Introduction
The currently available optimum treatment for
glioblastoma consists of cytoreductive surgery followed by
radiotherapy and chemotherapy (1).
This conventional therapeutic strategy results in a median survival
of 12–15 months in consecutive, non-selected glioblastoma patients
(2–4). Most large-scale clinical studies on
chemotherapy for malignant gliomas have utilized nitrosoureas
(2,3), and have usually produced negative
results regarding the survival gain for glioblastoma patients.
Although temozolomide chemotherapy contributes to a significant
improvement in patient survival, its benefit is usually restricted
to tumors without O6-methylguanine-DNA
methyltransferase (MGMT) expression (4,5).
Since the level of MGMT expression strongly influences the efficacy
of nitrosoureas or temozolomide (5–9), the
establishment of novel therapeutic strategies for MGMT-positive
glioblastoma is one of the main issues in contemporary
neurooncology.
Current cancer treatments for categories of patients
generally require the selection of therapy made on the basis of
clinical trials conducted on large populations. However, the
heterogeneity in drug sensitivity partly reduces the clinical
success gained with these empiric chemotherapeutic regimens used
for the general patient population (1). A therapeutic strategy with a protocol
modified case by case according to drug sensitivity is termed
‘individualized’ or ‘tailor-made’ chemotherapy (10). Published clinical studies using
in vitro drug sensitivity tests (DST) have shown improved
patient response rates as compared with empiric regimens (11–15).
The lessons learned from individualized chemotherapy may be
valuable in planning chemotherapy regimens for glioblastoma as
various anticancer drugs are actually administered in clinics.
We treated glioblastoma patients with various
anticancer agents according to individualized protocols selected by
DST. However, individualization of chemotherapy cannot easily be
adopted in every institution, since it is both time-consuming and
non-economical. In this report, the efficacy of each anticancer
agent for glioblastoma was retrospectively examined in relation to
the MGMT expression status by immunohistochemistry. This
information provides a clue for the selection of anticancer drugs
against glioblastoma expressing a high level of MGMT or those
harboring unmethylated promoter of the MGMT gene.
Materials and methods
Patients
Seventy-four consecutive patients newly diagnosed
with glioblastoma according to WHO classification were treated with
individualized chemotherapy at Chiba University Hospital or Chiba
Cancer Center Hospital from 1995 to 2004. All of the patients
treated during this period were evaluated and included in the study
without exclusion. The study protocol was approved by the
institutional review board, and a written informed consent was
obtained from all of the patients or a guardian. The patient
characteristics are summarized in Table I. Magnetic resonance imaging (MRI),
with and without gadolinium enhancement, was performed
preoperatively and postoperatively before the initiation of
radio-chemotherapy. Regarding extent of resection, the
total/subtotal resection was defined as 90% or more reduction of
the tumor volume in the postoperative MRI. The biopsy meant the
CT-guided stereotactic needle biopsy, and partial removal covered
all other situations. Toxicity was graded according to the National
Cancer Institute’s Common Toxicity Criteria version 3.0.
 | Table I.Patient characteristics (n=74). |
Table I.
Patient characteristics (n=74).
| Age (years) | |
| Mean | 51.5 |
| Range | 15–77 |
| Gender | |
| Male | 49 (66%) |
| Female | 25 (34%) |
| Karnofsky performance
score | |
| ≥70 | 42 (57%) |
| <70 | 32 (43%) |
| Tumor location | |
| Left | 37 (50%) |
| Right | 28 (38%) |
| Midline | 9 (12%) |
| Extent of
surgery | |
| Total/Subtotal | 46 (62%) |
| Partial/Biopsy | 28 (38%) |
Drug sensitivity test (DST)
Direct quantification of apoptosis by means of flow
cytometric DNA analysis is widely used in basic research and has
been successfully utilized for clinical DST (15–17).
Cell suspensions prepared from surgically resected tumor tissues
were incubated with each of 25 different anticancer drugs already
being used in clinical practice (cyclophosphamide, ifosphamide,
nimustine, ranimustine, cisplatin, carboplatin, adriamycin,
daunomycin, pirarubicin, epirubicin, aclarubicin, mitoxantrone,
etoposide, camptothecin, methotraxate, 5-fluorouracil, thioinosine,
cytosine arabinoside, mitomycin C, bleomycin, vincristine,
vinblastine, vindesine, paclitaxel and docetaxel). The in
vitro drug concentrations were set both at the peak plasma
concentration when the clinically recommended doses were provided
and at 1/10 of that level (18).
Drug-induced apoptosis was quantified with a flow cytometer
(FACScan; Becton Dickinson, Mountain View, CA, USA) as the sub-G1
population. To confirm the presence of drug-induced apoptosis,
morphological examinations of the nuclei were also performed on the
same samples. DNA integrity assessed by the FCM analysis correlated
well with the morphological changes in the nuclei.
Treatment protocols
For individualization of chemotherapy, the most
effective drug in vitro was routinely selected as the key
drug for each individual patient. In addition, one or two drugs
were selected for combination with the key drug according to their
degree of effectiveness and their mechanism of pharmaceutical
action. The doses and schedules of chemotherapy regimens were
determined on the basis of clinically recommended doses. When no
agent was positive in vitro, the patients were treated with
a modified PCV chemotherapy with substitution of lomustine with
nimustine (nimustine 75 mg/m2, vincristine 1
mg/m2 and procarbazine 100 mg/day) (19). For all patients, the conventional
60-Gy radiotherapy with a megavoltage machine was started within 2
weeks of surgical removal in conjunction with the chemotherapy.
MGMT immunohistochemistry
For immunohistochemical analysis, paraffin-embedded
samples were sliced and mounted on glass slides. Mouse monoclonal
anti-MGMT antibody MT3.1 (1:200 dilution; Chemicon, Inc., Temecula,
CA, USA) was used as the primary antibody. A heat-induced epitope
was formed using microwaves in 10 mM citric acid buffer at pH 7.2.
The samples were incubated with the antibody overnight in the same
buffer followed by incubation with the biotinylated secondary
antibody (1:500 dilution; Dako, Tokyo, Japan). The bound antibodies
were visualized by the avidin biotinylated peroxidase complex
method and diaminobenzidine tetrachloride (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). Human liver was used as
the positive control, and the negative control was achieved by
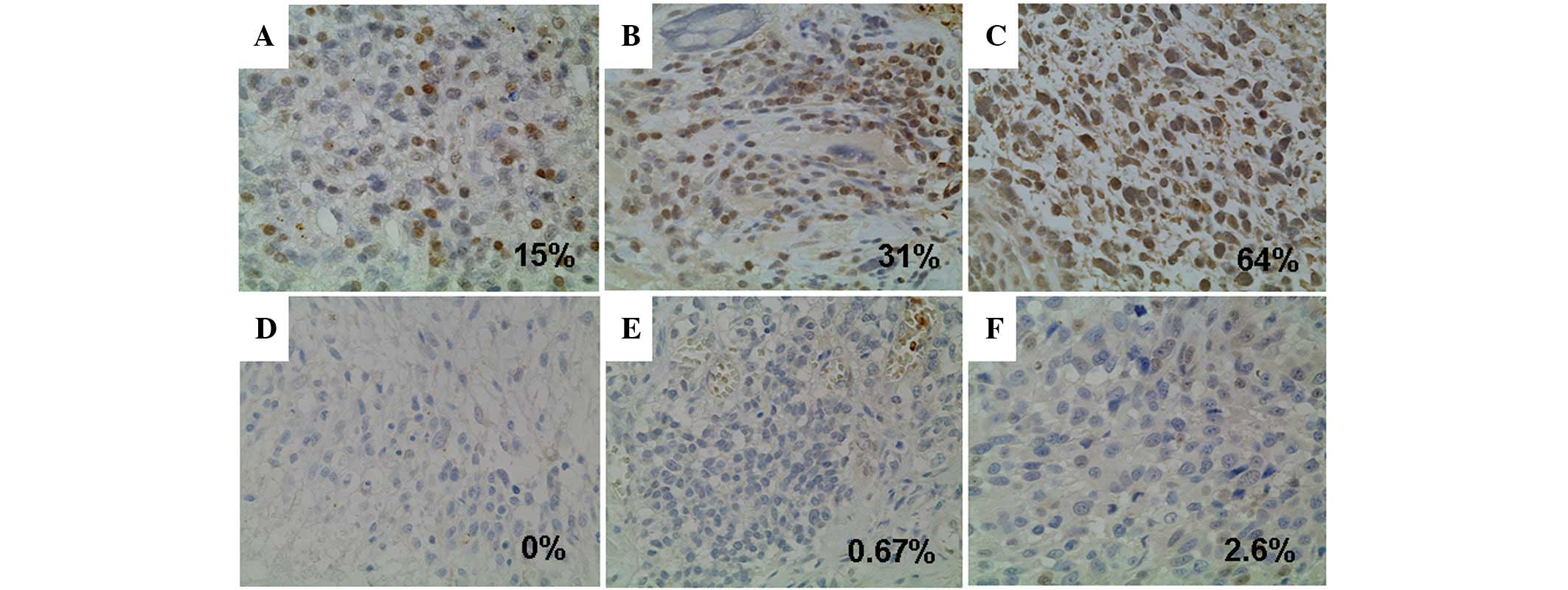
omitting the primary antibody from the procedure. Tissue specimens
that showed staining of >10% of the malignant cells were
considered positive for MGMT.
Statistical analysis
The primary end-point of this study was overall
survival and the secondary end-points were progression-free
survival and safety. Survival curves were generated using the
Kaplan-Meier method, and the survival rates were compared with the
log-rank test. The patient survival duration was calculated from
the date of surgery until the date of last follow-up or death, and
progression-free survival until the date of recurrence detection or
until the last follow-up. The Fisher’s exact probability test and
χ2 test were used to evaluate the statistical
significance of the differences between patient characteristics of
the two groups. Cox’s proportional hazard model was used to analyze
the prognostic variables. The hazard ratios for death were
calculated considering adjustment for age, Karnofsky performance
status score and the extent of resection.
Results
Efficacy of individualized
chemotherapy
All specimens from the 74 patients were examined for
their in vitro susceptibility to the 25 anticancer drugs. In
this series of newly diagnosed glioblastoma patients, the success
rate of the DST was 100%. There was remarkable heterogeneity in the
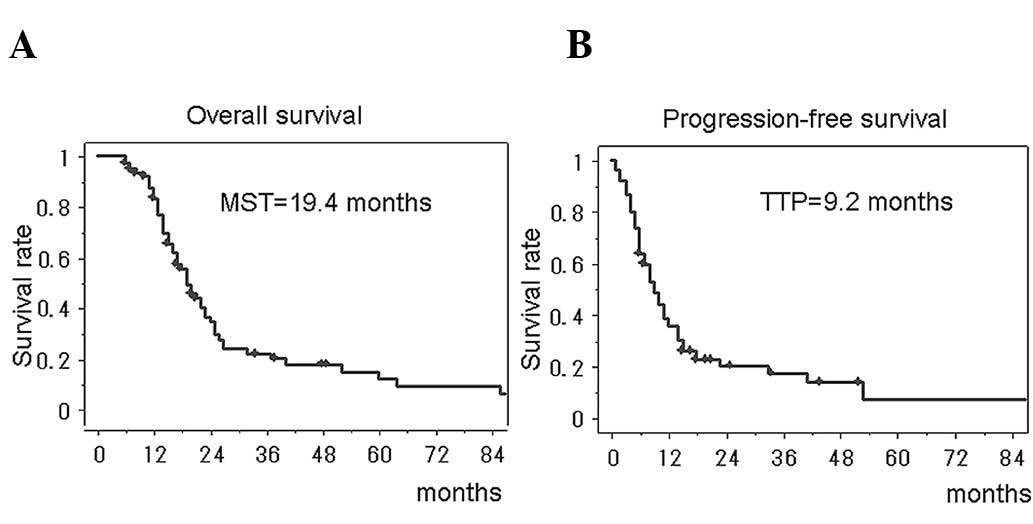
most effective drug. The median survival time of all of the 74
glioblastoma patients treated with the individualized chemotherapy
was 19.4 months (95% CI, 15.9–22.1), and the 2-year survival rate
was 36.5% (95% CI, 24.3–48.7). The median progression-free survival
was 9.2 months (95% CI, 7.6–12.3) (Fig. 1). The survival periods could be
favorably compared with those treated with temozolomide, the
present-day standard regimen for glioblastoma.
The univariate analysis showed that the clinical
factors previously known to affect the survival of patients with
glioblastoma were correlated with favorable prognosis in this
study; a Karnofsky performance status score of ≥70%, tumor
resection of ≥90% and <50 years of age. The multivariate
analysis showed that <50 years of age and tumor resection of
≥90% were significantly associated with favorable prognosis
(Table II, p=0.0002 and p=0.0211,
respectively).
 | Table II.Multivariate analyses for favorable
prognostic factors. |
Table II.
Multivariate analyses for favorable
prognostic factors.
| Hazard ratio (95%
CI) | P-value |
|---|
| Age (<50 vs.
≥50) | 0.41 (0.26–0.66) | 0.0002 |
| Karnofsky performance
score (≥70 vs. <70) | 0.69 (0.47–1.00) | 0.0607 |
| MGMT expression
(negative vs. positive) | 0.59 (0.41–0.88) | 0.0081 |
| Extent of resection
(≥90 vs. <90) | 0.60 (0.39–0.93) | 0.0211 |
MGMT expression and chemosensitivity
The MGMT-positive rate was 53.7% for the 74
glioblastomas (Fig. 2). According
to the DST, 58 tumors (78%) had at least one effective drug, and
the other 16 tumors (22%) were negative for all of the 25
anticancer drugs examined (all-drug-resistant tumors). The
relationship between the MGMT expression status and
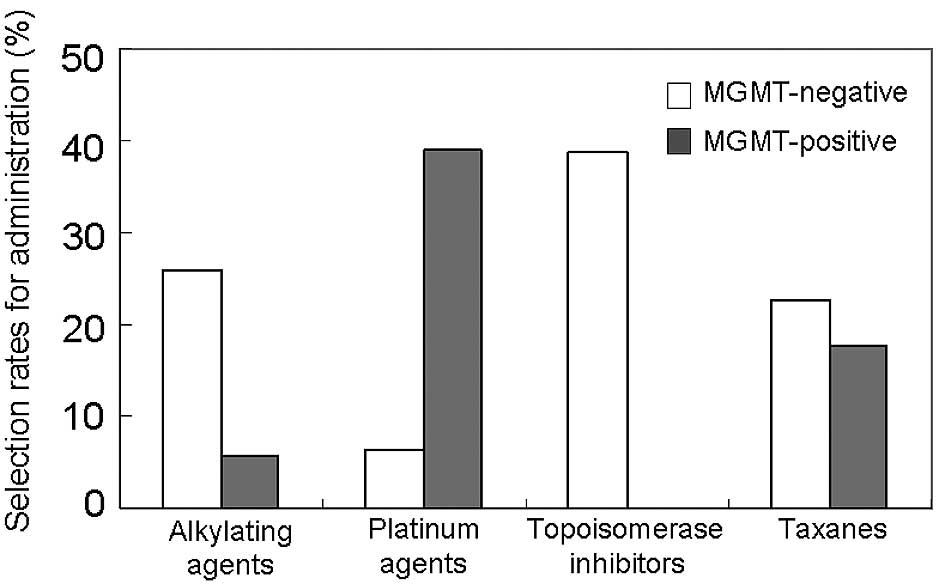
chemosensitivity was analyzed (Fig.
3). For the MGMT-positive tumors, the alkylating agents were
selected only in two cases, and the topoisomerase inhibitors were
never selected for administration as a key drug. The platinum
agents were more frequently effective against the MGMT-positive
tumors than against the MGMT-negative tumors. The taxanes were
equally selected either in the MGMT-negative or the MGMT-positive
group. Most of the all-drug-resistant tumors (14 out of 16 cases,
87.5%) were included in the MGMT-positive group (p=0.0019). Thus,
the MGMT expression status significantly influenced the in
vitro chemosensitivity of almost all categories of anticancer
agents except for the taxanes.
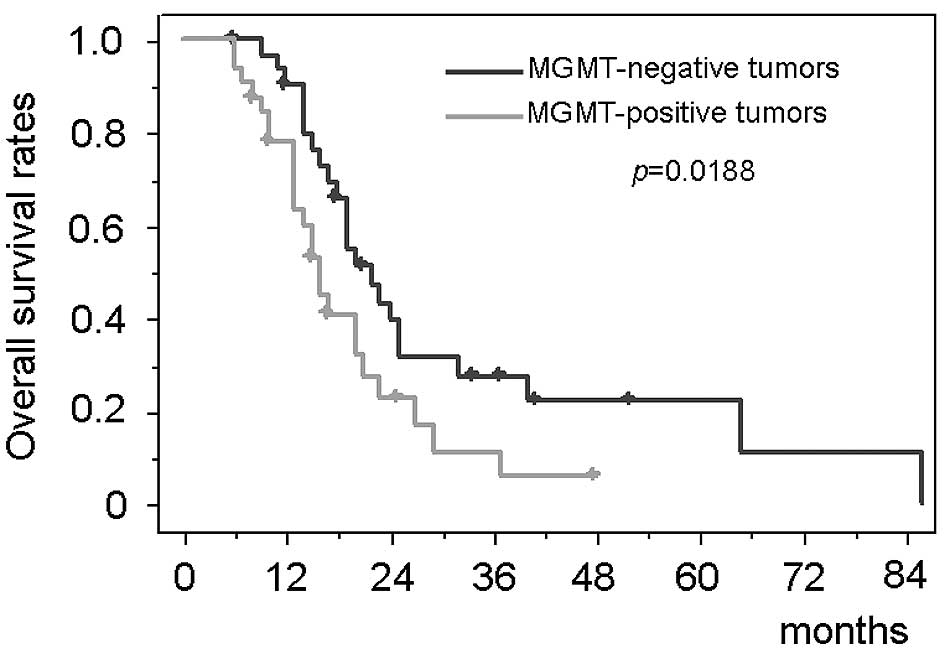
MGMT expression and survival
The patients with negative MGMT immunostaining had
significantly longer survival than those with positive MGMT [median
survival, 22.3 months (95% CI, 17.6–27.0) vs. 15.1 months (95% CI,
13.4–16.8); p=0.0188] (Fig. 4).
Immunohistochemical MGMT expression status had a significant impact
on the survival period in the multivariate analysis (Table II). The survival period of the
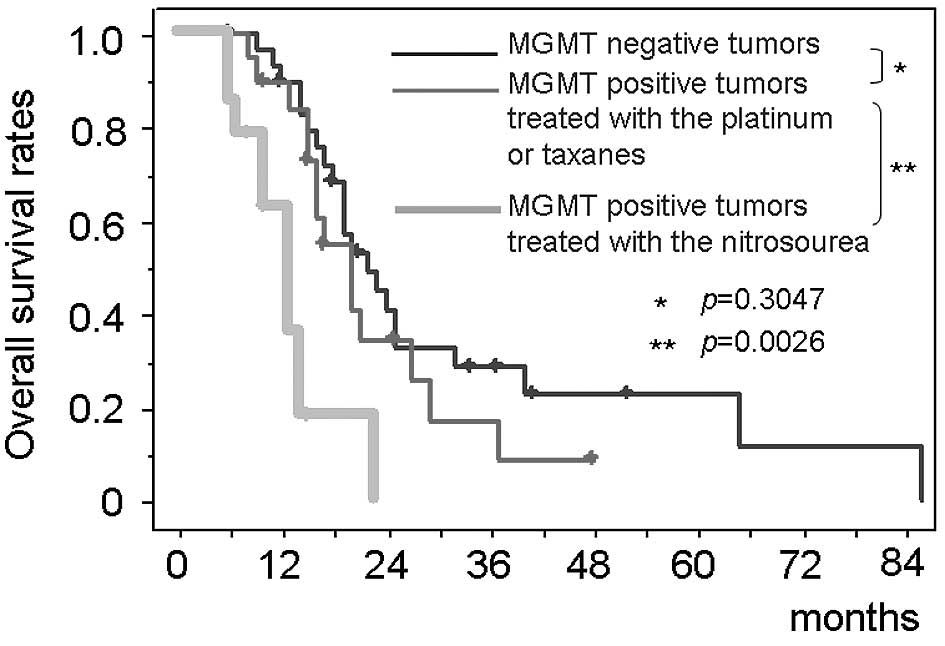
patients with MGMT-positive tumors treated with the platinum agents
or the taxanes [median survival, 20.1 months (95% CI, 18.0–22.7)]
was equivalent to that of the MGMT-negative tumors (p=0.3047)
(Fig. 5). In contrast, the
survival time of the patients with all-drug-resistant MGMT-positive
tumors (n=14) who were treated with the nitrosourea-based
chemotherapy (the modified PCV therapy) [median survival, 13.0
months (95% CI, 11.4–14.6)] was significantly shorter than both
that of the patients with MGMT-negative tumors (p=0.0007) and that
of MGMT-positive tumors treated with the platinum agents or the
taxanes (p=0.0026).
Safety evaluation
We monitored the adverse events, with special focus
on the hematological toxic effects. They were graded according to
the National Cancer Institute’s Common Toxicity Criteria version
3.0. Grade 3 or 4 neutropenia was observed in 9 patients (12.2%),
and severe pneumonia occurred in 3 patients (4.1%). However, there
was no treatment-related death in the present series.
Discussion
Our results suggest that individualized chemotherapy
with anticancer drugs prospectively selected based on in
vitro chemosensitivity tests for each glioblastoma patient
provides a median survival of 19.4 months which compares favorably
with most of the previously reported studies (2–4). The
potentially poor prognosis groups with age greater than 50 years,
Karnofsky performance status score less than 70% and surgical
resection less than 90% particularly benefited from the
individualized chemotherapy. Therefore, this study suggests an
important new direction in the chemotherapy for glioblastoma.
An important implication of this study is that a
large number of MGMT-positive glioblastomas were effectively
treated with individualized chemotherapy. Many studies have
indicated that MGMT is a significant prognostic factor for shorter
survival rates in glioblastoma patients (5–7),
whereas its prognostic value remains controversial (8,9).
Efficacy of temozolomide also depends significantly on the level of
MGMT expression (5). Therefore,
one of the most important issues in contemporary neurooncology is
how to treat glioblastoma with high MGMT expression. Therapeutic
strategies for MGMT-positive glioblastoma currently under
consideration have been designed to deplete MGMT and to combine
other agents which are not affected by MGMT (21). The present results demonstrate that
currently available anticancer drugs are much more effective when
administered to those most likely to respond. An individualized or
tailored strategy based on multiple biological information of the
tumor would be one of the effective approaches to treat
MGMT-positive glioblastoma.
However, an individualization strategy cannot easily
be adopted in every institution. The lessons learned from
individualized chemotherapy may be valuable in planning
chemotherapy regimens for MGMT-positive glioblastoma. The present
study suggests that the platinum agents and the taxanes can
potentially prolong the survival of patients with MGMT-positive
glioblastoma. The platinum agents as well as temozolomide and
O6-benzylguanine can abrogate MGMT activity
(22). Several studies have also
shown that the antitumor activity of platinum agents is not
affected by MGMT activity (23–25).
This knowledge has led to Phase II clinical trials with promising
results (26–28). Although MGMT affected the
efficacies of diverse anticancer drugs, only the taxanes were
independent from the MGMT status. Taxanes were clinically used in
some trials without marked improvement in the efficacy for
gliobastoma (29–31). However, multiple molecular
mechanisms were reported to affect their efficacies for glioma
cells (32). It is preferable to
treat MGMT-positive glioblastoma with multi-modality regimens
including platinum agents or the taxanes.
Currently, MGMT expression is estimated mainly by
methylation-specific PCR or immunohistochemistry (5–9).
Methylation-specific PCR is highly sensitive but is unable to
assess intratumoral heterogeneity and contaminating normal cells
such as endothelial cells (8,9). A
methylated band is observed even when cells that carry MGMT
promoter hypermethylation represent only a minor portion of the
tumor. Regulation of MGMT expression is a more complex phenomenon
in which abnormal promoter methylation is not the sole determining
factor. We employed immunohistochemistry to directly evaluate the
final functional molecule and the intratumoral heterogeneity,
although an objective threshold for evaluation may not be easily
set. We used a cut-off value of 10%, whereas the reported values
vary from 5 to 35% (8,9,33,34).
The results showed a tendency of polarization of the MGMT-positive
rate in the tumors with rates of less than 5% and those with more
than 35%. Consequently, the overall positive rate in our study is
consistent with published reports employing
immunohistochemistry.
This is the first report to show that
individualization of chemotherapy can potentially prolong the
survival of non-selected consecutive glioblastoma patients with
high MGMT expression without any additional toxicity. When the
stratification based on the MGMT expression is available, platinum
agents or the taxanes offer the highest probability for
effectiveness against MGMT-positive glioblastomas.
References
|
1.
|
Shapiro WR: Current therapy for brain
tumors: back to the future. Arch Neurol. 56:429–432. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Medical Research Council Brain Tumor
Working Party: Randomized trial of procarbazine, lomustine and
vincristine in the adjuvant treatment of high-grade astrocytoma: a
Medical Research Council trial. J Clin Oncol. 19:509–518.
2001.PubMed/NCBI
|
|
3.
|
Glioma Meta-analysis Trialist (GMT) Group:
Chemotherapy in adult high-grade glioma: a systematic review and
meta-analysis of individual patient data from 12 randomised trials.
Lancet. 359:1011–1018. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Stupp R, Mason WP, van den Bent MJ, et al:
European Organisation for Research and Treatment of Cancer Brain
Tumor and Radiotherapy Groups; National Cancer Institute of Canada
Clinical Trials Group: Radiotherapy plus concomitant and adjuvant
temozolomide for glioblastoma. N Engl J Med. 352:987–996. 2005.
View Article : Google Scholar
|
|
5.
|
Hegi ME, Diserens AC, Gorlia T, et al:
MGMT gene silencing and benefit from temozolomide in glioblastoma.
N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Jaeckle KA, Eyre HJ, Townsend JJ, et al:
Correlation of tumor O6-alkylguanine-DNA
methyltransferase levels with survival of malignant astrocytoma
patients treated with bis-chloroethylnitrosourea: a Southwest
Oncology Group study. J Clin Oncol. 16:3310–3315. 1998.
|
|
7.
|
Esteller M, Garcia-Foncillas J, Andion E,
et al: Inactivation of the DNA-repair gene MGMT and the clinical
response of gliomas to alkylating agents. N Engl J Med.
343:1350–1354. 2000. View Article : Google Scholar
|
|
8.
|
Brell M, Tortosa A, Verger E, et al:
Prognostic significance of O6-alkylguanine-DNA
methyltransferase determined by promoter hypermethylation and
immunohistochemical expression in anaplatic gliomas. Clin Cancer
Res. 11:5167–5174. 2005.
|
|
9.
|
Chinot OL, Barrie M, Fuentes S, et al:
Correlation between O6-alkylguanine-DNA
methyltransferase and survival in inoperable newly diagnosed
glioblastoma patients treated with neoadjuvant temozolomide. J Clin
Oncol. 25:1470–1475. 2007.PubMed/NCBI
|
|
10.
|
Cortazar P and Johnson BE: Review of the
efficacy of individualized chemotherapy selected by in vitro drug
sensitivity testing for patients with cancer. J Clin Oncol.
17:1625–1631. 1999.PubMed/NCBI
|
|
11.
|
Alonso K: Human tumor stem cell assay. A
prospective clinical trial. Cancer. 54:2475–2479. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Von Hoff DD, Sandbach JF, Clark GM, et al:
Selection of cancer chemotherapy for a patient by an in vitro assay
versus a clinician. J Natl Cancer Inst. 82:110–116. 1990.PubMed/NCBI
|
|
13.
|
Gazdar AF, Steinberg SM, Russell EK, et
al: Correlation of in vitro drug-sensitivity testing with response
to chemotherapy and survival in extensive-stage small cell lung
cancer: a prospective clinical trial. J Natl Cancer Inst.
82:117–124. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Cortazar P, Gazdar AF, Woods E, et al:
Survival of patients with limited-stage small cell lung cancer
treated with individualized chemotherapy selected by in vitro drug
sensitivity testing. Clin Cancer Res. 3:741–747. 1997.PubMed/NCBI
|
|
15.
|
Iwadate Y, Fujimoto S, Namba H and Yamaura
A: Promising survival for patients with glioblastoma multiforme
treated with individualised chemotherapy based on in vitro drug
sensitivity testing. Br J Cancer. 89:1896–1900. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Iwadate Y, Fujimoto S, Sueyoshi K, et al:
Prediction of drug cytotoxicity in 9L rat brain tumor by using flow
cytometry with a deoxyribonucleic acid-binding dye. Neurosurgery.
40:782–788. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Iwadate Y, Fujimoto S and Yamaura A:
Differential chemosensitivity in human intracerebral gliomas
measured by flow cytometric DNA analysis. Int J Mol Med.
10:187–192. 2002.PubMed/NCBI
|
|
18.
|
Alberts DS and Chen HSG: Tabular Summary
of Pharmacokinetic Parameters Relevant to In Vitro Drug Assay. Alan
R. Liss. New York: 351–359. 1980.
|
|
19.
|
Higuchi Y, Iwadate Y and Yamaura A:
Treatment of low-grade oligodendroglial tumors without
radiotherapy. Neurology. 63:2384–2386. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Iwadate Y, Namba H and Sueyoshi K:
Intra-arterial ACNU and cisplatin chemotherapy for the treatment of
glioblastoma multiforme. Neurol Med Chir. 35:598–603. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Mason WP and Cairncross JG: Drug insight:
temozolomide as a treatment for malignant glioma – impact of a
recent trial. Nat Clin Pract Neurol. 1:88–95. 2005.
|
|
22.
|
Tanaka S, Kobayashi I, Utsuki S, Oka H,
Yasui Y and Fujii K: Down-regulation of
O6-methylguanine-DNA methyltransferase gene expression
in gliomas by platinum compounds. Oncol Rep. 14:1275–1280.
2005.
|
|
23.
|
Beith J, Hartley J, Darling J and Souhami
R: DNA interstrand cross-linking and cytotoxicity induced by
chloroethylnitrosoureas and cisplatin in human glioblastoma cell
lines which vary in cellular concentration of
O6-alkylguanine-DNA methyltransferase. Br J Cancer.
75:500–505. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Preuss I, Thust R and Kaina B: Protective
effect of O6-methylguanine-DNA methyltransferase (MGMT)
on the cytotoxic and recombinigenic activity of different
antineoplastic drugs. Int J Cancer. 65:506–512. 1996.
|
|
25.
|
Fruehauf JP, Brem H, Parker R, et al: In
vitro drug response and molecular markers associated with drug
resistance in malignant gliomas. Clin Cancer Res. 12:4523–4532.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Balana C, Ramirez JL, Rosell R, et al:
O6-methylguanine-DNA methyltransferase methylation in
serum and tumor DNA predicts response to
1,3-bis(2-chloroethyl)-1-nitrosourea but not to temozolomide plus
cisplatin in glioblastoma multiforme. Clin Cancer Res. 9:1461–1468.
2003.
|
|
27.
|
Watanabe T, Katayama Y, Ogino A, et al:
Preliminary individualized chemotherapy for malignant astrocytomas
based on O6-methylguanine-deoxyribonucleic acid
methyltransferase methylation analysis. Neurol Med Chir.
46:387–394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Silvani A, Eoli M, Salmaggi A, et al:
Phase II trial of cisplatin plus temozolomide, in recurrent and
progressive malignant glioma patients. J Neurooncol. 66:203–208.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Rosenthal MA, Gruber ML, Glass J, et al:
Phase II study of combination taxol and estramustine phosphate in
the treatment of recurrent glioblastoma multiforme. J Neurooncol.
47:59–63. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Langer CJ, Ruffer J, Rhodes H, et al:
Phase II Radiation Therapy Oncology Group trial of weekly
paclitaxel and conventional external beam radiation therapy for
supratentorial glioblastoma multiforme. Int J Radiat Oncol Biol
Phys. 51:113–119. 2001. View Article : Google Scholar
|
|
31.
|
Ashamalla H, Zaki B, Mokhtar B, et al:
Fractionated stereotactic radiotherapy boost and weekly paclitaxel
in malignant gliomas clinical and pharmacokinetics results. Technol
Cancer Res Treat. 6:169–176. 2007. View Article : Google Scholar
|
|
32.
|
Karmakar S, Banik NL and Ray SK:
Combination of all-trans retinoic acid and paclitaxel-induced
differentiation and apoptosis in human gliobastoma U87MG xenografts
in nude mice. Cancer. 112:596–607. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Friedman HS, McLendon RE, Kerby T, et al:
DNA mismatch repair and O6-alkylguanine-DNA
alkyltransferase analysis and response to Temodal in newly
diagnosed malignant glioma. J Clin Oncol. 16:3851–3857.
1998.PubMed/NCBI
|
|
34.
|
Mollemann M, Wolter M, Feisberg J, et al:
Frequent promoter hypermethylation and low expression of the MGMT
gene in oligodendroglial tumors. Int J Cancer. 113:379–385. 2005.
View Article : Google Scholar : PubMed/NCBI
|