Introduction
Worldwide, over one million women are diagnosed
annually with breast cancer, equating to a tenth of all new cancers
and 23% of female cancers. Eighty percent of cases occur in women
over 50 years of age, although it is also the most commonly
diagnosed cancer in women under the age of 35 (1). The diagnosis and treatment of breast
cancer is commonly associated with considerable psychiatric
morbidity (2). For example, Hall
et al (3) reported that of
269 women with early breast cancer, 49.6% were clinically anxious
and 37.2% were clinically depressed in the first 3 months following
surgery, whereas Burgess et al (4) found that of 222 women with early
breast cancer, 48% were clinically anxious and/or depressed in the
first year.
Complementary and alternative medicines (CAM) are
widely used by patients with cancer to help them cope with the
stress of the diagnosis and treatment of the disease (5,6);
with an annual expenditure exceeding £1.6 billion in the UK
(7). A recent study found that 69%
of breast cancer survivors reported using some form of CAM, and of
these, 73% changed or initiated use due to cancer diagnosis
(8). Of the many forms of CAM
available, reflexology has been reported to be the most popular
amongst cancer patients in the UK (used by over 35% of those
receiving CAM treatment) (9).
Stress-induced immunosuppression, including that
associated with the diagnosis and treatment of cancer, is now a
well established immunological phenomenon (10,11).
A meta-analysis by Herbert and Cohen (12) revealed a relationship between
stress and decreased functional immune measures, and a more recent
meta-analysis of over 300 studies also showed that the immune
outcomes were dependent on the types of stress involved, e.g.,
acute vs. chronic stress (13).
Various parameters of the immune system are
adversely affected by stress. These include natural killer (NK)
cell activity, the numbers and percentages of circulating white
blood cells and immunoglobulin levels (12). NK cells, and their more active
IL2-stimulated counterparts, lymphokine activated killer (LAK)
cells, have anti-tumour properties, but are generally suppressed in
cancer patients (14); the
relationship of such effects to the development and/or progression
of cancer has been widely discussed but remains unresolved
(15–17).
T helper cells play a key role in controlling the
immune response. These can be subdivided further into T-helper 1
(Th1)- and T-helper 2 (Th2)-like cells; defined by the cytokine
repertoire they produce and the responses they induce. Th1-like
cells are principally involved in promoting cell-mediated immunity,
initiating a cytotoxic response and generally are considered as the
host’s main anti-cancer mechanism (18), whereas Th2-like cells stimulate a
humoral or antibody-mediated response, involved principally against
extracellular pathogens. A stress response induces a shift in
favour of Th2-like cells (10),
which is observed in different types of cancer by changes in the
concentrations of specific serum cytokines (19–21).
Stress can also alter the circulating levels of
neuroendocrine hormones, in particular cortisol and to a lesser
extent prolactin and growth hormone, whose effects on the immune
system are widespread (22,23).
These are caused, at least in part, through direct activation of
specific cell surface receptors expressed by immune cells (24–26).
The hypothalamic-pituitary-adrenal (HPA) axis is generally regarded
as the most probable pathway by which the effects of psychosocial
and complementary interventions on the immune system are mediated
(27–29). The stress of diagnosis and
treatment of cancer is also likely to act via the HPA axis and be
involved in the progression of cancer (30).
Several studies suggest that psychosocial
interventions aimed at promoting coping can alter the levels of HPA
hormones (31–33). In patients with cancer,
psychosocial interventions can normalize (34–36),
or reduce (37) cortisol levels,
as well as reduce prolactin levels (36). There is potential, therefore, for
stress-reducing CAM interventions to influence the immune system
via this neuroendocrine pathway.
A number of previous randomised trials evaluating
the effects of various behavioural, psychosocial and complementary
therapies in cancer patients have demonstrated both improvements in
quality of life as well as changes in biological parameters
(38–43). However, diagnostic and therapeutic
heterogeneity, as well as the use of different tumour types and
outcome measures, limit the conclusions that can be drawn.
Previous research on breast cancer patients has
shown that patients do not always comply with relaxation and guided
imagery (38,42); therefore, the present study was
designed to evaluate an alternative well-received intervention to
promote relaxation. Hence, the current randomised controlled trial
evaluated the effects of reflexology and scalp massage on host
defences and neuroendocrine function. Reflexology was compared with
two comparator conditions, namely treatment as usual, which
involves self-initiated support (SIS) in the Oncology Health
Centres (44), and scalp massage,
as a control for physical and social contact inherent in
reflexology. Patients receiving reflexology or scalp massage had
similar access to the Oncology Health Centres. It was hypothesised
that, compared with SIS, women with breast cancer randomised to
reflexology or to scalp massage would show reduced immunological
and endocrine signs of stress. Immunologically this would include
increases in the percentages of T helper cells expressing Th1
cytokines (i.e., IFNγ, IL2) and decreases in those expressing Th2
cytokines (i.e., IL4, IL10); increases in the percentage of overall
T cells, NK cells, T cytotoxic cells and activated T lymphocytes
with no change in B cell or monocyte number; and increases in
NK/LAK activity. From a neuroendocrine perspective, it was
hypothesised that decreases in cortisol, prolactin and growth
hormone levels, along with decreases in the number of lymphocytes
expressing their corresponding receptors would be observed.
Materials and methods
Design, approval and registration
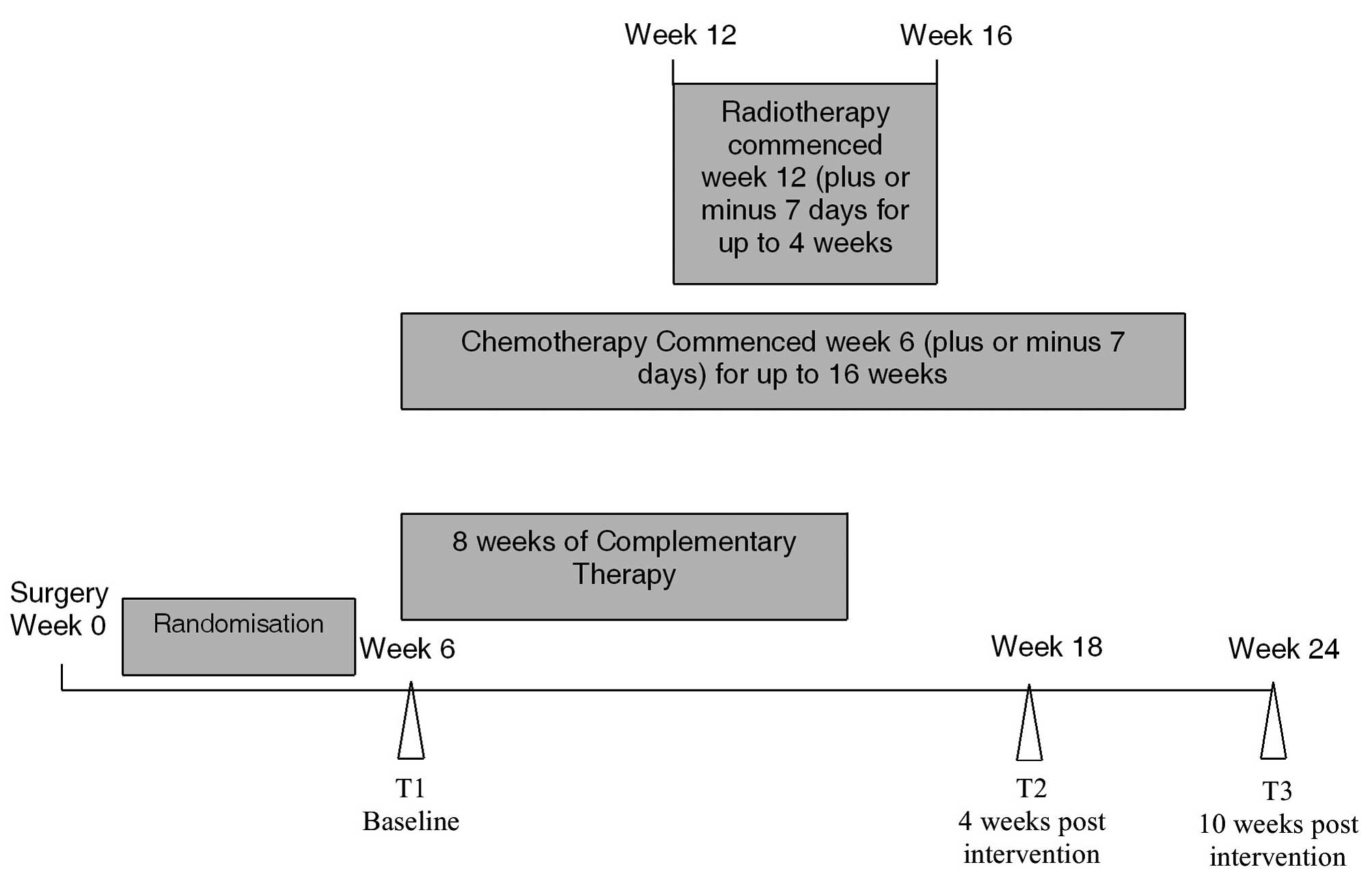
This was a three-armed randomised, controlled trial.
Data were collected at three time points: T1 (6±1 week post breast
surgery), T2 and T3 (4 and 10 weeks post completion of CAM,
respectively) (Fig. 1).
Ethical approval was obtained from Hull and East
Yorkshire Local Research Ethics Committee (reference 01/01/010),
and the study was registered with the International Standard
Randomized Controlled Trial Registry (ISRCTN 87652313).
Patients
Women over 18 years of age with early breast cancer
[T1, T2 (<3 cm), N0, N1a, M0], awaiting adjuvant therapy, were
recruited consecutively after surgery. A diagnostically homogeneous
group of patients was chosen to minimise the effects of disease and
stage-related variables (Table I)
(45). Patients with a previous
cancer diagnosis or more advanced disease were not eligible for
recruitment, as were those participating in other clinical trials
and those suffering from clinically significant cognitive
impairment or dementia.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Total (n=183) | Reflexology
(n=60) | Massage (n=61) | SIS (n=62) | P-value |
|---|
| Mean age
(years) | 58.78 | 59.37 | 57.70 | 59.26 | 0.61 |
| SD | 10.31 | 10.47 | 10.12 | 10.23 | |
| Age range | 32–81 | 32–81 | 36–76 | 36–77 | |
| Ethnicity | | | | | 1.00 |
| Caucasian | 183 | 60 | 59 | 62 | |
| Other | 2 | 0 | 2 | 0 | |
| ER status | | | | | 0.78 |
| Positive | 164 | 53 | 56 | 55 | |
| Negative | 18 | 6 | 5 | 7 | |
| Unknown | 1 | 1 | 0 | 0 | |
| PR status | | | | | 0.65 |
| Positive | 150 | 47 | 52 | 51 | |
| Negative | 30 | 11 | 8 | 11 | |
| Unknown | 3 | 2 | 1 | 0 | |
| T stage | | | | | 0.42 |
| DCIS | 3 | 2 | 0 | 1 | |
| T1 | 124 | 40 | 43 | 41 | |
| T2 | 52 | 15 | 18 | 19 | |
| T3 | 4 | 3 | 0 | 1 | |
| Breast Surgery | | | | | 0.87 |
| Wide local
excision | 144 | 47 | 46 | 51 | |
|
Quadrantectomy | 1 | 1 | 0 | 0 | |
| Mastectomy | 26 | 8 | 11 | 7 | |
| Mast +
reconstruction | 12 | 4 | 4 | 4 | |
| Radiotherapy
planned | | | | | 0.31 |
| Yes | 149 | 52 | 50 | 47 | |
| No | 34 | 8 | 11 | 15 | |
| Chemotherapy
planned | | | | | 0.88 |
| Yes | 30 | 10 | 11 | 9 | |
| No | 153 | 50 | 50 | 53 | |
Randomisation
Patients who gave written informed consent (n=183)
were randomised to one of three interventions in the Oncology
Health Centres at Castle Hill or Princess Royal Hospitals in Hull:
self-initiated support (SIS) plus foot reflexology (n=60), SIS plus
scalp massage (identical amount of comparator physical and social
contact intervention from the same therapists who administered
reflexology; n=61), or SIS alone (treatment as usual; n=62)
(44).
A permuted block randomisation sequence for each
stratum (menopausal status, chemotherapy and radiotherapy) was
generated using Graph-Pad (http://www.graphpad.com); block size was 8 and was
concealed. The sequences were stored in sealed, opaque, numbered
envelopes. Randomisation was carried out remotely at the Clinical
Trials Section of the Institute of Rehabilitation, University of
Hull. Biological assessments were carried out in a completely
blinded manner.
Interventions
Patients randomised to reflexology or massage
received 8 sessions at weekly intervals for 8 weeks commencing 7
weeks after surgery. Eight sessions at weekly intervals was chosen
on the recommendation of an external consultant who was formerly
the Secretary of the Scottish Institute of reflexology.
Reflexology was administered by two part-time staff
who had been trained to the standards required for membership of
the Scottish Institute of Reflexology. Their performance and
adherence to the reflexology protocol was monitored at regular
intervals during the study by an external consultant experienced in
administering reflexology to patients with cancer.
Scalp massage was used as a control for attention,
physical contact and non-specific therapist effects. Patients
randomised to massage received gentle scalp massage from the same
therapists according to a quality assured protocol. Since
reflexologists believe that the ears and neck have ‘terminals’,
care was taken to avoid these areas. Scalp massage was chosen,
rather than foot or hand massage, because any manipulation or
pressure to the feet and hands, according to reflexology theory,
will stimulate pressure points and, therefore, will be a weak form
of reflexology rather than an appropriate ‘placebo’.
Women randomised to SIS were invited to attend, or
telephone, one of the Oncology Health Centres whenever they wished.
They received ‘treatment as usual’ in the Centres when they
attended, as did those randomised to reflexology or massage. The
Oncology Health Centres are staffed by clinical health
psychologists and nurses, and provide psychosocial support services
for more than 1,500 new patients per year and almost as many new
relatives. Emphasis is placed on the prevention of psychological
and psychiatric morbidity, and evidence-based psychopharmacological
and psychotherapeutic interventions are offered to patients who
develop clinically significant problems. Patients can access the
service without referral, and appointments are not necessary.
In order to control for practitioner variables, each
practitioner saw a similar number of patients in each of the two
physical contact arms of the study. As far as possible, each
patient had the same therapist throughout, and both treatments were
given in the same rooms.
Conventional treatment
All patients underwent conventional treatment
according to current best practice (surgery, radiotherapy,
chemotherapy and hormone therapy) as clinically indicated.
Biological assays
Peripheral blood mononuclear cell
(PBMC) isolation
Venous blood (50 ml) was collected into syringes
containing 1,250 IU heparin from all patients at T1, T2 and T3 and
transported to the Centre for Biomedical Research at the University
of Hull. PBMCs were isolated using Ficoll-Hypaque (Sigma), density
gradient centrifugation (46). The
PBMCs were washed with phosphate-buffered saline (PBS; pH 7.4),
enumerated using a haemocytometer and assessed for viability by
trypan blue exclusion, before resuspension in foetal bovine serum
(Invitrogen) containing 10% (v/v) dimethylsulphoxide (Sigma).
Aliquots were then frozen at 1°C/min and stored in liquid nitrogen
until use.
Serum separation. Venous blood (8 ml) was
collected into serum separator tubes at approximately the same time
of day (to control for diurnal variation) for each patient at T1,
T2 and T3. These were incubated at 4°C for 30 min before
centrifugation at 1,500 × g for 10 min. The top serum layer was
aliquoted and stored at −80°C until ELISA analysis.
Immunophenotyping of peripheral blood mononuclear
cells. An aliquot of PBMCs from each of the three time points
was resuspended in complete medium [RPMI-1640 medium supplemented
with 10% (v/v) foetal bovine serum, penicillin (100
U/ml)/streptomycin (100 μg/ml) and L-glutamine (2 mM), all
purchased from Invitrogen]. Approximately 2×105 PBMCs
were labelled with 5 μl (0.1 mg/ml) of one of a panel of
fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies
(AbD Serotec, Oxford, UK) for 30 min in the dark at room
temperature. These antibodies were specific for the surface
markers: CD2 and CD3 (T cells), CD4 (T helper cells), CD8 (T
cytotoxic cells), CD16 and CD56 (NK cells), CD14 (monocytes), CD19
(B cells) and CD25 (activated lymphocytes/regulatory T cells).
Purified mouse IgG1-FITC was used as an irrelevant control.
Following labelling, the cells were washed with PBS, pH 7.4,
containing 0.1% (w/v) bovine serum albumin and 10 mM
NaN3 (PBS/BSA/azide; Sigma) and recovered by
centrifugation before immediate acquisition of 10,000 cells/sample
using a FACS Calibur™ machine (Becton Dickinson, Biosciences,
Oxford, UK). Analysis was performed using CellQuest Pro V software
(Becton Dickinson) with gates being set around the lymphocytes and
the monocytes based on forward scatter/side scatter distribution.
Histograms were drawn for each antibody using the lymphocyte gate,
except for CD14 which used the monocyte gate. The plots using the
irrelevant control were used to set a marker whereby ≤3% of cells
were positive with this reagent. To calculate the percentage of
specific binding, the irrelevant value was subtracted from the
percentage of cells staining with the test antibody.
Th1/Th2 cellular determination. The method
used was a modification of that by Jung et al (47). Briefly, PBMCs were incubated for 4
h at 37°C with 5% CO2 in either activation medium
[complete RPMI containing ionomycin (2 μg/ml), brefeldin A (20
μg/ml) and phorbol 12-myristate 13-acetate (20 ng/ml), all from
Sigma] or control medium [complete RPMI containing solely brefeldin
A (20 μg/ml)]. Approximately 2×105 PBMCs, were then
incubated for 30 min in the dark with 5 μl (0.1 mg/ml) of FITC
labelled monoclonal antibody specific for the surface markers CD3
or CD8; purified mouse IgG1 provided the irrelevant control (AbD
Serotec). After incubation, the cells were washed with
PBS/BSA/azide, and fixed using Leucoperm A (AbD Serotec) for 15 min
in the dark. Following further washes, the cells were permeabilised
with Leucoperm B before being incubated with 5 μl r-phycoerythrin
(RPE)-labelled anti-cytokine antibody [IL2, IFNγ (Becton
Dickinson), IL4, IL10 (AbD Serotec), 0.1 mg/ml] for 30 min at room
temperature in the dark. TNFα (Becton Dickinson) provided a
positive activation control, and purified mouse IgG1 provided the
negative control. Cells were acquired and analysed as described
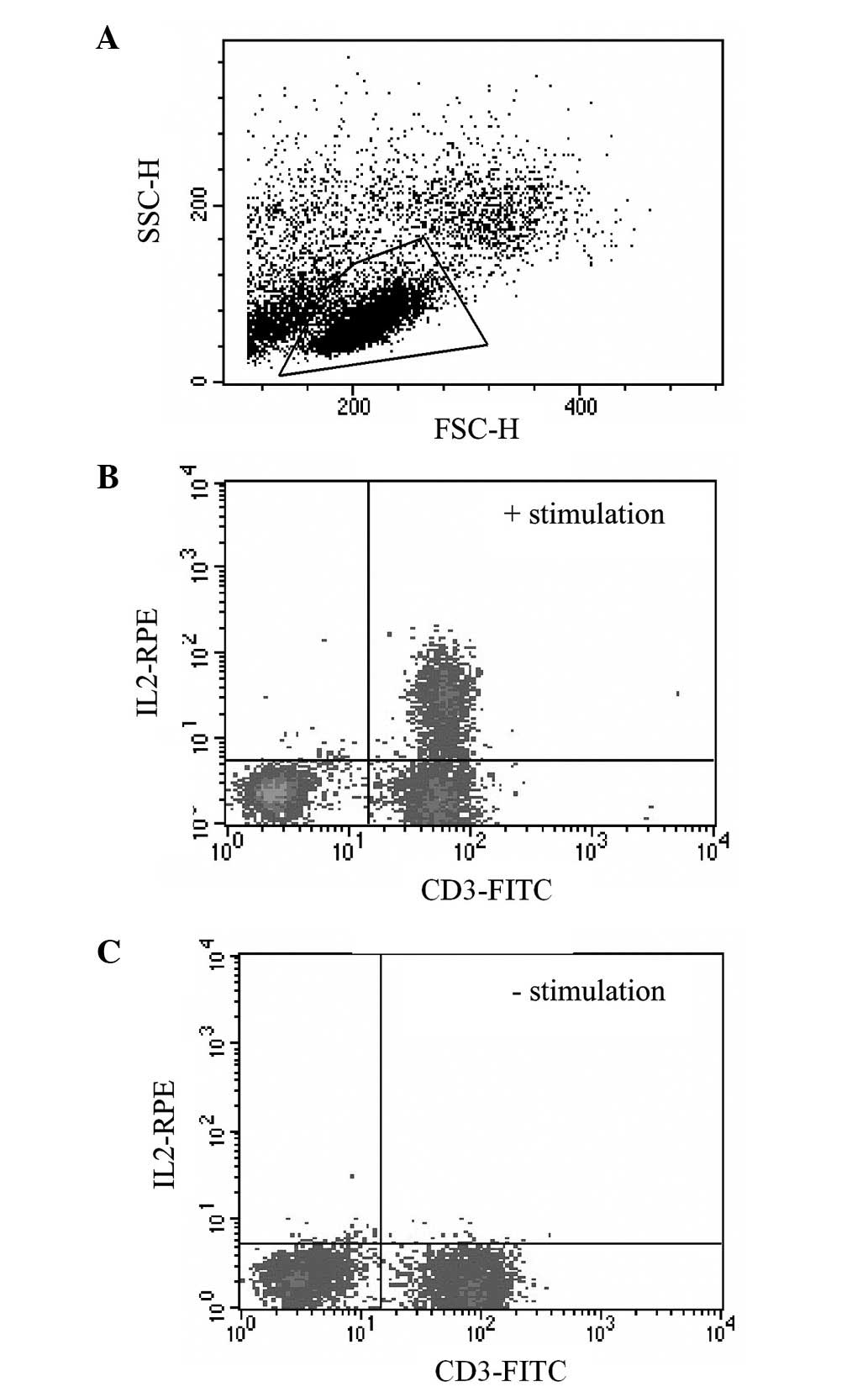
previously using a FACS Calibur™. The lymphocyte subset was gated
on the basis of forward and side scatter characteristics (Fig. 2A). Stimulated lymphocytes produced
Th1 (IL2/IFNγ) and Th2 (IL4/IL10) cytokines (Fig. 2B). CD3 was used as a total
lymphocyte marker, and the CD4 fraction was determined by
subtraction of the percentage of CD8+ cells.
Unstimulated controls (Fig. 2C)
were used to set the quadrants so that <1% of these cells were
positive for both the surface and the cytokine antibodies (upper
right quadrant).
NK and LAK cell cytotoxicity. The cytotoxic
activity of both NK and LAK cells within the PBMC population was
determined using a modification of the Live/Dead Cell-mediated
cytotoxicity kit (Molecular Probes/Invitrogen) (48). Briefly, 2×106 log-phase
growing target cells [erythroleukaemic cell line (K562)] for NK and
Burkitt lymphoma cell line (Daudi) for LAK] were prelabelled with a
green fluorescent membrane dye (DiOC18(3), 30 μM) for 1 h at 37°C. Once thawed
PBMCs were incubated for 48 h in complete RPMI medium with or
without recombinant IL2 (500 U/ml; AbD Serotec) (49) to stimulate LAK formation and for NK
determination respectively; this also allows for the adherence and
removal of monocytes. Following washing, viable effector cells were
enumerated using trypan blue exclusion, and 1.5 or 3×105
cells were added to 3×104 target cells to give a 5:1 and
10:1 ratio, respectively, in a total volume of 140 μl. An equal
volume of the membrane impermeable dye, propidium iodide (PI; 150
μM; Sigma), was added to each tube before centrifuging briefly at
1,000 × g for 1 min and incubation at 37°C for 3.5 h in a
humidified atmosphere. Appropriate controls of target and effector
cells alone were also prepared. Following incubation, the cytotoxic
activity was analysed by flow cytometry; samples were acquired for
45 sec with no gating and data were obtained for both the green
(FL-1, DiOC18(3)) and
red (FL-3, PI) fluorescence, as well as forward and side scatter
characteristics. Data analysis was performed on dot plots of FL-1
vs. FL-3. Quadrants were set using the appropriate controls to
exclude the effector cells from the analysis, and the lysis of
target cells was determined from the percentage of cells present in
the upper right (UR) quadrant (green and red positive, i.e., dead
target cells) divided by the total number of green target cells (UR
+ LR).
Serum hormone measurements. The hormones
prolactin, cortisol and growth hormone were all measured in
duplicate, using Enzyme Linked Immunosorbant Assay (ELISA, DRG
Instruments GmbH, Germany). All samples from the same patient were
analysed on the same ELISA plate to minimise intra-patient
variability. Prolactin levels were measured using a standard solid
phase sandwich ELISA technique, with a lower detection limit of 2
ng/ml, according to the manufacturer’s protocol. Cortisol levels
were analysed using a competitive ELISA with a lower detection
limit of 2.5 ng/ml, according to the manufacturer’s protocol.
Growth hormone levels were determined using a solid phase Enzyme
Amplified Sensitivity Immunoassay, in which monoclonal antibodies
against distinct epitopes of human growth hormone are used to
create the sandwich; with a lower detection limit of 0.11 μIU/ml.
Data for each sample were extracted from the standard curve.
Hormone receptor measurements. Following
activation of PBMCs as described above, the cells which were to be
used for the detection of the prolactin receptor (PRL-R) and the
glucocorticoid receptor (Gluc-R) were permeabilised. PBMCs were
then incubated for 30 min at 4°C with 5 μl (1 mg/ml) mouse
anti-human antibodies: GH-R unconjugated, Gluc-RFITC conjugated
(both AbD Serotec) and PRL-R Ab-1 (B6.2) unconjugated (Neomarkers,
Fremont, CA). Purified mouse IgG1 was used as the negative control.
Following washing with PBS/BSA/azide unconjugated antibodies were
detected using a secondary rabbit anti-mouse F(ab’), IgG:FITC
antibody (AbD Serotec) for 30 min at 4°C. PBMCs were washed again
before flow cytometric analysis.
Statistics
Data were analysed using SPSS v13 for MS Windows. α
was set at 0.05 (two-tailed). The comparability of the three groups
at baseline (T1; clinical, socio-demographic and psychosocial
variables) was assessed using one-way analyses of variance (ANOVA)
for continuous variables, and the Chi-square exact test for
categorical variables. All data were included in the analyses of
categorical variables, and missing data were not inputed, as cohort
averages would not have been appropriate.
An intention to treat analysis was carried out
(50), and continuous variables
were analysed using univariate analyses of covariance (ANCOVA),
with age, tumour stage and baseline (T1) values as covariates. To
minimise the risk of a Type I error, paired comparisons were only
considered when the f-value for the three-group comparison was
significant, and Bonferroni corrections were applied for subsequent
paired comparisons. Data were log transformed when the
distributions differed significantly from the normal.
Results
Recruitment and use of samples
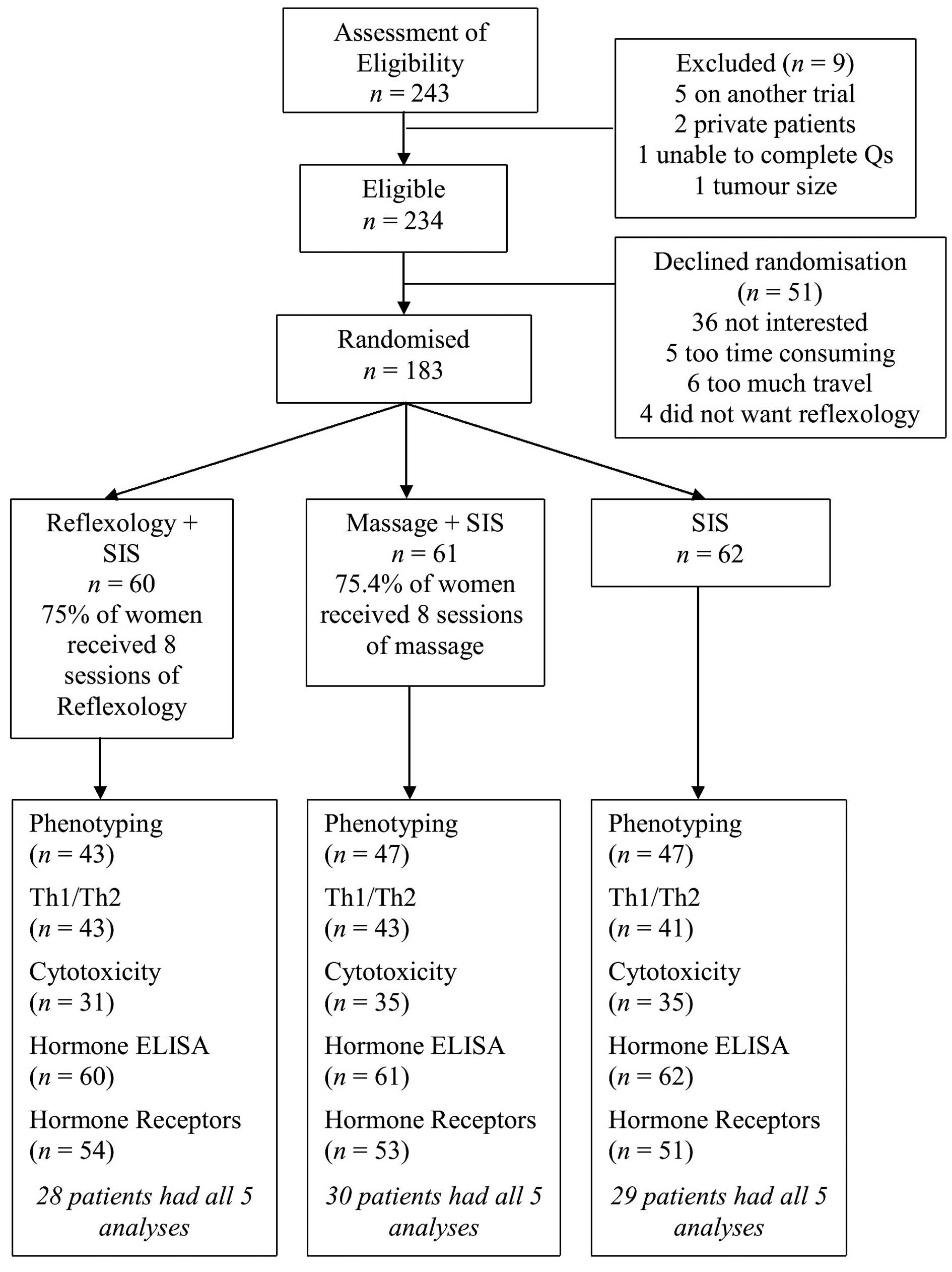
A consecutive series of 243 women was assessed for
eligibility (Fig. 3). Of the 234
who were eligible, 183 (78.2%) agreed to be randomised. The most
common reason for not wishing to participate was lack of interest,
often because women expressed a desire to ‘get on with their
lives’. Four (1.7%) eligible patients refused randomisation because
they did not wish to partake in reflexology.
Sixty patients were randomised to reflexology, 61 to
massage and 62 to SIS alone. The characteristics of the women by
randomisation are shown in Table
I. The three groups did not differ significantly for any of the
demographic or clinical variables, including radiotherapy and/or
chemotherapy.
The CONSORT diagram (Fig. 3) indicates the samples used for the
different biological assays. The number of aliquots of PBMCs
obtained from each patient varied, and there were insufficient
aliquots from each patient to be used for all techniques. Samples
were selected for each analysis on the basis that there were
sufficient aliquots of PBMCs present for each of the three time
points to enable a full dataset to be collected.
Effects of reflexology and massage on
phenotypic distribution of PBMCs
Flow cytometry was used to determine the changes
which occurred in the distribution of the mononuclear cell
populations (Table II). At T3,
ANCOVA showed that the percentage of CD25+ lymphocytes
in the patients receiving massage was significantly higher than for
those in the SIS group (p=0.05). No significant between-group
differences were found for the remainder of the phenotypic
markers.
 | Table II.Mean percentage of PBMCs from early
breast cancer patients expressing phenotypic markers at T2 and
T3. |
Table II.
Mean percentage of PBMCs from early
breast cancer patients expressing phenotypic markers at T2 and
T3.
| T2 |
|
| A
Reflexology + SIS | B
Massage + SIS | C
SIS | A vs. B vs.
C
f-test
p-value | A vs. B
f-test
p-value | A vs. C
f-test
p-value | B vs. C
f-test
p-value |
|
| CD3 | 69.6±2.8 | 70.2±2.6 | 66.3±2.7 | 0.56 | 1.0 | 1.00 | 0.93 |
| CD2 | 75.3±3.6 | 75.0±3.5 | 70.2±3.5 | 0.51 | 1.0 | 0.92 | 0.97 |
| CD4 | 47.0±2.3 | 45.7±2.2 | 44.2±2.2 | 0.69 | 1.0 | 1.00 | 1.00 |
| CD8 | 20.6±1.1 | 19.7±1.1 | 22.5±1.1 | 0.17 | 1.0 | 0.64 | 0.19 |
| CD16 | 16.7±1.2 | 16.8±1.1 | 17.6±1.1 | 0.83 | 1.0 | 1.00 | 1.00 |
| CD56 | 9.9±1.2 | 11.1±1.1 | 11.4±1.1 | 0.62 | 1.0 | 1.00 | 1.00 |
| CD14 | 7.8±1.1 | 8.2±1.1 | 8.4±1.1 | 0.93 | 1.0 | 1.00 | 1.00 |
| CD19 | 6.5±0.8 | 7.4±0.8 | 7.5±0.8 | 0.63 | 1.0 | 1.00 | 1.00 |
| CD25 | 7.3±1.0 | 6.5±1.0 | 5.3±1.0 | 0.36 | 1.0 | 0.47 | 1.00 |
| T3 |
|
| CD3 | 71.8±2.0 | 69.8±1.9 | 69.9±1.9 | 0.71 | 1.00 | 1.00 | 1.00 |
| CD2 | 75.8±3.1 | 74.7±2.9 | 74.7±2.9 | 0.96 | 1.00 | 1.00 | 1.00 |
| CD4 | 47.8±1.8 | 48.0±1.8 | 47.5±1.8 | 1.00 | 1.00 | 1.00 | 1.00 |
| CD8 | 21.8±1.5 | 21.9±1.4 | 21.1±1.4 | 0.92 | 1.00 | 1.00 | 1.00 |
| CD16 | 18.2±1.8 | 20.2±1.8 | 17.8±1.7 | 0.59 | 1.00 | 1.00 | 1.00 |
| CD56 | 13.5±1.7 | 14.8±1.6 | 13.2±1.6 | 0.76 | 1.00 | 1.00 | 1.00 |
| CD14 | 9.3±1.0 | 9.3±1.0 | 8.1±1.0 | 0.60 | 1.00 | 1.00 | 1.00 |
| CD19 | 6.8±0.9 | 9.3±0.9 | 7.4±0.9 | 0.11 | 0.14 | 1.00 | 0.38 |
| CD25 | 10.1±1.5 | 10.8±1.5 | 5.7±1.5 | 0.03a | 1.00 | 0.12 | 0.05a |
The percentages of NK, B cells, CD3+ and
CD4+ T cells were very similar to those previously
reported both pre- and post-psychosocial intervention by Carlson
et al in a cohort of patients with either breast or prostate
cancer (51).
Effects of reflexology and massage on
Th1/Th2 cell balance in PBMCs
Table III shows the
results from the flow cytometry method used to determine the
percentage of lymphocytes producing Th1 (IL2/IFNγ) and Th2
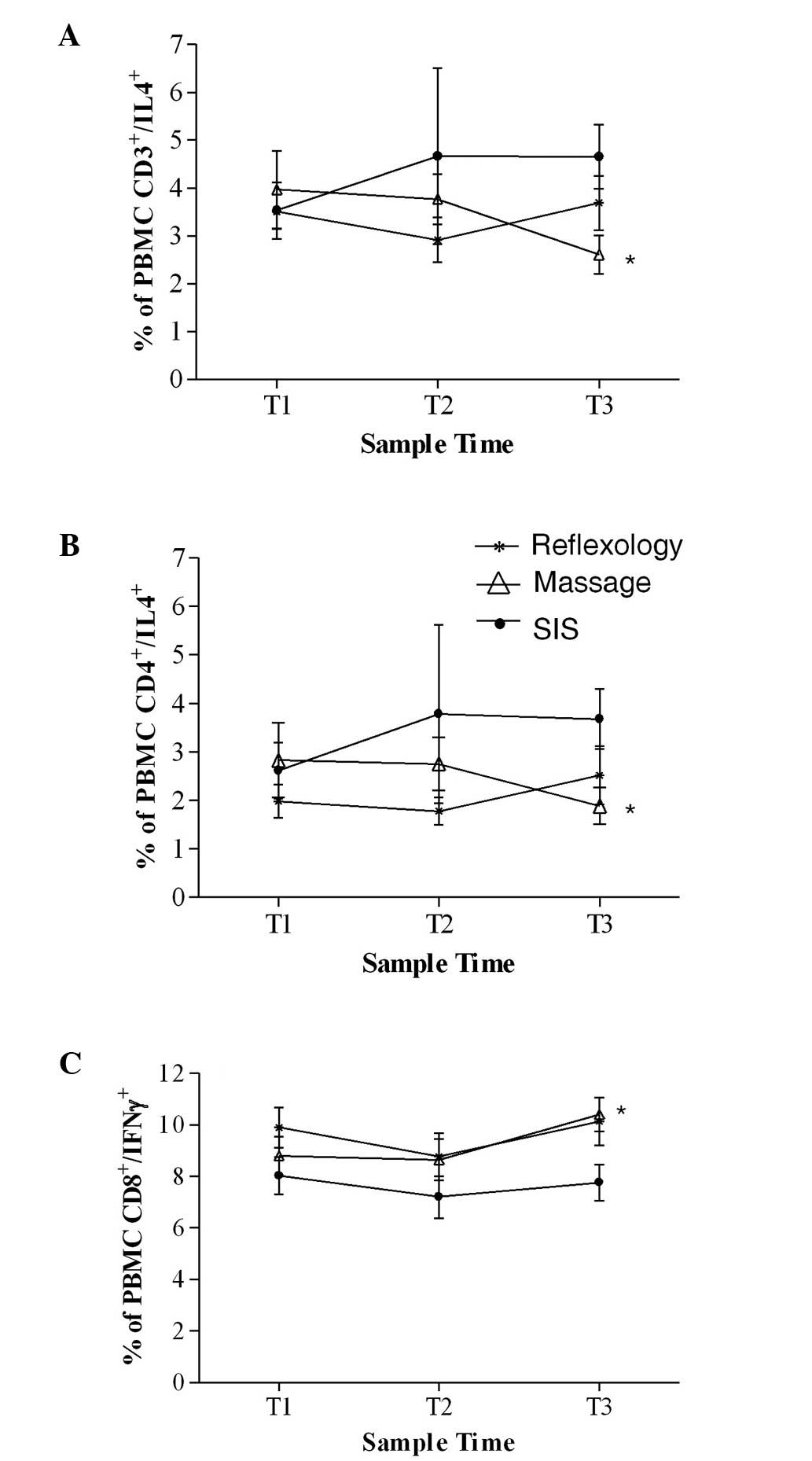
(IL4/IL10) cytokines. ANCOVA showed a significantly lower
percentage of CD3+ cells expressing IL4 at T3 in the
massage patients compared with the SIS patients (p=0.02, Fig. 4A). The same was true in the
CD4+ subset of T cells expressing IL4 which mirrored the
results of the CD3+ cells (Fig. 4B; p=0.02).
 | Table III.Mean percentage of Th1 (IL2, IFNγ)
and Th2-like (IL4, IL10) T (CD3), cytotoxic (CD8) and helper (CD4)
cell proportions in PBMCs from breast cancer patients at T2 and
T3. |
Table III.
Mean percentage of Th1 (IL2, IFNγ)
and Th2-like (IL4, IL10) T (CD3), cytotoxic (CD8) and helper (CD4)
cell proportions in PBMCs from breast cancer patients at T2 and
T3.
| T2 |
|
| A
Reflexology + SIS | B
Massage + SIS | C
SIS | A vs. B vs.
C
f-test
p-value | A vs. B
f-test
p-value | A vs. C
f-test
p-value | B vs. C
f-test
p-value |
|
| CD3/IL2 | 23.5±1.5 | 25.2±1.5 | 22.5±1.6 | 0.45 | 1.00 | 1.00 | 0.64 |
| CD3/IFNγ | 19.3±1.1 | 20.3±1.1 | 17.2±1.2 | 0.17 | 1.00 | 0.61 | 0.19 |
| CD3/IL4 | 2.9±1.1 | 3.9±1.1 | 4.9±1.2 | 0.48 | 1.00 | 0.69 | 1.00 |
| CD3/IL10 | 1.2±0.4 | 1.5±0.4 | 0.9±0.4 | 0.63 | 1.00 | 1.00 | 1.00 |
| CD8/IL2 | 2.6±0.2 | 2.8±0.2 | 2.5±0.2 | 0.59 | 1.00 | 1.00 | 0.93 |
| CD8/IFNγ | 8.1±0.6 | 9.2±0.6 | 8.0±0.6 | 0.26 | 0.51 | 1.00 | 0.45 |
| CD8/IL4 | 0.8±0.17 | 1.2±0.2 | 1.0±0.2 | 0.21 | 0.25 | 0.92 | 1.00 |
| CD8/IL10 | 0.4±0.15 | 0.4±0.2 | 0.5±0.2 | 0.82 | 1.00 | 1.00 | 1.00 |
| CD4/IL2 | 20.9±1.4 | 22.5±1.4 | 20.0±1.5 | 0.46 | 1.00 | 1.00 | 0.66 |
| CD4/IFNγ | 10.7±0.8 | 11.2±0.8 | 9.6±0.8 | 0.32 | 1.00 | 0.87 | 0.43 |
| CD4/IL4 | 1.8±1.1 | 2.9±1.2 | 4.1±1.2 | 0.38 | 1.00 | 0.49 | 1.00 |
| CD4/IL10 | 0.7±0.4 | 1.1±0.4 | 0.6±0.4 | 0.57 | 1.00 | 1.00 | 1.00 |
| T3 |
|
| CD3/IL2 | 26.7±1.7 | 26.7±1.8 | 26.0±1.8 | 0.94 | 1.00 | 1.00 | 1.00 |
| CD3/IFNγ | 20.5±1.0 | 21.2±1.1 | 19.5±1.1 | 0.52 | 1.00 | 1.00 | 0.76 |
| CD3/IL4 | 3.7±0.6 | 2.5±0.6 | 4.8±0.6 | 0.03a | 0.44 | 0.56 | 0.02a |
| CD3/IL10 | 0.9±0.2 | 0.8±0.2 | 0.9±0.2 | 0.72 | 1.00 | 1.00 | 1.00 |
| CD8/IL2 | 3.0±0.2 | 2.8±0.3 | 2.5±0.3 | 0.48 | 1.00 | 0.70 | 1.00 |
| CD8/IFNγ | 9.4±0.6 | 10.6±0.6 | 8.3±0.6 | 0.03a | 0.45 | 0.59 | 0.02a |
| CD8/IL4 | 1.7±0.3 | 1.0±0.3 | 1.0±0.3 | 0.07 | 0.12 | 0.17 | 1.00 |
| CD8/IL10 | 0.3±0.1 | 0.2±0.1 | 0.3±0.1 | 0.86 | 1.00 | 1.00 | 1.00 |
| CD4/IL2 | 24.9±1.6 | 24.1±1.7 | 23.5±1.7 | 0.82 | 1.00 | 1.00 | 1.00 |
| CD4/IFNγ | 11.6±0.7 | 11.1±0.7 | 11.2±0.7 | 0.89 | 1.00 | 1.00 | 1.00 |
| CD4/IL4 | 2.7±0.6 | 1.7±0.6 | 3.7±0.6 | 0.02a | 0.9 | 0.20 | 0.02a |
| CD4/IL10 | 0.8±0.2 | 0.6±0.2 | 0.7±0.2 | 0.85 | 1.00 | 1.00 | 1.00 |
At T3, ANCOVA showed that a significantly higher
percentage of CD8+ cells were expressing IFNγ in the
massage group compared with the SIS group (p=0.02, Fig. 4C). No significant between-group
differences were found for any of the other T cell subsets
expressing Th1- or Th2-like cytokines.
Effects of reflexology and massage on
the cytotoxic activity of PBMCs
The NK and LAK cell activity was determined at
effector:target ratios of 5:1 and 10:1 using a flow cytometry-based
method. These ratios were chosen since they provided the most
reproducible results in initial studies and spared sufficient PBMCs
for use in other experiments. There were no significant
between-group differences in the cytotoxic activity of either NK or
LAK cells at any time point (Table
IV).
 | Table IV.Mean percentage K562 and Daudi cell
death induced by NK and LAK cells respectively, in PBMCs from
breast cancer patients at 5:1 and 10:1 (effector:target) ratios at
T2 and T3. |
Table IV.
Mean percentage K562 and Daudi cell
death induced by NK and LAK cells respectively, in PBMCs from
breast cancer patients at 5:1 and 10:1 (effector:target) ratios at
T2 and T3.
| T2 |
|
| Ratio | A
Reflexology + SIS | B
Massage + SIS | C
SIS | A vs. B vs.
C
f-test
p-value | A vs. B
f-test
p-value | A vs. C
f-test
p-value | B vs. C
f-test
p-value |
|
| NK | 5:1 | 5.2±1.1 | 6.1±1.0 | 5.5±1.1 | 0.83 | 1.00 | 1.00 | 1.00 |
| NK | 10:1 | 5.4±1.4 | 7.8±1.3 | 7.6±1.8 | 0.42 | 0.65 | 1.00 | 1.00 |
| LAK | 5:1 | 5.8±0.9 | 4.6±0.9 | 6.2±1.0 | 0.44 | 1.00 | 1.00 | 0.69 |
| LAK | 10:1 | 5.8±1.6 | 4.5±1.4 | 5.2±1.8 | 0.82 | 1.00 | 1.00 | 1.00 |
| T3 |
|
| NK | 5:1 | 5.5±1.1 | 5.5±1.1 | 8.2±1.1 | 0.12 | 1.0 | 0.22 | 0.23 |
| NK | 10:1 | 5.5±1.4 | 5.3±1.3 | 9.8±1.6 | 0.09 | 1.0 | 0.16 | 0.12 |
| LAK | 5:1 | 5.3±1.0 | 4.9±1.0 | 6.2±1.0 | 0.62 | 1.0 | 1.00 | 1.00 |
| LAK | 10:1 | 5.3±1.6 | 6.2±1.6 | 7.1±1.8 | 0.78 | 1.0 | 1.00 | 1.00 |
Effects of reflexology and massage on
serum hormone levels and receptor expression in PBMCs
Analysis of the serum hormones or hormone receptors
(cortisol, prolactin and growth hormone) also found no significant
between-group differences (Tables
V and VI). All the values
obtained for the serum hormone concentrations were within the
normal range described in the manufacturer’s protocols.
 | Table V.Mean concentration of cortisol
(ng/ml), prolactin (ng/ml) and growth hormone (μIU/ml) in serum
from breast cancer patients at T2 and T3. |
Table V.
Mean concentration of cortisol
(ng/ml), prolactin (ng/ml) and growth hormone (μIU/ml) in serum
from breast cancer patients at T2 and T3.
| T2 |
|
| Serum hormones | A
Reflexology + SIS | B
Massage + SIS | C
SIS | A vs. B vs.
C
f-test
p-value | A vs. B
f-test
p-value | A vs. C
f-test
p-value | B vs. C
f-test
p-value |
|
| Cortisol | 123.2±5.6 | 110.4±5.6 | 117.9±5.8 | 0.28 | 0.33 | 1.00 | 1.00 |
| Prolactin | 6.4±1.1 | 5.3±1.1 | 4.3±1.1 | 0.39 | 1.00 | 0.51 | 1.00 |
| Growth hormone | 2.6±0.5 | 1.5±0.5 | 2.5±0.5 | 0.25 | 0.40 | 1.00 | 0.50 |
| T3 |
|
| Cortisol | 131.7±6.3 | 117.8±6.1 | 122.5±6.3 | 0.29 | 0.36 | 0.91 | 1.00 |
| Prolactin | 6.3±0.9 | 4.4±0.9 | 4.7±0.9 | 0.27 | 0.39 | 0.61 | 1.00 |
| Growth hormone | 3.0±1.1 | 3.2±1.0 | 3.6±1.1 | 0.92 | 1.00 | 1.00 | 1.00 |
 | Table VI.Mean percentages of PBMCs from breast
cancer patients expressing the receptors for cortisol (Gluc-R),
prolactin (PRL-R) and growth hormone (GH-R) at T2 and T3. |
Table VI.
Mean percentages of PBMCs from breast
cancer patients expressing the receptors for cortisol (Gluc-R),
prolactin (PRL-R) and growth hormone (GH-R) at T2 and T3.
| T2 |
|
| Hormone
receptors | A
Reflexology + SIS | B
Massage + SIS | C
SIS | A vs. B vs.
C
f-test
p-value | A vs. B
f-test
p-value | A vs. C
f-test
p-value | B vs. C
f-test
p-value |
|
| Gluc-R | 72.6±2.8 | 77.3±2.8 | 68.2±3.1 | 0.09 | 0.70 | 0.89 | 0.09 |
| PRL-R | 70.8±2.8 | 63.7±2.8 | 67.5±3.1 | 0.21 | 0.23 | 1.00 | 1.00 |
| GH-R | 1.8±0.7 | 1.4±0.7 | 1.8±0.8 | 0.91 | 1.00 | 1.00 | 1.00 |
| T3 |
|
| Gluc-R | 73.9±3.2 | 73.9±3.0 | 74.4±3.2 | 1.00 | 1.00 | 1.00 | 1.00 |
| PRL-R | 74.0±3.1 | 66.5±2.9 | 67.4±3.0 | 0.16 | 0.22 | 0.37 | 1.00 |
| GH-R | 1.1±1.0 | 3.4±0.9 | 1.7±1.0 | 0.23 | 0.30 | 1.00 | 0.71 |
Discussion
This study of 183 women with early breast cancer is
the largest randomised, controlled trial of reflexology reported to
date. Over 78% of a consecutive series of eligible women consented
to participate in the study, which suggests that the results are
representative and generalisable.
Scalp massage was chosen to control for the effects
of extra physical and social contact, both of which could enhance
relaxation and act as a buffer against stress. Massage is often
combined with aromatherapy, and beneficial effects have been
reported previously (52).
Research designed to evaluate the relative contributions of the
extra physical and social contact would be of considerable
interest, especially in light of this study’s findings.
Imbalances in proportions of immune cells in
patients with cancer have been previously documented (53,54),
resulting in generalised and/or specific immunosuppression. Here
the only change observed in lymphocyte subsets was the increase
over time in the percentage of CD25+ cells from patients
receiving either massage or reflexology, and by T3 the difference
was significantly greater in the massage patients compared with the
SIS group. The current finding is consistent with previous results
from the present group who reported that the percentage of
CD25+ cells was significantly greater in breast cancer
patients receiving relaxation training and guided imagery compared
with SIS (38). Research in the
1990s showed that activated CD25+ T lymphocytes could
induce tumour cell death and play a role in inhibiting tumour
growth in animal models (55,56),
suggesting that the enhanced percentage of CD25+ cells
in the present study could be beneficial to breast cancer patients.
However, more recent work has focused intensely on a subpopulation
of CD25+ cells, namely CD4+CD25+
cells, now commonly known as T regulatory cells. These cells are
frequently increased in patients with several types of malignancies
and are correlated positively with disease stage and poor prognosis
(57). They also play a role in
immune evasion mechanisms employed by cancer cells (58), and can decrease the activity of
CD8+ T cells and NK cells (59). Further characterisation of the
CD25+ subpopulations was not possible due to the lack of
cells for the analysis of T regulatory cell markers such as FoxP3,
GITR and CD127.
Previously, a small scale study of breast cancer
patients found increases in lymphocyte and NK cell numbers over
time following massage therapy and progressive muscle relaxation
(60). Hypnotic guided imagery in
breast cancer patients has also been reported to increase absolute
NK cell numbers (61), but this
increase was not maintained after a 3-month follow-up. In the
current study, no between-group differences were observed in NK
cell numbers, and changes in CD25+ lymphocytes were only
apparent at 6 months.
In support of a delayed NK cell response, a
randomised controlled study by Fawzy et al (43) evaluating the effect of a 6-week
structured psychiatric group intervention in melanoma patients who
had undergone surgery, demonstrated an increase in absolute NK and
large granular lymphocyte numbers which only became evident at the
6-month follow-up. Other studies, which have demonstrated no
changes in overall lymphocyte cell numbers and subsets, have
usually had relatively brief follow-up periods (51). In contrast, van der Pompe et
al (36) demonstrated lower
percentages of NK, CD8 and CD4 cells following 13 weeks of
experiential-existential group psychotherapy in breast cancer
patients who had undergone surgery at least 4 months prior to the
study. Thus, changes in lymphocyte subsets may well occur following
a CAM intervention, but effects could be delayed most probably due
to the suppressive effects associated with the proximity to surgery
and adjuvant chemotherapy and/or radiotherapy treatments.
Natural cytotoxicity can be reduced in certain types
of cancer patients, and conventional treatments can suppress this
further (62); however, the
ability of NK and LAK cells to kill human cancer cells efficiently
ex vivo has led to much work assessing their potential as a
form of immunotherapy (63,64).
Using effector:target ratios of 5:1 and 10:1 we found no
significant differences between groups for the interventions, which
is in agreement with other studies (61,43).
This suggests that the mechanism by which reflexology and massage
enhance quality of life does not directly involve the increased
activation of the cytotoxic NK and LAK cells, but could however be
mediated by increased cell number. Numerous effector:target ratios
have been used ranging from 1:1 to 50:1 (61,65),
however if ratios above 10:1 were used in the current study there
would have been insufficient cells to study the breadth of immune
parameters.
The most notable findings with respect to the
Th1/Th2 balance were that, in the massage patients at T3, there was
a significantly lower percentage of both CD3+ and
CD4+ cells expressing the Th2 cytokine IL4 compared with
SIS, and this was accompanied by a significant increase in the
percentage of CD8+ cells expressing the Th1 cytokine
IFNγ. The fact that different cell populations are both changing in
a manner that produces a Th1-like response is highly intriguing. It
also suggests that the commonly observed increase in circulating
IL-10 in cancer patients, produced by Th2-like cells, is a later
phenomenon caused as a consequence of changes in other T cell
subsets. Overall the results strongly suggest that there has been
some form of rebalancing of the Th1/Th2 system in the patients
receiving scalp massage.
In the present study, IL4 proved to be a good marker
for Th2-like cells; however, IL10 was detected at very low levels,
in accordance with previous studies, which found percentages of
approximately 0.2–0.6% in caregivers and controls (66). A further practical limitation of
the study, in addition to the lack of PBMCs, was that the
neuroendocrine factors were only measured at a single time-point,
and it is well known that some of these have pronounced circadian
rhythms. This was a practical constraint due to the inability to
take multiple blood or other biological samples in a day. However,
variations were minimized by taking blood at similar time-points.
Other studies have commonly used saliva, an easier fluid to sample;
however, this was not available in the current trial.
The practical limitations described above are
possibly responsible for the fact that no effect of massage or
reflexology was observed on hormone concentrations or receptor
levels. These results contrast with previous studies in cancer
patients which demonstrated that psychotherapeutic treatment and
greater social support can normalise or reduce cortisol levels, as
well as lowering prolactin levels. In breast and colorectal cancer
patients, mindfulness-based stress reduction has not only been
shown to improve quality of life, but was also associated with
decreased afternoon cortisol levels (67).
A quality of life study conducted on the same
patients showed a high level of satisfaction and compliance with
both reflexology and massage, and demonstrated that at T2, massage
improved quality of life, but reflexology did not have an effect
until T3 (68). This is partly in
accordance with the immune factors as many did not become
significant until the final endpoint (T3). The primary end point
for the quality of life study, T2, was chosen as 4 weeks after the
end of the final session of reflexology or massage. The
effectiveness of the support provided during SIS could provide one
explanation for the lack of differences found between the groups,
as we have previously shown that the provision of a fully
integrated oncology health service with drop-in facilities and
trained staff to identify and resolve concerns immediately, is
associated with very low levels of psychosocial morbidity in women
with locally advanced breast cancer (42).
This study has demonstrated that in women with early
breast cancer, scalp massage, the active control condition, but not
reflexology, the treatment of interest, administered according to
standardised protocols induced a range of immunological changes
including an increase in the percentage of CD25+ cells
and a shift towards a Th1-like response. Further studies in other
cancer populations should now be undertaken, and attempts made to
evaluate the underlying biopsychosocial mechanisms, as well as the
possible clinical consequences of these changes.
Acknowledgements
Funding support for the trial was
provided by the National Health Service, National Cancer Research
and Development programme NCP2/X229. Hull and East Yorkshire
Hospitals NHS Trust Endowments and the University of Hull provided
support for the laboratory work. We wish to thank all the patients
who participated, members of the Trial Management Group and the NHS
R&D Cancer Programme. We also wish to acknowledge the
therapists Mrs. A. Grantham and Mrs. S. Waters and the Clinical and
Research Nurse Specialists (Behavioural Oncology) in the Oncology
Health Service (Mrs. J. Bateman, Mrs. K. Ellwood, Mrs. C.
Hebblewhite, Mrs. T. Hope and Mr. M. Lines). We are grateful to the
clinicians who referred patients; in addition to those clinicians
listed in the Trial Management Group, we wish to acknowledge the
help of Dr Sunil K. Upadhyay and Dr A. Abdel-Hamid. Trial
Management Group (excluding authors): Professor M.J. Lind,
Foundation Professor of Oncology, University of Hull; Dr A.
Chaturvedi, Consultant Clinical Oncologist, Hull and East Yorkshire
Hospitals NHS Trust; Mr. J. Wood, Divisional Manager (Cancer
Services), Hull and East Yorkshire Hospitals NHS Trust; Mrs. J.
Jenkinson, Breast Care Clinical Nurse Specialist, Hull and East
Yorkshire Hospitals NHS Trust; Mr. W. Brown, Superintendent
Radiographer, Hull and East Yorkshire Hospitals NHS Trust;
Professor J.R.T. Monson, Professor of Surgery, University of Hull;
Professor P.J. Drew, Professor in Tissue Engineering and Wound
Healing, Hull York Medical School; Mr. J. Fox, Consultant Breast
Surgeon, Hull and East Yorkshire Hospitals NHS Trust; Mr. T.
Mahapatra, Consultant Breast Surgeon, Hull and East Yorkshire
Hospitals NHS Trust; Ms P. McManus, Consultant Breast Surgeon, Hull
and East Yorkshire Hospitals NHS Trust.
References
|
1.
|
Cancer Research UK: News and Resources
(http://info.cancerresearchuk.org/cancerstats/types/breast/incidenceuri).
|
|
2.
|
Zabora J, Brintzenhofenszoc K, Curbow B,
Hooker C and Piantadosi S: The prevalence of psychological distress
by cancer site. Psychooncology. 10:19–28. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Hall A, A’Hern R and Fallowfield L: Are we
using appropriate self-report questionnaires for detecting anxiety
and depression in women with early breast cancer? Eur J Cancer.
35:79–85. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Burgess C, Cornelius V, Love S, Graham J,
Richards M and Ramirez A: Depression and anxiety in women with
early breast cancer: five year observational cohort study. BMJ.
330:7022005.PubMed/NCBI
|
|
5.
|
Cassileth BR and Vickers AJ: High
prevalence of complementary therapy and alternative medicine use
among cancer patients. J Clin Oncol. 23:2590–2592. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Molassiotis A, Fernadez-Ortega P, Pud D,
et al: Use of complementary and alternative medicine in cancer
patients: a European survey. Ann Oncol. 16:655–663. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Ernst AR and White A: The BBC survey of
complementary medicine use in the UK. Complement Ther Med. 8:32–36.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Matthews AK, Sellergren MA, Huo D, List M
and Fleming G: Complementary and alternative medicine use among
breast cancer survivors. J Altern Complement Med. 13:555–562. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Corner J, Cawley N and Hildebrand S: An
evaluation of the use of massage and essential oils on the
wellbeing of cancer patients. Int J Palliat Nurs. 1:67–73.
1995.
|
|
10.
|
Elenkov IJ and Chrousos GP: Stress system
– Organization, physiology and immunoregulation.
Neuroimmunomodulation. 13:257–267. 2006.
|
|
11.
|
Walker LG, Green VL, Greenman J, Walker A
and Sharp DM: PNI and Chronic Malignant Disease: Cancer. Human
Psychoneuroimmunology (PNI). Irwin M and Vedhara K: Oxford
University Press; UK: pp. 137–163. 2005
|
|
12.
|
Herbert TB and Cohen S: Stress and
immunity in humans: a meta-analytic review. Psychosom Med.
55:364–379. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Segerstrom SC and Miller GE: Psychological
stress and the immune system: a meta-analytic study of 30 years of
enquiry. Psychol Bull. 130:601–630. 2004.PubMed/NCBI
|
|
14.
|
Ghiringhelli F, Menard C, Puig PE, et al:
Metronomic cyclophosphamide regimen selectively depletes
CD4+CD25+ regulatory T cells and restores T
and NK effector functions in end stage cancer patients. Cancer
Immunol Immunother. 56:641–648. 2007.PubMed/NCBI
|
|
15.
|
Spiegel D and Giese-Davis J: Depression
and cancer: mechanisms and disease progression. Biol Psychiatry.
54:269–282. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Yang EV and Glaser R: Stress-induced
immunomodulation: implications for tumorigenesis. Brain Behav
Immun. 17(Suppl 1): 37–40. 2003. View Article : Google Scholar
|
|
17.
|
Penninx BW, Guralnik JM, Pahor M, Ferrucci
L, Cerhan JR, Wallace RB and Havlik RJ: Chronically depressed mood
and cancer risk in older persons. J Natl Cancer Inst. 90:1888–1893.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Ikeda H, Old LJ and Schreiber RD: The
roles of IFNγ in protection against tumour development and cancer
immunoediting. Cytokine Growth Factor Rev. 13:95–109. 2002.
|
|
19.
|
Nelson EL, Wenzel LB, Osann K, et al:
Stress, immunity and cervical cancer: biobehavioural outcomes of a
randomized clinical trial. Clin Cancer Res. 14:2111–2118. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Neuner A, Schindel M, Wildenberg U, Muley
T, Lahm H and Fischer JR: Prognostic significance of cytokine
modulation in non-small cell lung cancer. Int J Cancer.
101:2872–2892. 2002. View Article : Google Scholar
|
|
21.
|
O’Hara RJ, Greenman J, MacDonald AW, et
al: Advanced colorectal cancer is associated with impaired
interleukin 12 and enhanced interleukin 10 production. Clin Cancer
Res. 4:1943–1948. 1998.PubMed/NCBI
|
|
22.
|
Webster Marketon JI and Glaser R: Stress
hormones and immune function. Cell Immunol. 252:16–26.
2008.PubMed/NCBI
|
|
23.
|
Lightman SL: The neuroendocrinology of
stress: a never ending story. J Neuroendocrinol. 20:880–884. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Dohi K, Kraemer WJ and Mastro AM: Exercise
increases prolactin-receptor expression on human lymphocytes. J
Appl Physiol. 94:518–524. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Gotovac K, Sabioncello A, Rabatic S, Berki
T and Dekaris D: Flow cytometric determination of glucocorticoid
receptor (GCR) expression in lymphocyte subpopulations: lower
quantity of GCR in patients with post-traumatic stress disorder
(PTSD). Clin Exp Immunol. 131:335–339. 2003. View Article : Google Scholar
|
|
26.
|
Rapaport R, Sills IN, Green L, et al:
Detection of human growth hormone receptors on IM-9 cells and
peripheral blood mononuclear cell subsets by flow cytometry:
correlation with growth hormone-binding protein levels. J Clin
Endocrinol Metab. 80:2612–2619. 1995.
|
|
27.
|
Malarkey WB and Mills PJ: Endocrinology:
the active partner in PNI research. Brain Behav Immun. 21:161–168.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Besedovsky HO and Rey AD: Physiology of
psychoneuroimmunology: a personal view. Brain Behav Immun.
21:34–44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Leonard BE: The HPA and immune axes in
stress: the involvement of the serotonergic system. Eur Psychiatry.
20(Suppl 3): 302–306. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Ross K: Mapping pathways from stress to
cancer progression. J Natl Cancer Inst. 100:914–917. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Facchinetti F, Tarabusi M and Volpe A:
Cognitive-behavioral treatment decreases cardiovascular and
neuroendocrine reaction to stress in women waiting for assisted
reproduction. Psychoneuroendocrinology. 29:162–173. 2004.
View Article : Google Scholar
|
|
32.
|
Gaab J, Blattler N, Menzi T, Pabst B,
Stoyer S and Ehlert U: Randomized controlled evaluation of the
effects of cognitive-behavioral stress management on cortisol
responses to acute stress in healthy subjects.
Psychoneuroendocrinology. 28:767–779. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
McKinney CH, Antoni MH, Kumar M, Tims FC
and McCabe PM: Effects of guided imagery and music (GIM) therapy on
mood and cortisol in healthy adults. Health Psychol. 16:390–400.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Cruess DG, Antoni MH, McGregor BA, et al:
Cognitive-behavioral stress management reduces serum cortisol by
enhancing benefit finding among women being treated for early stage
breast cancer. Psychosom Med. 62:304–308. 2000. View Article : Google Scholar
|
|
35.
|
Turner-Cobb JM, Sephton SE, Koopman C,
Blake-Mortimer J and Spiegel D: Social support and salivary
cortisol in women with metastatic breast cancer. Psychosom Med.
62:337–345. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Van der Pompe G, Duivenvoorden HJ, Antoni
MH, Visser A and Heijnen CJ: Effectiveness of a short-term group
psychotherapy program on endocrine and immune function in breast
cancer patients: an exploratory study. J Psychosom Res. 42:453–466.
1997.PubMed/NCBI
|
|
37.
|
Heinrichs M, Baumgartner T, Kirschbaum C
and Ehlert U: Social support and oxytocin interact to suppress
cortisol and subjective responses to psychosocial stress. Biol
Psychiatry. 54:1389–1398. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Eremin O, Walker MB, Simpson E, et al:
Immuno-modulatory effects of relaxation training and guided imagery
in women with locally advanced breast carcinoma undergoing
multimodality treatment. Breast. 18:17–25. 2008. View Article : Google Scholar
|
|
39.
|
Andersen BL, Farrar WB, Golden-Kreutz DM,
et al: Psychological, behavioral and immune changes after a
psychological intervention: a clinical trial. J Clin Oncol.
22:3570–3580. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
McGregor BA, Antoni MH, Boyers A, Alferi
SM, Blomberg BB and Carver CS: Cognitive-behavioural stress
management increases benefit finding and immune function among
women with early stage breast cancer. J Psychosom Res. 56:1–8.
2004. View Article : Google Scholar
|
|
41.
|
Hernandez-Reif M, Ironson G, Field T, et
al: Breast cancer patients have improved immune and neuroendocrine
functions following massage therapy. J Psychosom Res. 57:45–52.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Walker LG, Walker MB, Ogston K, et al: The
psychological, clinical and pathological effects of relaxation
training and imagery during primary chemotherapy. Br J Cancer.
80:262–268. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Fawzy FI, Kemeny ME, Fawzy NW, Elashoff R,
Morton D, Cousins N and Fahey JL: A structured psychiatric
intervention for cancer patients. II Changes over time in
immunological measures. Arch Gen Psychiatry. 47:729–735. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Walker LG: Psychosocial Oncology in Hull
and the East Riding of Yorkshire. Newsletter of the British
Psychosocial Oncology Society. March;10–12. 2000.
|
|
45.
|
Anderson BL: Psychological interventions
for cancer patients to enhance quality of life. J Consult Clin
Psychol. 60:552–568. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Boyum A: Isolation of leucocytes from
human blood. Further observations. Methylcellulose, dextran and
ficoll as erythrocyte-aggregating agents. Scand J Clin Lab Invest.
97:31–50. 1968.
|
|
47.
|
Jung T, Schauer U, Heusser C, Neuman C and
Rieger C: Detection of intracellular cytokines by flow cytometry. J
Immunol Methods. 159:197–207. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Piriou L, Chilmonczyk S, Genetet N and
Albina E: Design of a flow cytometric assay for the determination
of natural killer and cytotoxic T-lymphocyte activity in human and
in different animal species. Cytometry. 41:289–297. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Carlens S, Gilljam M, Chambers BJ, et al:
A new method for in vitro expansion of cytotoxic human CD3–CD56+
natural killer cells. Hum Immunol. 62:1092–1098. 2001.
|
|
50.
|
Vickers AJ and Altman DG: Statistics
notes: analysing controlled trials with baseline and follow-up
measurements. BMJ. 323:1123–1124. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Carlson LE, Speca M, Patel KD and Goodey
E: Mindfulness-based stress reduction in relation to quality of
life, mood, symptoms of stress, and immune parameters in breast and
prostate cancer outpatients. Psychosom Med. 65:571–581. 2003.
View Article : Google Scholar
|
|
52.
|
Wilkinson SM, Love SB, Westcombe AM, et
al: Effectiveness of aromatherapy massage in the management of
anxiety and depression in patients with cancer: a multicentre
randomized controlled trial. J Clin Oncol. 25:532–539. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Kuss I, Hathaway B, Ferris RL, Gooding W
and Whiteside TL: Imbalance in absolute counts of T lymphocyte
subsets in patients with head and neck cancer and its relation to
disease. Adv Otorhinolaryngol. 62:161–172. 2005.PubMed/NCBI
|
|
54.
|
Hong WS, Min Y, Son YS and Hong S:
Peripheral blood lymphocyte subsets in patients with stomach
cancer. J Korean Med Sci. 10:164–168. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Heys SD, Gough DB and Eremin O:
Immunotherapy with interleukin-2, recent developments. Expert Opin
Investig Drugs. 5:269–288. 1996. View Article : Google Scholar
|
|
56.
|
Al Sarireh B and Eremin O: Tumour
associated macrophage (TAMS): disordered function, immune
suppression and progressive tumour growth. J R Coll Surg Edinb.
45:1–16. 2000.PubMed/NCBI
|
|
57.
|
Beyer M and Schultze JL: Regulatory T
cells in cancer. Blood. 108:804–811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Curiel TJ: Tregs and rethinking cancer
immunotherapy. J Clin Invest. 117:1167–1174. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Trzonkowski P, Szmit E, Myśliwska J and
Myśliwski A: CD4+CD25+ T regulatory cells
inhibit cytotoxic acitivity of CTL and NK cells in humans – impact
of immunosenescence. Clin Immunol. 119:307–316. 2006.
|
|
60.
|
Hernandez-Reif M and Field T: Natural
killer cells and lymphocytes increase in women with breast cancer
following massage therapy. Int J Neurosci. 115:495–510. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Bakke AC, Purtzer MZ and Newton P: The
effect of hypnotic-guided imagery on psychological well-being and
immune function in patients with prior breast cancer. J Psychosom
Res. 53:1131–1137. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Brittenden J, Heys SD, Ross J and Eremin
O: Natural killer cells and cancer. Cancer. 77:1226–1243. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
63.
|
Malmberg KJ, Bryceson YT, Carlsten M, et
al: NK cell-mediated targeting of human cancer and possibilities
for new means of immunotherapy. Cancer Immunol Immunother.
57:1541–1552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64.
|
Joshi AD, Clark EM, Wang P, et al:
Immunotherapy of human neuroblastoma using umbilical cord
blood-derived effector cells. J Neuroimmune Pharmacol. 2:202–212.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
65.
|
Godoy-Ramirez K, Franck K and Gaines H: A
novel method for the simultaneous assessment of natural killer cell
conjugate formation and cytotoxicity at the single-cell level by
multiparameter flow cytometry. J Immunol Methods. 239:35–44. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
66.
|
Glaser R, Maccallum RC, Laskowski BF,
Malarkey WB, Sheridan JF and Kiecolt-Glaser JK: Evidence for a
shift in the Th-1 to Th-2 cytokine response associated with chronic
stress and ageing. J Gerontol A Biol Sci Med Sci. 56:477–482. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
67.
|
Carlson LE, Speca M, Patel KD and Goodey
E: Mindfulness-based stress reduction in relation to quality of
life, mood, symptoms of stress and levels of cortisol,
dehydroepiandrosterone sulfate (DHEAS) and melatonin in breast and
prostate cancer outpatients. Psychoneuroendocrinology. 29:448–474.
2004. View Article : Google Scholar
|
|
68.
|
Walker AA, Walker MB, Greenman J, Sharp DM
and Walker LG: Does reflexology improve quality of life in women
who have undergone surgery for early breast carcinoma?
Psychooncology. 15:S1372006.
|