Introduction
Telomeres are specialized structures at the ends of
eukaryotic chromosomes that protect genomic DNA from degradation
and end-to-end fusion, thereby maintaining chromosome stability
(1). In humans, this telomeric DNA
consists of tandem repeated hexanucleotids TTAGGG (2). These sequences are diminished with
each round of normal somatic cell division (3). After a certain number of cell
divisions, normal somatic cells eventually stop dividing. This
senescent stage is thought to be the result of the reduction in
telomeres (4).
Telomerase is a ribonucleoprotein enzyme that
maintains telomeric repeats at the end of telomeres to compensate
for sequence loss during DNA replication. Telomerase activity is
assumed to be involved in the maintenance of telomere length, and
its reactivation is required for cell immortalization. Telomerase
activity has been reported in many human cancers (5). One study reported a correlation
between telomerase activity in bladder cancer tissue and tumor
grade or stage (6). However,
little is known regarding the association between telomerase
activity in bladder cancer tissue and prognosis. Telomerase
activity was measured using a PCR-enzyme-linked immunosorbent assay
(ELISA) in bladder lesions with or without carcinoma. The
correlation between telomerase activity and grade, stage or
cancer-specific survival was also evaluated, and the association
between telomerase activity and recurrence-free survival was
analyzed in primary superficial bladder cancer patients to
determine the usefulness of telomerase activity for predicting
recurrence after a transurethral resection.
Materials and methods
The present study was conducted on 81 subjects
including 75 patients (57 males and 18 females; range 26–87 years)
with bladder cancer and 6 patients (5 males and 1 female; range
59–79 years) with dysplasia or an inflammatory bladder lesion.
Tissue specimens were obtained by cold-cup cystoscopic biopsies.
Necrotic lesions were avoided and the biopsy was obtained from
lesions located as deep as possible, particularly in advanced-stage
bladder cancer. Each sample was sliced in two, with one part taken
for a pathological examination and the other immediately frozen in
liquid nitrogen to avoid RNA degradation or telomerase denaturation
until the proteins were extracted. If a pathological analysis of
the specimen revealed degenerative changes, the specimen obtained
for the telomerase assay was abandoned. The tissue specimens were
stored at −80°C until assay. The bladder carcinomas were staged and
graded according to the World Health Organization classification
(7). Clinicopathological
characteristics of the bladder cancer patients are shown in
Table I.
 | Table I.Clinicopathological characteristics of
the 75 bladder cancer patients. |
Table I.
Clinicopathological characteristics of
the 75 bladder cancer patients.
| Follow up in months
(mean ± SD, range) | 37.2±30.9
(1–114) |
| Age (years) | 71.6±12.3
(26–87) |
| Gender (no., %) | |
| Male | 57 (76.0) |
| Female | 18 (24.0) |
| Stage (no., %) | |
| Ta | 18 (24.0) |
| T1 | 29 (38.7) |
| T2 | 18 (24.0) |
| T3 | 9 (12.0) |
| T4 | 1 (1.3) |
| Grade (no., %) | |
| G1 | 9 (12.0) |
| G2 | 41 (54.7) |
| G3 | 25 (33.3) |
Extraction
Bladder tissues specimens (10–100 mg) were washed
once in ice-cold buffer (10 mmol/l HEPES-KOH pH 7.5, 1.5 mmol/l
MgCl2, 10 mmol/l KCl and 1 mmol/l dithiothreitol) then
homogenized with 50–200 μl of cold lysis buffer (0.5% CHAPS, 10
mmol/l Tris-HCl pH 7.5, 1 mmol/l EGTA, 1 mmol/l MgCl2,
10% glycerol, 5 mmol/l β-mercaptoethanol and 0.1 mmol/l
phenylmethylsulfonylfluoride) until complete lysis, when the
extracts were quickly chilled in liquid N2. After 25 min
at 4°C, the lysates were centrifuged at 16,000 g for 20 min at 4°C.
The supernatants were aliquoted, quickly frozen, and stored at
−80°C until assay. The protein concentrations of the extracts were
measured using the Bradford method, with bovine serum albumin (BSA)
as the standard (Bio-Rad, Ivry, France).
Telomerase assay
Extracts containing 6 μg of protein were analyzed
using a non-radioactive test based on the method described by Kim
et al (8). A telomerase
PCR-ELISA was performed according to the manufacturer’s
instructions (Boehringer Mannheim, Meylan, France). This
non-radioactive method was based on the recently developed
telomeric repeat amplification protocol (TRAP) assay (8). All extracts were treated with RNase.
The results were visualized using a colorimetric method. Absorbance
values were reported as the absorbance at 450 nm red against the
blank (reference wavelength 630 nm). The specimens were considered
to be telomerase-positive when the difference in absorbance (ΔA;
A450–A630) was >0.2 optical density (OD)
units.
Statistical analysis
Statistical analysis was performed using the
Mann-Whitney U test and χ2-test to evaluate the
significance of the differences between the patient groups. The
Kaplan-Meyer method was used to summarize the distribution of
cancer-specific survival and recurrence-free survival, and the
log-rank test was used to evaluate individual factors with respect
to these outcomes. P-values <0.05 were considered to be
significant.
Results
Forty-nine of the 75 bladder cancer patients (65.3%)
were found to be telomerase-positive, as was 1 out of the 6 control
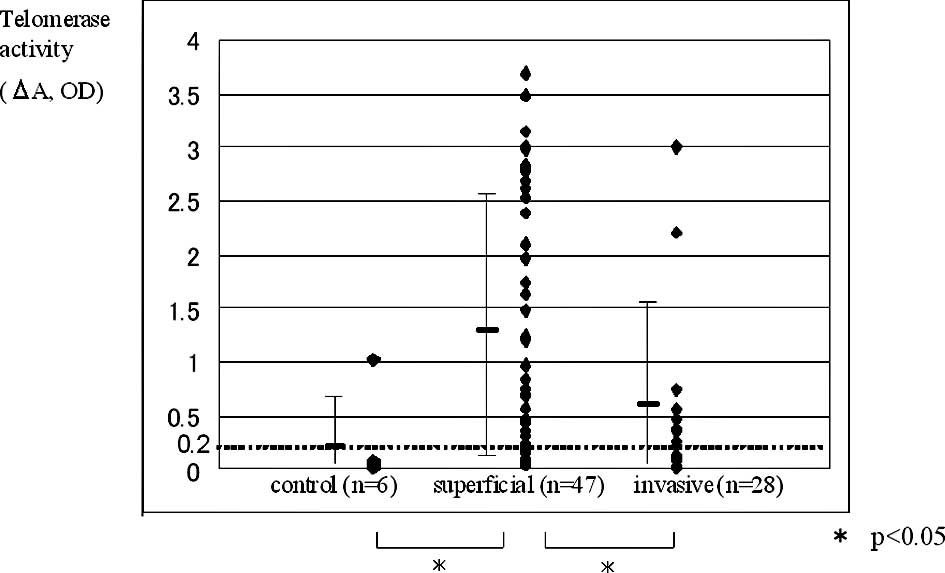
specimens. There were significant differences (P<0.05) in
telomerase activity among the control, superficial (pTa-pT1) and
invasive groups (pT2–pT4; Fig. 1),
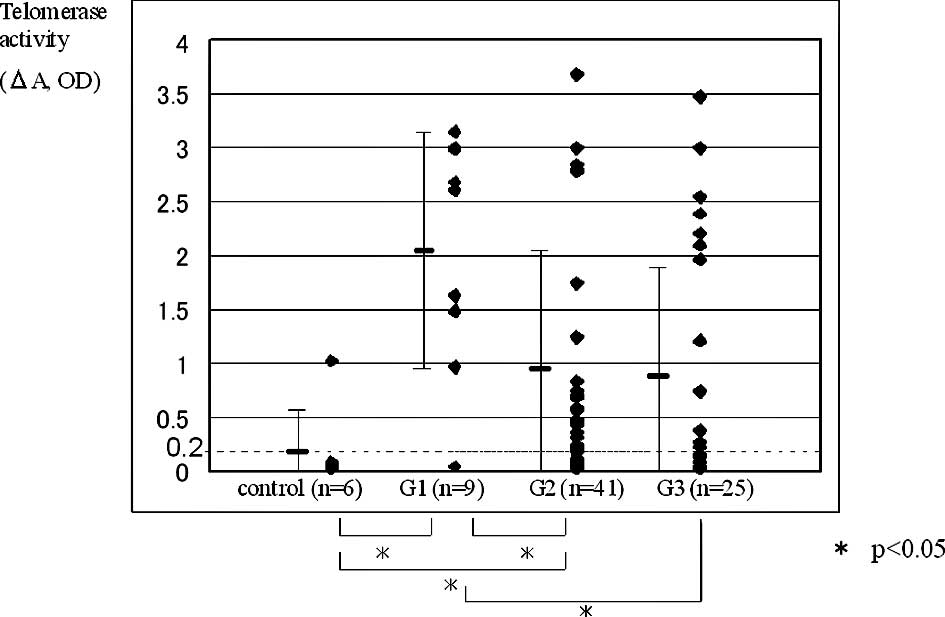
and among the control, low- and high-grade groups (control vs. G1;
control vs. G2; G1 vs. G2; G1 vs. G3; Fig. 2). Only 1 positive patient in the
control group had inflammatory lesions. Telomerase positivity
according to stage was 79.7% for superficial and 42.9% for invasive
carcinomas. Telomerase positivity according to grade was 88.9% for
G1, 70.7% for G2 and 48.0% for G3 (Table II). The G1 tumor group had
significantly higher telomerase positivity than the G3 tumor group,
and the superficial group had significantly higher telomerase
positivity than the invasive group.
 | Table II.Comparison of tumor grade and stage
with telomerase activity in bladder cancer using the
χ2-test (P<0.05). |
Table II.
Comparison of tumor grade and stage
with telomerase activity in bladder cancer using the
χ2-test (P<0.05).
| Tumor | No. | Telomerase activity
|
|---|
| Negative (%) | Positive %) |
|---|
| Superficial | 47 | 10 (21.3) | 37 (78.7) |
| Invasive | 28 | 16 (57.1) | 12 (42.9) |
| Grade 1 | 9 | 1 (11.1) | 8 (88.9) |
| Grade 2 | 41 | 12 (29.3) | 29 (70.7) |
| Grade 3 | 25 | 13 (52.0) | 12 (42.9) |
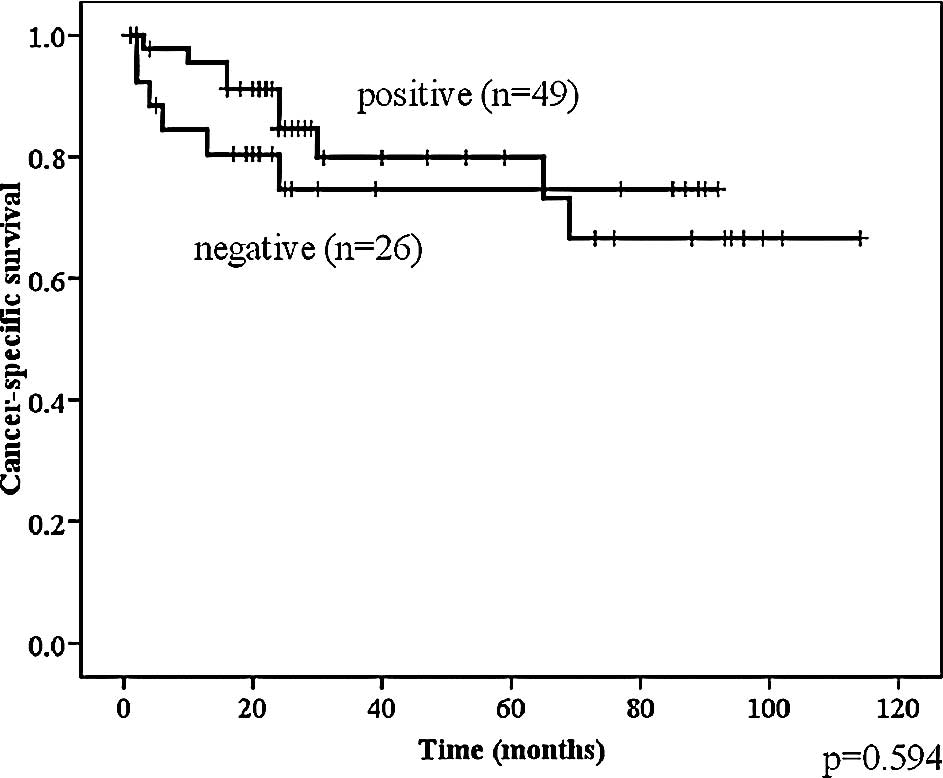
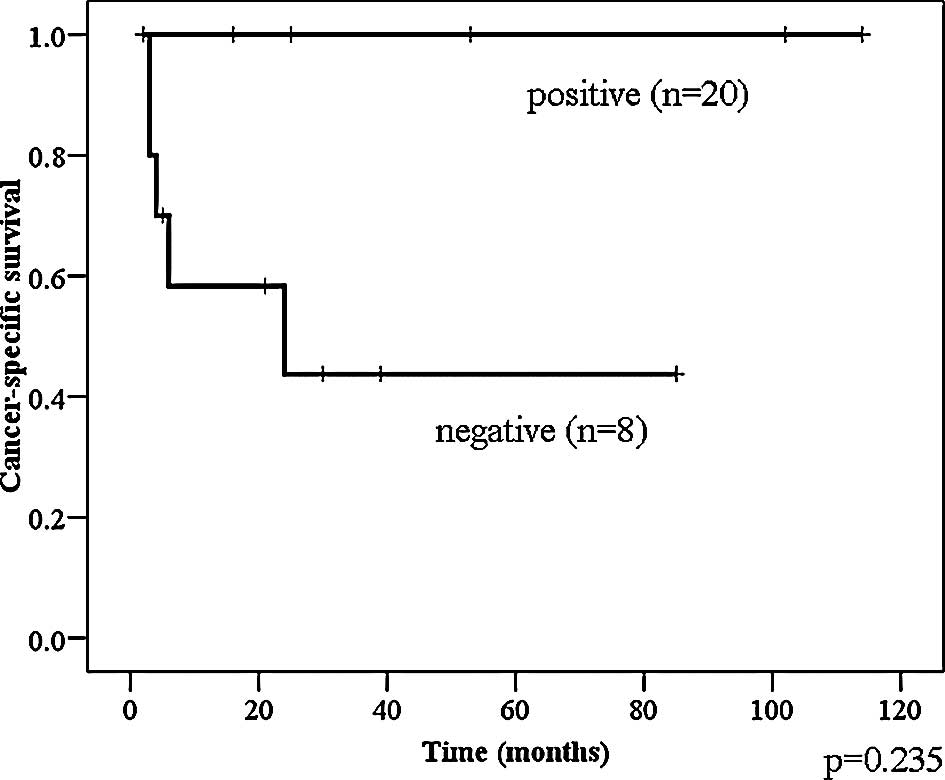
Telomerase activity was not significantly associated
with cancer-specific survival in the total population of bladder
cancer patients (Fig. 3).
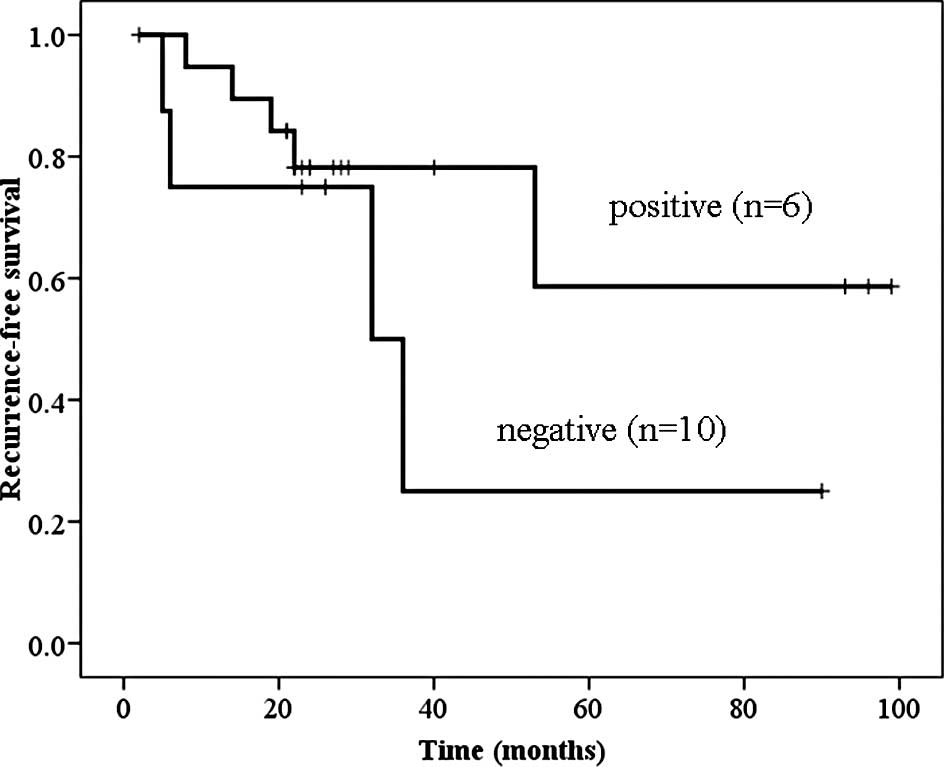
Telomerase-positive primary superficial bladder cancer patients
tended to have a good prognosis with regard to recurrence-free
survival; however, the difference was not significant (Fig. 4).
Six of 16 patients with invasive and grade 3 tumors
were telomerase-positive; all 6 patients had a relatively good
prognosis (Fig. 5).
Discussion
Telomerase activity is detected in a high percentage
of tumors and in over 80% of human tumor specimens in vivo
(9). Telomerase is apparently
reactivated in tumors, and telomerase activity may play a role in
the tumorigenic process (10).
Telomerase activity was observed in most tumor
samples, a finding consistent with previous studies on bladder
cancer (6,8,11–14).
The detection of telomerase activity in exfoliated urothelium is
useful in the diagnosis of bladder cancer, as well as in the early
detection of bladder cancer recurrence in the follow-up cases
(11,15).
An association between telomerase activity and
pathological grade or stage in bladder cancer patients has also
been demonstrated (5,6,12).
Lin et al reported that the expression of telomerase
activity was clearly associated with pathological grade and stage
(12). Tumors with high telomerase
activity were characteristically accompanied by a high grade and
advanced stage. Takihana et al also demonstrated that the
expression of human telomerase reverse transcriptase (hTERT) mRNA
was correlated with the progression of stage and grade in bladder
cancer (10).
However, in the present study, low telomerase
activity was found in advanced-stage and high-grade tumors, whereas
high telomerase activity occurred in early-stage and low-grade
tumors. Lancelin et al reported that the absence of
telomerase activity in three tumor extracts was not correlated with
low tumor grade (5). In their
report, an inhibition of telomerase activity was observed in the
PC3 (telomerase-positive) cell line upon its incubation with
telomerase-negative cancer extract during the telomere elongation
phase. The authors noted that this result indicated the presence of
telomerase inhibitor in the tumors. However, the mechanisms behind
this inhibition of telomerase activity remain unclear.
The present data were consistent with the findings
of Lancelin et al (5).
Telomerase activity may therefore play an important role in
malignant alteration, but is not necessarily associated with tumor
invasiveness and progression.
Certain studies have demonstrated that high
telomerase activity is correlated with tumor progression and poor
outcome in other types of tumors (16–18).
Tatsumoto et al reported that the up-regulation of
telomerase activity is an independent prognosis-associated factor
in patients with colorectal cancer (16). Marchetti studied telomerase
activity in 118 stage I non-small cell lung cancer patients
(17), and observed that
telomerase activity was a significant predictor of overall survival
in these patients. In addition, in the same series of patients,
telomerase activity was a marker of disease-free survival.
By contrast, other reports noted that tumors with
low telomerase activity had a poor prognosis (9,19,20).
Kawanishi et al analyzed telomerase activity in 122 surgical
Stage II specimens of colorectal carcinoma (19). The prognosis was found to be worse
for patients with telomerase-negative tumors than for patients with
telomerase-positive tumors. The authors assumed that the alternate
telomerase-independent pathway is activated in telomerase-negative
tumors, and that the alternative pathway represents the predominant
mechanism for cellular transformation. It has been suggested that
the mechanism by which this pathway leads to immortalization
involves telomere elongation and stabilization via chromosomal
translocation and recombination. Accordingly, telomerase-negative
cells are expected to possess a more aggressive, genetically
unstable phenotype.
In the present study, increased telomerase activity
was not significantly associated with cancer-specific survival in
the bladder cancer patients. However, patients positive for
telomerase activity did appear to demonstrate a good prognosis, in
particular those patients with invasive and grade 3 tumors.
Consequently, in these patients, telomerase activity may be useful
as prognostic marker. In this study, the number of patients with
invasive and grade 3 tumors was small. To elucidate the association
between telomerase activity and prognosis, future studies with a
large sample number are required.
In conclusion, telomerase activity may be useful for
predicting the prognosis in patients with a particularly high stage
or high grade of bladder cancer. Patients with low telomerase
activity tumors may have a poor prognosis.
References
|
1.
|
Blackburn EH: Structure and function of
telomerase. Nature. 350:569–573. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Moyzis RK, Buckingham JM, Cram S, et al: A
highly conserved repetitive DNA sequence (TTAGGG) n, present at the
telomere of human chromosomes. Proc Natl Acad Sci USA.
85:6622–6626. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Hastie ND, Dempster M, Dunlop MG, Thompson
AM, Green DK and Allshire RC: Telomerase reduction in human
colorectal carcinoma and with aging. Nature. 346:866–868. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Allsopp RC, Vaziri H, Patterson C, et al:
Telomere length predicts replicative capacity of human fibroblasts.
Proc Natl Acad Sci USA. 89:10114–10118. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Lancelin F, Anidjar M, Villette JM, et al:
Telomerase activity as a potential marker in a preneoplastic
bladder lesions. BJU Int. 85:526–531. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Okumura A, Mizuno I, Nagakawa O and Fuse
H: Telomerase activity is correlated with lower grade and lower
stage bladder carcinoma. Int J Urol. 11:1082–1086. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Mostofi FK, Davis CJ and Sesterhenn IA:
Histological Typing of urinary Bladder Tumours. 2nd edition.
Springer; Berlin: 1999, View Article : Google Scholar
|
|
8.
|
Kim NW, Piatyszek MA, Prowse KR, et al:
Specific association of human telomerase activity with immoral
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Rossi A, Russo G, Puca A, et al: The
antiretroviral nucleoside analogue Abacavir reduces cell growth and
promotes differentiation of human medulloblastoma cells. Int J
Cancer. 125:235–243. 2009. View Article : Google Scholar
|
|
10.
|
Takihana Y, Tsuchida T, Fukasawa M, Araki
I, Tanabe N and Takeda M: Real-time quantitative analysis for human
telomerase RNA component mRNA expression an markers for
clinicopathologic parameters in urinary bladder cancer. Int J Urol.
13:401–408. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Bravaccini S, Sanchini MA, Granato AM, et
al: Urine telomerase activity for the detection of bladder cancer
in females. J Urol. 178:57–61. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Lin Y, Miyamoto H, Fujinami K, et al:
Telomerase activity in human bladder cancer. Clin Cancer Res.
2:929–932. 1996.PubMed/NCBI
|
|
13.
|
Zhang B, Bai YX, Ma H, et al: Silencing
PinX1 compromises telomere length maintenance as well as
tumorigenicity in telomerase-positive human cancer cells. Cancer
Res. 69:75–83. 2009. View Article : Google Scholar
|
|
14.
|
Lee DH, Yang SC, Hong SJ, Chung BH and Kim
IY: Telomerase: a potential marker of bladder transitional cell
carcinoma in bladder washes. Clin Cancer Res. 4:535–538.
1998.PubMed/NCBI
|
|
15.
|
Eissa S, Swellam M, Ali-Labib R, Mansour
A, El-Malt O and Tash FM: Detection of telomerase in urine by 3
methods: evaluation of diagnostic accuracy for bladder cancer. J
Urol. 178:1068–1072. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Tathumoto N, Hiyama E, Murakami Y, Imamura
Y, Shay JW, Matsuura Y and Yokoyama T: High telomerase activity is
an independent prognostic indicator of poor outcome in colorectal
cancer. Clin Cancer Res. 6:2696–2701. 2000.PubMed/NCBI
|
|
17.
|
Marchetti A, Bertacca G, Buttitta F,
Chella A, Quattrocolo G, Angeletti CA and Bevilasqua G: Telomerase
activity as a prognostic marker indicator in stage I non-small cell
lung cancer. Clin Cancer Res. 5:2077–2081. 1999.PubMed/NCBI
|
|
18.
|
Hiyama E, Yokoyama T, Tatsumoto N, Hiyama
K, Imamura Y, Murakami Y, Kodama T, Piatyszek MA, Shay JW and
Matsuura Y: Telomerase activity in gastric cancer. Cancer Res.
55:3258–3262. 1995.PubMed/NCBI
|
|
19.
|
Kawanishi TR, Lopez F, Fratantonio S, Kim
N, Goldblum J, Tubbs R, Elson P, Lavery I, Bukowski RM, Ganapathi R
and Ganapathi MK: Telomerase activity in stage II colorectal
carcinoma: Telomerase negative tumors are correlated with poor
prognosis. Cancer. 95:1834–1839. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Nooredin Z, Lina F and Annika D: Weak
telomerase activity in malignant cells in metastatic serous
effusions. Acta Cytologica. 51:412–416. 2007. View Article : Google Scholar : PubMed/NCBI
|