cell motility and transcriptional regulation
(1). S100A4 is a member of this
family and is found as a highly expressed transcript in metastatic
tumor cell lines (2). S100A4 is
up-regulated in several malignancies including bladder cancer, and
plays a role in tumor aggressiveness (3,4).
However, the exact pathological role of S100A4 in bladder cancer
remains unclear; little is known about its role in tumor
invasiveness and prognostic significance in patients with
organ-confined tumors.
In general, few symptoms are apparent when bladder
cancer cells are localized to the submucosal layer (superficial
tumor). Such patients are usually treated by transurethral
resection (TUR) and/or intravesical therapy without any severe
negative effect on quality of life (QOL). Although threequarters of
newly diagnosed patients with bladder cancer have superficial
tumors, approximately 70% of these recur in the urinary tract and
4–45% of recurrent tumors develop muscle invasive disease (5,6).
Patient prognosis worsens when cancer cells reach surrounding
tissues and distant organs, and when TUR becomes an ineffective
method of treatment. Thus, it is conceivable that prevention of
muscle invasion is crucial for maintenance of QOL and improvement
of prognosis in patients with superficial bladder cancer.
The mechanisms at play early in bladder cancer
development are not fully understood, despite this step being the
most important determinant for choice of therapeutic strategy and
prognosis. In addition, treatment strategies for bladder cancer
vary markedly between patients with organ-confined tumors and those
with extravesical extension including metastasis. Although several
effective therapies are available for tumors ≤ pT2 stage (7,8),
only a few options are available with a satisfactory outcome in
patients with pT3 or pT4 and/or metastasis (8). Researchers and clinicians therefore
need better information regarding predictive factors for
progression to an advanced stage in patients with organ-confined
bladder cancer.
Various members of the matrix metalloproteinase
(MMP) family are reported targets of S100A4 (9). One of the most representative
functions of MMPs is proteolytic degradation of extracellular
matrix (ECM). Such degradation is important for cancer cell
invasion, and a variety of MMPs are associated with cancer cell
progression in many malignancies (10). MMP-2, -9 and -14 are also regulated
by S100A4 (11–13), while MMP-2 and -9 are of clinical
significance in bladder cancer (14). Several reports also cite the
clinical and pathological significance of MMP-14 in bladder cancer
(15–18), although its pathological role in
the early steps of invasion remains unknown. In addition, the
possible direct relationship between S100A4 and MMP-14 expression
in human bladder cancer is uncharted.
The aim of this retrospective study was to clarify
the pathological roles of S100A4 and MMP-14 in early-stage bladder
cancer cell invasion of muscle. In addition, predictive factors for
metastasis and survival in patients with organ-confined tumors were
also investigated. The findings identified S100A4 expression as a
useful prognostic marker in patients with organ-confined
disease.
Materials and methods
Patients and tumor samples
We reviewed consecutive surgical specimens of
bladder cancer obtained at our hospital from 1995 to 2003.
Furthermore, we also examined 15 normal tissue samples of the
urinary bladder obtained from apparently normal areas of the
bladder of patients with transitional cell carcinoma of the upper
urinary tract. None of these patients showed recurrence within a
follow-up period of 6–13 years. Patients who received neo-adjuvant
therapy were excluded. Tumors were staged according to the 2004
American Joint Committee on Cancer and graded according to the
World Health Organization and International Society for Urological
Pathology classification system. Patients diagnosed with metastatic
tumor and/or pT3 or pT4 were also excluded from the study. Fifteen
patients did not receive adjuvant therapy (17.6%). There were no
significant differences in S100A4 expression (p=0.783) and MMP-14
expression (p=0.165) between patients who received and did not
receive adjuvant therapy. The median duration of follow-up was 53
months (range, 2–195 months). The study protocol met the ethical
standards of the Human Ethics Review Committee of Nagasaki
University School of Medicine.
Immunohistochemistry
Anti-S100A4 (Zymed Laboratories, San Francisco, CA,
USA) and anti-MMP-14 (Lab Vision Corporation, Fremont, CA, USA)
antibodies were used for immunostaining. Sections (5 μm thick) were
deparaffinized and rehydrated. Antigen retrieval was performed at
95°C for 40 min in 0.01 M sodium citrate buffer (pH 6.0). All
sections were then immersed in 3% hydrogen peroxide for 30 min to
block endogenous peroxidase activity. Sections were incubated
overnight with the primary antibody at 4°C, and then incubated with
peroxidase using the labelled polymer method with Dako EnVision+™
Peroxidase (Dako Corp., Carpenteria, CA, USA) for 60 min. The
peroxidase reaction was visualized with the liquid
3,3-diaminobenzidine tetrahydrochloride (DAB) substrate kit (Zymed
Laboratories). Sections were counterstained in hematoxylin. Breast
cancer and prostate cancer tissues were used as positive controls
for S100A4 and MMP-14, respectively, according to previous reports
(19,20). A consecutive section from each
sample processed without the primary antibody was used as a
negative control.
The expression levels of each molecule were
evaluated semiquantitatively, taking into account the percentage of
positively stained cancer cells per field of microscopic view [at
least 300 carcinoma cells were examined in high-power fields
(×200)]. Expression was considered positive when the antibody
staining intensity was strong. The percentage of positively stained
cancer cells was determined using a continuous scale. Slides were
evaluated twice at different times by two investigators (Y.M. and
Y.S.), who were blinded to the clinical and pathological features
of the tumor.
Statistical analysis
All data are expressed as the median and
interquartile range (IQR). The Fisher’s exact test was used to
categorically compare data. Spearman’s rank correlation coefficient
was calculated to confirm Pearson’s correlation. Survival was
evaluated by Kaplan-Meier analysis and the log-rank test. To
quantify the survival analysis, patients were divided into two
groups: high S100A4 expression (>median) and low expression
(≤median), and pT stage was also divided into two groups: low (pTa
and 1) and high pT stage (pT2). Variables that achieved statistical
significance (p<0.05) by univariate analysis were subsequently
entered into a multivariate analysis [described as odds ratios (OR)
with 95% confidence intervals (95% CIs), together with the
p-values]. All statistical analyses were two-sided, and
significance was defined as p<0.05. All statistical analyses
were performed on a personal computer with the statistical package,
StatView for Windows (version 5.0, Abacus Concept, Inc., Berkeley,
CA, USA).
Results
Immunohistochemical expression and
pathological significance
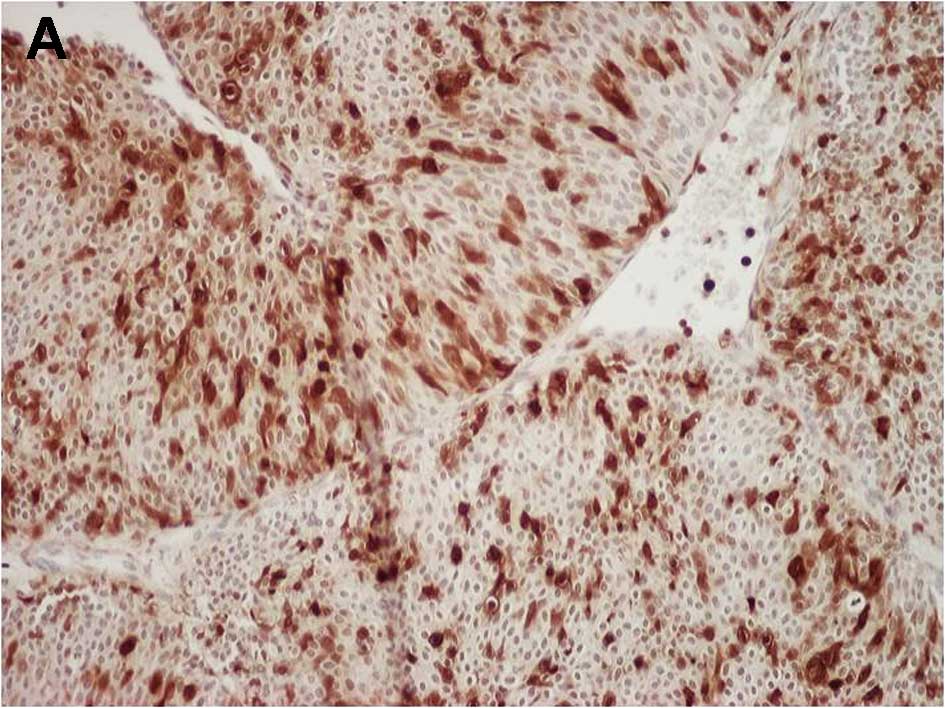
Fig. 1A and B show
representative examples of S100A4 and MMP-14 immunostaining,
respectively, in bladder cancer cells. The proportion of S100A4-
and MMP-14-positive cancer cells relative to the total cell number
was 41.6% (36.5–50.9%) and 22.3% (15.9–32.0%), respectively. In
stroma tissues, some fibroblast cells and infiltrating cells showed
positive staining for S100A4 and MMP-14, respectively. However,
such expression was not so strong and their frequencies were very
low. In normal urothelium, strong expression for both S100A4 and
MMP-14 were not found in all specimens.
This study consisted of 65 men and 20 women, and the
median (IQR) age was 70 (61–70) years. The gender was not
associated with expression of S100A4 or MMP-14 (p=0.316 or 0.812,
respectively). Likewise, age was not associated with expression of
S100A4 or MMP-14 (p=0.613 or 0.712, respectively). Table I summarizes the relationships
between clinicopathological features of the cancer and expression
levels of S100A4 or MMP-14. The proportion of S100A4-positive
cancer cells in pT2 tumors was significantly higher than in either
pTa or pT1 tumors, but similar in the latter two stages of tumors
(p=0.823). The findings were similar for MMP-14 expression and
tumor stage (Table I). With
respect to tumor grade, S100A4-positive cancer cells were
significantly more abundant in high grade than in low grade cases
(p=0.023). However, no such relationships were found with MMP-14
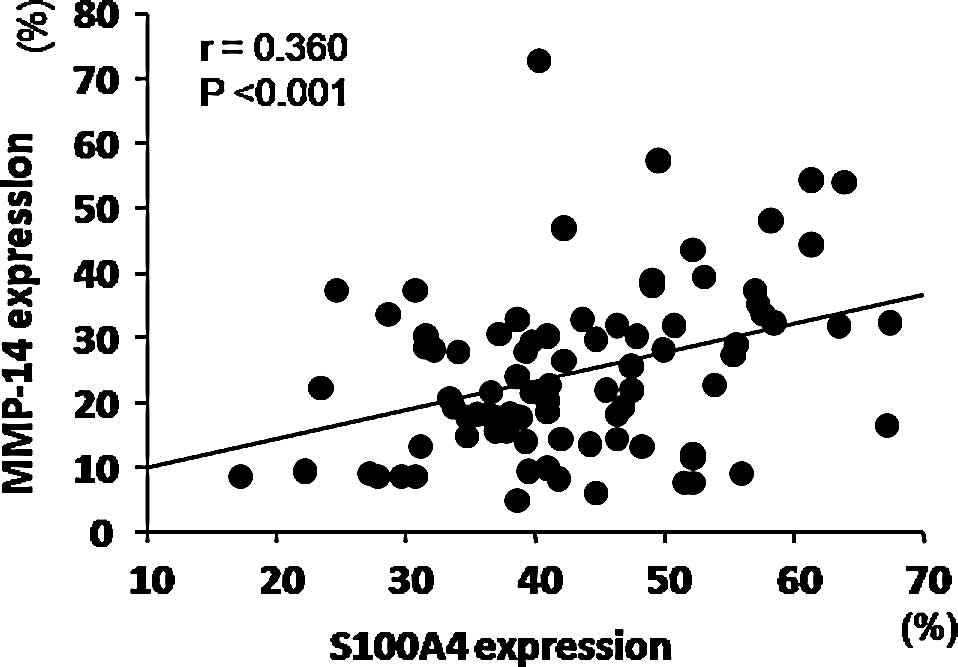
expression (p=0.338). Finally, S100A4 expression correlated
positively with MMP-14 expression (r=0.360, Fig. 2).
 | Table I.Relationships between expression
levels of S100A4 and MMP-14 and pathological features. |
Table I.
Relationships between expression
levels of S100A4 and MMP-14 and pathological features.
| No. | S100A4 expression
median (IQR) | P-value | MMP-14 expression
median (IQR) | P-value |
|---|
| Pathological
stage | | | | | |
| Ta | 28 | 38.7 (32.4–46.3) | 0.823 | 20.9 (13.5–29.0) | 0.912 |
| T1 | 36 | 40.9 (34.7–46.5) | <0.001 | 20.9 (14.6–28.7) | <0.001 |
| T2 | 21 | 53.0 (43.6–59.0) | | 32.7 (23.6–39.4) | |
| Grade | | | | | |
| Low | 42 | 40.4 (33.5–47.3) | 0.023 | 26.1 (17.8–31.9) | 0.338 |
| High | 43 | 45.5 (38.5–54.7) | | 18.7 (13.9–32.6) | |
Correlation with prognosis
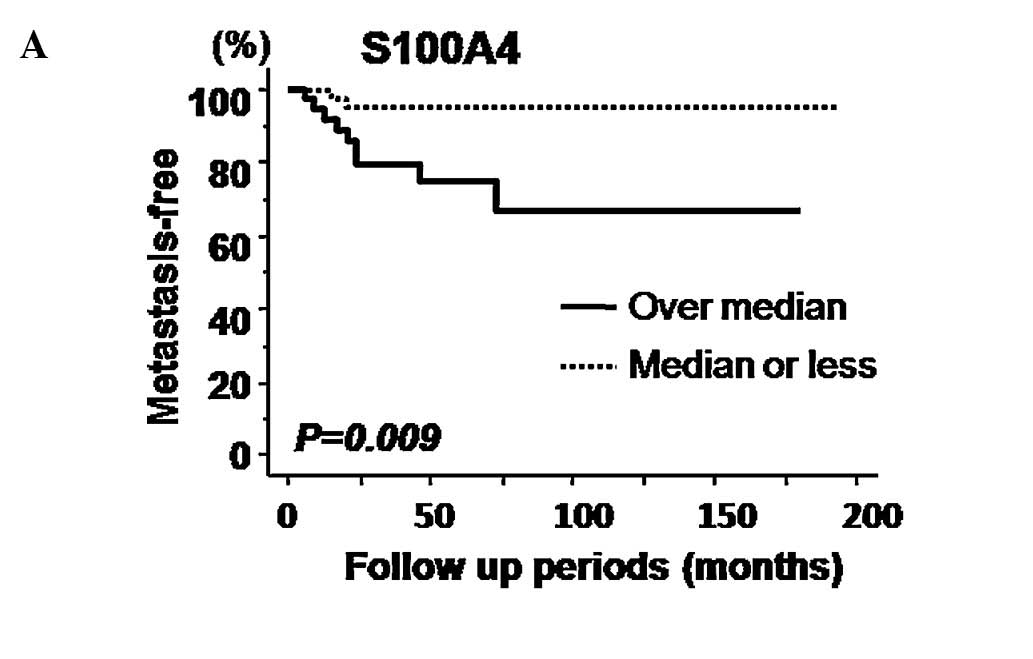
Kaplan-Meier curves for metastasis-free survival
relative to expression of S100A4 and MMP-14 are presented in
Fig. 3A and B, respectively.
Log-rank tests identified S100A4, but not MMP-14 expression, as a
significant predictive factor of metastasis-free survival. However,
neither S100A4 nor MMP-14 expression was significantly related to
cause-specific survival (Fig. 3C and
D). A potential role for S100A4 in distant metastasis was
investigated in more detail using a multivariate analysis model
including pT stage and grade. High pT stage was identified as a
significant predictive factor (OR=6.19, 95% CI=1.41–27.27,
p=0.016), however, S100A4 expression and high grade did not qualify
as a significant predictor by this analysis (OR=3.40, 95%
CI=0.59–19.47, p=0.170 and OR=1.16, 95% CI=0.21–6.53, p=0.869,
respectively).
Discussion
In this study, S100A4 expression in pT2 bladder
tumors was significantly higher than in superficial tumors (pTa and
pT1). Proportions of S100A4-positive bladder cancer cells in
invasive tumors (pT2-4) were previously reported to be higher than
in superficial tumors (4,21). However, these studies did not
discuss relative differences in S100A4 expression among pTa, pT1
and pT2 tumors. In general, invasiveness-related proteins tend to
be up-regulated in proportion to cancer stage. Thus, it is possible
that comparing S100A4 expression in superficial tumors with the
entire pT2-4 spectrum does not always reflect the exact role of
this protein in the early invasion stages. Spread of cancer cells
into surrounding muscle is one of the most important determinants
for successful QOL and prognosis, justifying the importance of
comparing superficial tumors with pT2, but not with the whole tumor
spectrum. S100A4 was found to be important in the early stages of
muscle invasion in the current study, providing important new
information for planning treatment strategies for patients with
superficial bladder tumors.
The results presented herein also showed that
proportions of MMP-14-positive cancer cells in pT2 tumors were
significantly higher than in superficial tumors. Only one published
study previously examined the relationship between MMP-14
expression and pT stage, showing no significant association
(15). However, this analysis only
measured MMP-14 expression in whole samples obtained at surgery,
and not in selected ones. In another study of samples obtained by
laser capture microdissection, MMP-14 expression differed between
tumor epithelium and stroma (18).
From this result, the use of whole sample in the former study might
not reflect the invasive function of MMP-14 in bladder cancer
cells, as might different sample sizes (41 vs. 85). We speculate
that MMP-14 is indeed associated with muscle invasion of bladder
cancer cells.
Of additional interest is the positive correlation
shown between S100A4 and MMP-14 expression. Several
metastasisrelated molecules are regulated by S100A4. DeLassus et
al (22) recently showed that
overexpression of S100A4 in human breast cancer cells correlated
positively with MMP-14 expression. The present study also showed a
similar relationship between pT stage and S100A4 or MMP-14.
Although the expression of both proteins in invaded muscle tissue
differed significantly from those in superficial tumors, there was
no significant difference between pTa and pT1 with regard to gene
expression of S100A4 and MMP-14. These findings implicate S100A4 in
bladder cancer cell invasion into muscle layer via the regulation
of MMP-14.
Univariate survival analyses identified S100A4
expression as a significant predictor of metastasis-free survival
in patients with organ-confined bladder cancer, supporting previous
reports of S100A4 expression and metastasis in bladder cancer
(4,19,23).
However, S100A4 expression was not identified as a significant
predictor in multivariate analyses. In this study, S100A4
expression was associated with both pT stage and grade and these
factors were also significant predictors for metastasis-free
survival. So, we hypothesized that S100A4 was not a independent
predictor in multivariate analysis. On the other hand, several
investigators reported S100A4 expression to be a significant
predictor of bladder cancer-specific survival (4,23),
although the current study found no such association. None of our
patients had extravesical tumor extension (pT3 or pT4) and/or
metastasis unlike these earlier studies, possibly accounting for
the discrepancy. On the other hand, MMP-14 expression was not
associated with metastasis-free survival. S100A4 was reported to
regulate MMP-2 and MMP-9, in addition to MMP-14 (11–13).
We speculate that MMP-14 plays minimum roles in the steps of
metastasis.
In conclusion, S100A4 is potentially important
during early muscle invasion in bladder cancer via its regulation
of MMP-14. The present study identified S100A4 expression as a
significant prognostic factor for metastasis-free survival in
patients with organ-confined tumors.
Acknowledgements
We are grateful to Mr Yoshikazu Tsuji
and Mr Takumi Shimogama for the outstanding support. This study was
not supported by any grants and funds.
References
|
1.
|
Heizmann CW, Fritz G and Schafer BW: S100
proteins: structure, functions and pathology. Front Biosci.
7:d1356–d1368. 2002.
|
|
2.
|
Ebralidze A, Tulcinsky E, Grigorian M,
Afanasyeva A, Senin V, Revazova E and Lukanidin E: Isolation and
characterization of a gene specifically expressed in different
metastatic cells and whose deduced gene product has a high degree
of homology to a Ca2+-binding protein family. Genes Dev.
3:1086–1093. 1989.
|
|
3.
|
Yao R, Davidson DD, Lopez-Beltran A,
MacLennan GT, Montironi R and Cheng L: The S100 proteins for
screening and prognostic grading of bladder cancer. Histol
Histopathol. 22:1025–1032. 2007.
|
|
4.
|
Matsumoto K, Irie A, Satoh T, Ishii J,
Iwabuchi K, Iwamura M, Egawa S and Baba S: Expression of S100A2 and
S100A4 predicts for disease progression and patient survival in
bladder cancer. Urology. 70:602–607. 2007.
|
|
5.
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2007.
|
|
6.
|
Heney NM, Ahmed S, Flanagan MJ, Frable W,
Corder MP, Haffermann MD and Hawkins IR: Superficial bladder
cancer: progression and recurrence. J Urol. 130:1083–1086.
1983.
|
|
7.
|
Sternberg S, Donat J, Bellmunt R, Millikan
W, Stadler P, De Mulder A, Sherif H, von der Maase T, Tsukamoto M
and Soloway M: Chemotherapy for bladder cancer: treatment
guidelines for neoadjuvant chemotherapy and metastatic cancer.
Urology. 69:62–79. 2007.
|
|
8.
|
Bellmunt J, Albiol S and Kataja V; EMSO
Guidelines Working Group: Invasive bladder cancer: ESMO clinical
recommendations for diagnosis, treatment and follow-up. Ann Oncol.
19(Suppl 2): 47–48. 2008.
|
|
9.
|
Garrett SC, Varney KM, Weber DJ and
Bresnick AR: S100A4, a mediator of metastasis. J Biol Chem.
281:677–680. 2006.
|
|
10.
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006.
|
|
11.
|
Bjørnland K, Winberg JO, Odegaard OT,
Hoving E, Loennechen T, Aasen AO, Fodstad O and Maelandsmo GM:
S100A4 involvement in metastasis: degradation of matrix
metalloproteinases and tissues inhibitors of matrix
metalloproteinases in osteosarcoma cells transfected with an
anti-S100A4 ribozyme. Cancer Res. 59:4702–4708. 1999.
|
|
12.
|
Mathisen B, Lindstad RI, Hansen J,
El-Gewely SA, Maelandsmo GM, Hoving E, Fodstad O, Loennechen T and
Winberg JO: S100A4 regulates membrane induced activation of matrix
metalloproteinase-2 in osteosarcoma cells. Clin Exp Metastasis.
20:701–711. 2003.
|
|
13.
|
Saleem M, Kweon M-H, Johnson JJ, Adhami
MA, Elcheva A, Khan N, Hataluri V and Mukhtar H: S100A4 accelerates
tumorigenesis and invasion of human prostate cancer through the
transcriptional regulation of matrix metalloproteinase 9. Proc Natl
Acad Sci USA. 103:14825–14830. 2006.
|
|
14.
|
Gontero P, Banisadr S, Frea B and Brausi
M: Metastasis markers in bladder cancer; a review of literature and
clinical considerations. Eur Urol. 46:296–311. 2004.
|
|
15.
|
Kanayama H, Yokota K, Kurokawa Y, Murakami
Y, Nishitani M and Kagawa S: Prognostic values of matrix
metalloproteinase-2 and tissue inhibitor of matrix
metalloproteinase-2 expression in bladder cancer. Cancer.
82:1359–1366. 1998.
|
|
16.
|
Furukawa A, Tsuji M, Nishitani T, Kanda K,
Inoue Y, Knayama H and Kagawa S: Role of the matrix
metalloproteinase and tissue inhibitors of metalloproteinase
families in noninvasive and invasive tumors transplanted in mice
with severe combined immunodeficiency. Urology. 51:849–853.
1998.
|
|
17.
|
Hara I, Miyake H, Hara S, Arakawa S and
Kamidono S: Significance of matrix metalloproteinase and tissue
inhibitors of metalloproteinase expression in the recurrence of
superficial transitional cell carcinoma of the bladder. J Urol.
165:1769–1772. 2001.
|
|
18.
|
Wallard MJ, Pennington CJ,
Veerakumarasivam A, Burtt G, Mills IG, Warren A, Leung HY, Murphy
G, Edwards DR, Neal DE and Kelly JD: Comprehensive profiling and
localization of the matrix metalloproteinases in urothelial
carcinoma. Br J Cancer. 94:569–757. 2006.
|
|
19.
|
Ismail NI, Kaur G, Hashim H and Hassan MS:
S100A4 overexpression proves to be independent marker for breast
cancer progression. Cancer Cell Int. 5:8–12. 2008.
|
|
20.
|
Maruta S, Sakai H, Kanda S, Hayashi T,
Kanetake H and Miyata Y: E1AF expression is associated with
extra-prostatic growth and matrix metalloproteinase-7 expression in
prostate cancer. APMIS. 117:791–796. 2009.
|
|
21.
|
Davies BR, O’Donnell M, Durkan GC, Rudland
PS, Barraclough R, Neal DE and Mellon JK: Expression of S100A4
protein is associated with metastasis and reduced survival in human
bladder cancer. J Pathol. 196:292–299. 2002.
|
|
22.
|
DeLassus GS, Cho H, Park J and Eliceiri
GL: New pathway links from cancer-progression determinants to gene
expression of matrix metalloproteinases in breast cancer cells. J
Cell Physiol. 217:739–744. 2008.
|
|
23.
|
Agerbaek M, Alsner J, Marcussen N,
Lundbeck F and von der Maase H: Focal S100A4 protein expression is
an independent predictor of development of metastatic disease in
cystectomized bladder cancer patients. Eur Urol. 50:777–785.
2006.
|