Introduction
Cisplatin is a commonly used drug in head and neck
cancer chemotherapy in combination with 5-FU or docetaxel. However,
one of the major limitations to its use in the treatment of head
and neck cancer is natural or acquired resistance to cisplatin
(1). The mechanism of resistance
to cisplatin is unclear, but several hypotheses have been suggested
in previous reports. Resistance to cisplatin is generally
multi-factorial and has been shown to be the result of reduced drug
accumulation, inactivation by thiol-containing species, increased
repair/tolerance of platinum-DNA adducts, and alterations in
proteins involved in apoptosis (2,3).
It is generally accepted that the futile attempt to
repair cisplatin-induced DNA damage may finally result in the
triggering of apoptosis (4). The
mismatch repair (MMR) system, one of the signal transduction
pathways, is involved in inducing apoptosis. Many studies have
demonstrated that the loss of MMR in cisplatin resistance is
associated with microsatellite instability (MSI) and reduced
apoptosis (5). Cells in which the
MMR system has been inactivated display an MSI phenotype identified
in tumors of several different origins, both heredity and sporadic
(6). Microsatellite sequences are
tandem repeat sequences of 1–4 nucleotide units, and more than tens
of thousands of different microsatellite sequences are distributed
throughout human chromosomes. MSI characterizes the mutator
phenotype and is the hallmark of MMR deficiency (7).
In this study, cisplatin-resistant UM-SCC 23 C/R and
UM-SCC 81B cells were isolated from the head and neck squamous cell
carcinoma UM-SCC 23 cell line. In cisplatin-resistant cells, hMLH1
gene and protein expression levels were decreased in the
cisplatin-resistant cell lines. The MSI phenotype was absent in all
the cell lines. Our data support the hypothesis that hMLH1 is an
important predictor of cisplatin sensitivity, while MSI was not
involved in cisplatin sensitivity.
Materials and methods
Cells and cell culture
The UM-SCC 23 and UM-SCC 81B cells (head and neck
squamous cell carcinoma cell lines) were kindly donated by Dr
Thomas E. Carey, Laboratory of Head and Neck Cancer Biology at the
University of Michigan. The cells were maintained in Dulbecco’s
modified Eagle’s medium (DMEM; Sigma, MO, USA) supplemented with
10% fetal bovine serum (FBS; Invitrogen, CA, USA) in a humidified
atmosphere of 5% CO2 at 37°C.
Isolation of cisplatin-resistant
cells
UM-SCC 23 cells (10×106) were inoculated
into a 10-cm dish and cultured for 24 h in DMEM with 10% FBS. Cells
were then treated with cisplatin (Nihonkayaku, Tokyo, Japan) at a
concentration of 0.5 mg/ml for 24 h, then cultured in DMEM without
cisplatin until returning to stable growth. The concentration of
cisplatin treatment was stepwisely increased from 1.0, 2.0, 3.0,
4.0 to 5.0 mg/ml.
Colony formation assay for cisplatin
sensitivity
The appropriate number of cells were inoculated in a
6-cm dish, and treated with each concentration of cisplatin for 24
h. The cells were washed twice with PBS, and the culture medium was
exchanged for a fresh one. Seven to fourteen days after
inoculation, colonies were stained with 0.05% crystal violet.
Colonies of ≥50 cells were scored as originating from a single
clonogenic cell.
Analysis of hMLH1 and hMSH2 mRNA
expression
Expression of hMLH1 and hMSH2 mRNA in each cell line
was determined by real-time RT-PCR. Total RNA was extracted with
TRIzol reagent (Invitrogen) from the cell lines, and first cDNA
strand synthesis, performed with ThermoScript™ (Invitrogen) for the
detection of hMLH1 and hMSH2 mRNA, was amplified under the
following conditions: 10 min at 95°C and 40 cycles of 5 sec at
95°C, 20 sec at 60°C and 40 sec at 72°C. The LightCycler System
(Roche Diagnostics, Sandhoferstrase, Mannheim, Germany) with SYBR
Green PCR Core Reagents (PE Biosystems, Werrinton, UK) was used.
Expression levels of hMLH1 and hMSH6 mRNA for each sample were
determined by standardizing with the expression level of
β-actin.
Western blot analysis
To observe the expression of hMLH1 and hMSH2,
proteins were extracted with RIPA solution (1% NP-40, sodium
deoxycholate and 0.05% SDS in PBS). Total protein (10 μg) was
loaded onto a 10% SDS gel and blotted onto a nitrocellulose
membrane after electrophoresis. The primary mouse polyclonal
anti-hMLH1 (Pharmingen, CA, USA), polyclonal anti-hMSH2 (Serotec
Ltd., Oxford, UK) and polyclonal anti-β-actin (Abcam, Cambridge,
UK) were used in 1:200, 1:200 and 1:10,000 dilutions,
respectively.
The secondary antibodies were peroxidase-conjugated
anti-mouse IgG used in a 1:10,000 dilution. Immunoreactive proteins
were detected using enhanced chemiluminescence (ECL; Amersham
Pharmacia Biotech Inc., NJ, USA).
DNA preparation and microsatellite
analysis
Approximately 50–100 cells were seeded onto a 10-cm
dish and cultured at 37°C in 5% CO2 for 7 days, and the
colony was prepared. Using small cloning cylinders, each single
clone was isolated, inoculated into a well of a 48-well plate, and
grown to confluence. DNA samples for PCR amplification were
prepared by treating the cells with cytolytic solutions (10 mM
Tris-HCl of 100 μl, 1 mM EDTA, 5 μg/ml Proteinase K) for 2 h at
65°C and 15 min at 95°C. Extracted DNA was amplified on
microsatellite loci D9S171 and D13S175 by PCR using microsatellite
primer (ABI PRISM® Linkage Mapping Sets, version 2.5;
Applied Biosystems, CA, USA). The reaction was conducted under the
following conditions: 12 min at 95°C, 10 cycles of 15 sec at 95°C,
15 sec at 55°C, 15 sec at 72°C and 20 cycles of 15 sec at 89°C, 15
sec at 55°C, 15 sec at 72°C. Microsatellite analysis was performed
with the ABI PRISM 310 Genetic Analyzer (Applied Biosystems).
Results
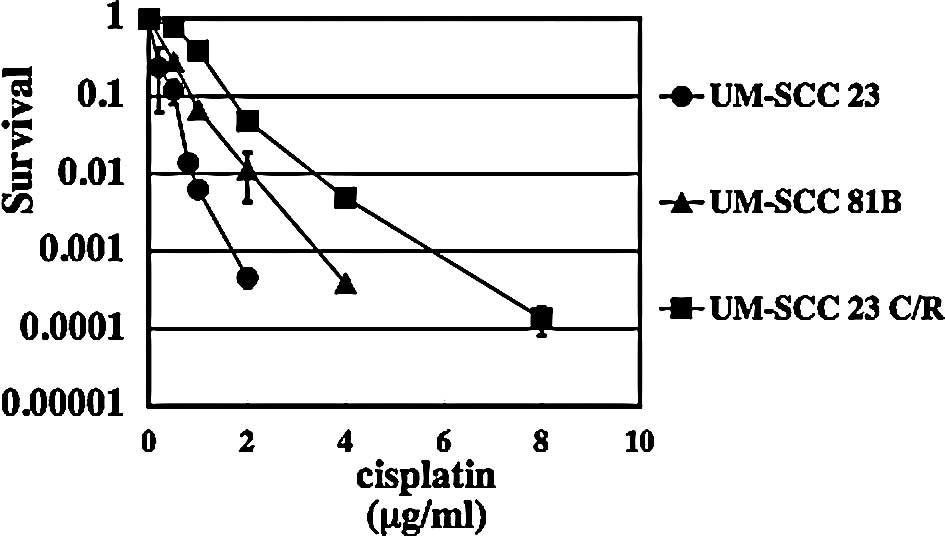
Cisplatin sensitivity of each cell
line
The cisplatin sensitivity of the UM-SCC 23 cells,
and of the UM-SCC 81B and UM-SCC 23 C/R cells isolated from the
UM-SCC 23 cell line by colony formation assay, was analyzed. The
results are shown in Fig. 1.
UM-SCC 81B cells, the intrinisic cisplatin-resistant cell line for
cisplatin, were ∼2-fold more resistant than UM-SCC 23 cells. UM-SCC
23 C/R cells were ∼3.5-fold more resistant to cisplatin than UM-SCC
23 cells.
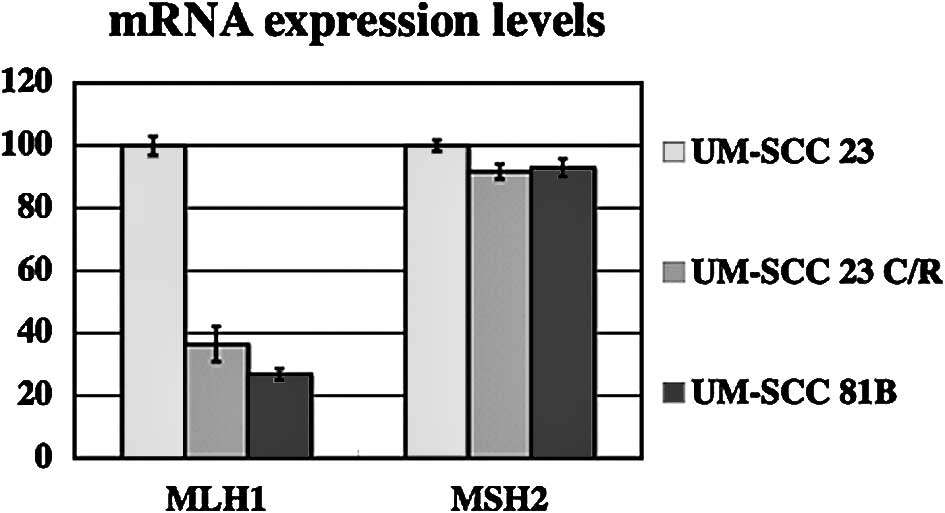
hMLH1 and hMSH2 mRNA expression
levels
Expression of hMLH1 and hMSH2 mRNA in UM-SCC 23 and
UM-SCC 23 C/R cells was analyzed by real-time RT-PCR. Expression
levels of hMLH1 mRNA in UM-SCC 81B and UM-SCC 23 C/R cells were
decreased ∼60% as compared with UM-SCC 23 cells. A difference in
hMSH2 mRNA expression level was not found among the three cell
lines (Fig. 2).
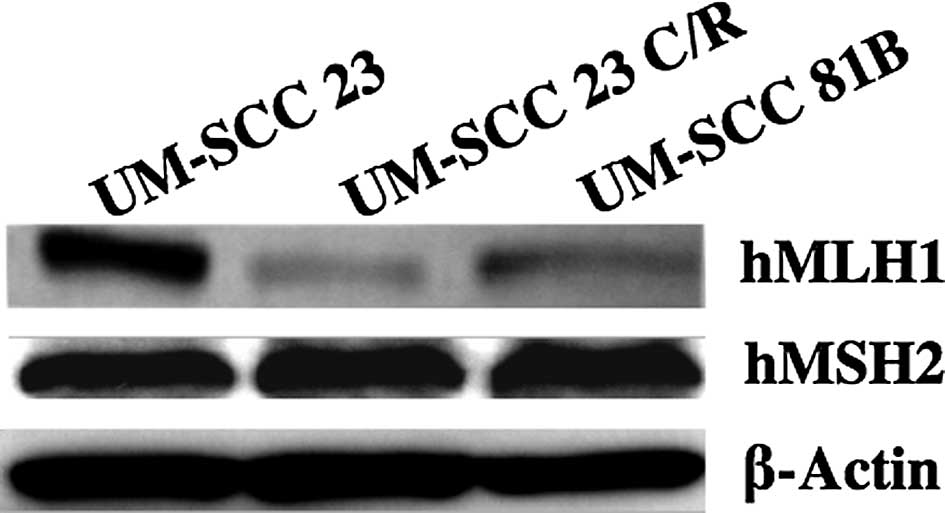
hMLH1 and hMSH2 protein expression
level
hMLH1 and hMSH2 mismatch repair proteins were
analyzed by Western blot analysis. The hMLH1 protein expression
level was decreased to a greater extent in the UM-SCC 23 C/R than
in the UM-SCC 23 cells. The hMLH1 expression level was further
decreased in the UM-SCC 81B cells. hMSH2 was examined using the
same method, but no change was found among the three cell lines
(Fig. 3).
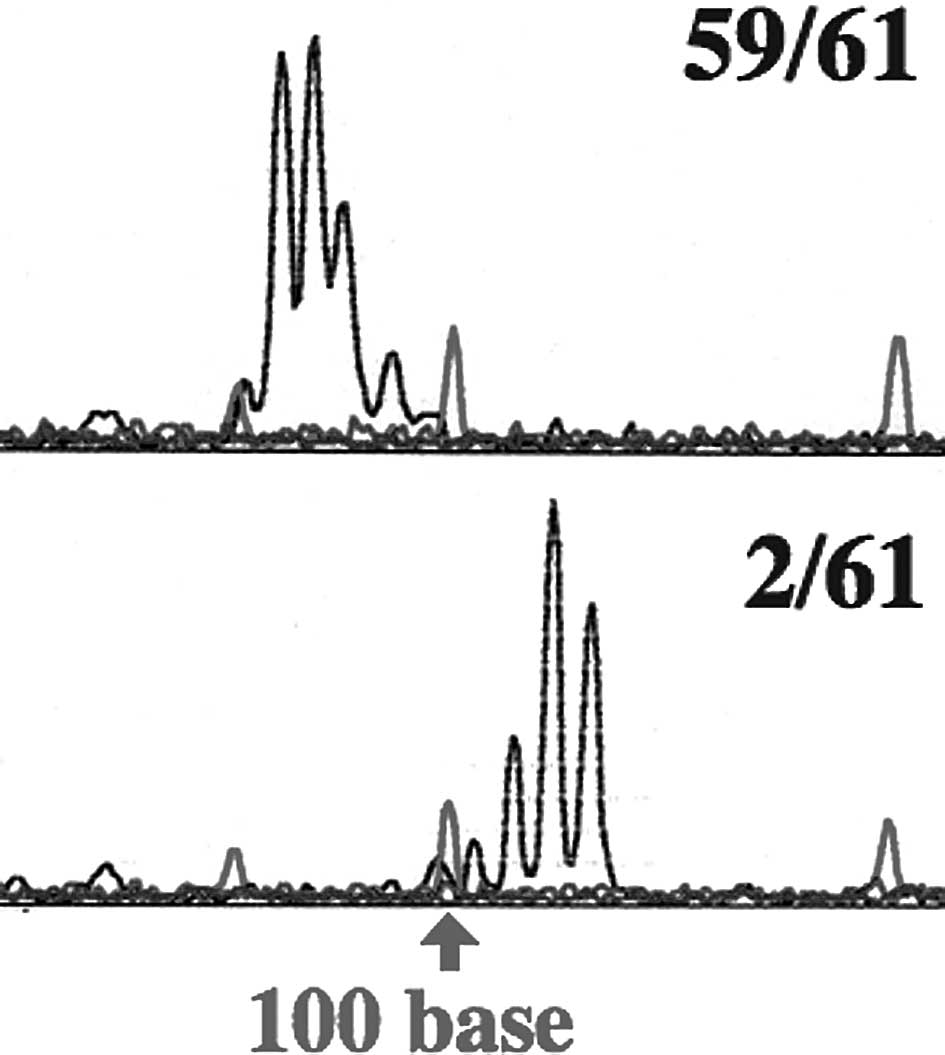
Microsatellite instability
Microsatellite instability was analyzed with Gene
Scan. A change in the microsatellite was found in 1 of 55 samples
in D9S171 for the UM-SCC 23 cells. Sixty-six samples of UM-SCC 23
C/R cells were analyzed, but changes in the microsatellite were not
observed. The micro-satellite changes were found in 2 samples each
in D9S171 and D13S175 among 61 samples for UM-SCC 81B cells
(Fig. 4; Table I).
 | Table I.The ratio of the microsatellite change
in each cell line. |
Table I.
The ratio of the microsatellite change
in each cell line.
| Change
|
|---|
| D9S171 | D13S175 |
|---|
| UM-SCC 23 | 1/55 (1.81%) | 0/55 (0.00%) |
| UM-SCC 23 C/R | 0/66 (0.00%) | 0/66 (0.00%) |
| UM-SCC 81B | 2/61 (3.03%) | 2/61 (3.03%) |
Discussion
The MMR system plays an important role in the
control of genomic instability in cells. In order to ensure genomic
stability, it is necessary that the repair of DNA occurs prior to
DNA replication (8). Before repair
is initiated, the damage to DNA must be recognized by specific
proteins. Indeed, a number of DNA damage recognition proteins have
been identified, but studies to define their involvement in
cisplatin-resistant tumor cells have largely been confined to the
MMR complex (9). MMR serves a
critical purpose in maintaining the integrity of the genome through
the repair of DNA mismatch lesions, but does not actually repair
cisplatin adducts. One proposed theory is that MMR attempts to
repair the lesion, but in failing to do so activates the apoptotic
signal (10).
In this study, no difference was observed in hMSH2
mRNA and protein expression levels among the three cell lines.
However, hMLH1 mRNA and protein expression levels were
significantly decreased in the cisplatin-resistant cells. The MMR
system involves at least five proteins (hMLH1, hMSH2, hMSH3, hMSH6
and hPMS2) and functions as an ATP-dependent repair process that
corrects misincorporated nucleotides. hMSH2/hMSH6 heterodimers
directly bind, as the first mismatch recognizing complex, to GpG
intrastrand adducts of cisplatin, and hMLH1/hPMS2 heterodimers are
subsequently recruited to play an important role as the
hMSH2/hMSH6/hMLH1/hPMS2 complex, and induce the stabilization and
pro-apoptotic activation of p73 (11). This process requires both the MMR
system and the c-Abl kinase. Previous studies have shown a
decreased hMLH1 expression level or a defect caused by methylation
of the promoter domain of hMLH1 in cisplatin-resistant cells
(12). It is thought that the
expression level of hMLH1 involved in apoptotic induction is
decreased through recognition of the cisplatin adduct in the
process in which cells acquire cisplatin resistance. Therefore, the
cisplatin-DNA-adduct is recognized by hMSH2; however, the decrease
of the apoptosis signal pathway mediated by the mismatch repair
system decreases hMLH1 expression levels, resulting in the
development of cisplatin resistance (13). In light of this observation
regarding cisplatin sensitivity, hMLH1 mRNA and gene product
expression levels may be predictors of natural and acquired
cisplatin resistance.
Analysis of the microsatellite sequence in UM-SCC
23, UM-SCC 81B and UM-SCC 23 C/R cells indicated changes in the
microsatellite in 2 samples each of D9S171 and D13S175 among 61
samples in UM-SCC 81B cells, a natural cisplatin-resistant cell
line. The frequency of microsatellite changes was much lower than
in other reports of MSI. In addition, a microsatellite change was
not found in UM-SCC 23 C/R cells established as cisplatin-resistant
cells. MSI was hardly evident in the three cell lines. The absence
of MSI in sporadic colon cancer can be a predictive marker of
sensitivity for the first post-operative adjuvant chemotherapy
(14), and sensitivity of
cisplatin-based chemotherapy is not associated with MSI in cervical
cancer (15,16).
In conclusion, since hMLH1 mRNA and protein
expression levels were decreased in the UM-SCC 81B and UM-SCC 23
C/R natural and acquired cisplatin-resistant cell lines, cisplatin
adduct recognition was deduced to be involved in the acquisition of
cisplatin resistance. Therefore, hMLH1 gene and gene product
expression levels are effective predictors of the sensitivity to
cisplatin in head and neck cancer chemotherapy. In addition, MSI
was not present in conjunction with decreased hMLH1 expression
levels; therefore, cisplatin-based chemotherapy is not associated
with the frequency of MSI.
References
|
1.
|
Choong N and Vokes E: Expanding role of
the medical oncologist in the management of head and neck cancer.
CA Cancer J Clin. 58:32–53. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Wozniak K and Blasiak J: Recognition and
repair of DNA-cisplatin adducts. Acta Biochim Pol. 49:583–596.
2002.PubMed/NCBI
|
|
3.
|
Siddik ZH: Cisplatin: mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Yang Z, Faustino PJ, Andrews PA, Monastra
R, Rasmussen AA, Ellison CD and Cullen KJ: Decreased cisplatin/DNA
adduct formation is associated with cisplatin resistance in human
head and neck cancer cell lines. Cancer Chemother Pharmacol.
46:255–262. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Papouli E, Cejka P and Jiricny J:
Dependence of the cytotoxicity of DNA-damaging agents on the
mismatch repair status of human cells. Cancer Res. 64:3391–3394.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Colella G, Marchini S, D’Incalci M, Brown
R and Broggini M: Mismatch repair deficiency is associated with
resistance to DNA minor groove alkylating agents. Br J Cancer.
80:338–343. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Geisler JP, Goodheart MJ, Sood AK, Holmes
RJ, Hatterman-Zogg MA and Buller RE: Mismatch repair gene
expression defects contribute to microsatellite instability in
ovarian carcinoma. Cancer. 98:2199–2206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ishizaki K, Nishizawa K, Kato T, Kitao H,
Han ZB, Hirayama J, Suzuki F, Cannon TF, Kamigaichi S, Tawarayama
Y, Masukawa M, Shimazu T and Ikenaga M: Genetic changes induced in
human cells in Space Shuttle experiment (STS-95). Aviat Space
Environ Med. 72:794–798. 2001.PubMed/NCBI
|
|
9.
|
Manic S, Gatti L, Carenini N, Fumagalli G,
Zunino F and Perego P: Mechanisms controlling sensitivity to
platinum complexes: role of p53 and DNA mismatch repair. Curr
Cancer Drug Targets. 3:21–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Pani E, Stojic L, El-Shemerly M, Jiricny J
and Ferrari S: Mismatch repair status and the response of human
cells to cisplatin. Cell Cycle. 15:1796–1802. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Shimodaira H, Yoshioka-Yamashita A,
Kolodner RD and Wang JY: Interaction of mismatch repair protein
PMS2 and the p53-related transcription factor p73 in apoptosis
response to cisplatin. Proc Natl Acad Sci USA. 100:2420–2425. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Cejka P, Stojic L, Mojas N, Russell AM,
Heinimann K, Cannavó E, di Pietro M, Marra G and Jiricny J:
Methylation-induced G(2)/M arrest requires a full complement of the
mismatch repair protein hMLH1. EMBO J. 22:2245–2254. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Stojic L, Brun R and Jiricny J: Mismatch
repair and DNA damage signaling. DNA Repair. 3:1091–1101. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Ribic CM, Sargent DJ, Moore MJ, Thibodeau
SN, French AJ, Goldberg RM, Hamilton SR, Laurent-Puig P, Gryfe R,
Shepherd LE, Tu D, Redston M and Gallinger S: Tumor
microsatellite-instability status as a predictor of benefit from
fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J
Med. 349:247–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Helleman J, van Staveren IL, Dinjens WN,
van Kuijk PF, Ritstier K, Ewing PC, van der Burg ME, Stoter G and
Berns EM: Mismatch repair and treatment resistance in ovarian
cancer. BMC Cancer. 31:2012006. View Article : Google Scholar
|
|
16.
|
Magnowska M, Surowiak P, Nowak-Markwitz E,
Michalak M, Magnowski P, Rokita W, Kedzia H, Zabel M and Spaczyński
M: Analysis of hMLH1 and hMSH2 expression in cisplatin-treated
ovarian cancer patients. Ginekol Pol. 79:826–834. 2008.PubMed/NCBI
|