Introduction
By discriminating between self and non-self, our
immune system protects us from invading microbes, including
bacteria, viruses and parasites. The immune system has several
cellular components that can theoretically be recruited for
protection from tumors. It can detect early lesions through
immunosurveillance and eliminate them, and is thought to maintain
tumors in a state of equilibrium. Yet, tumor cells with either
reduced immunogenicity or with increased capacity to attenuate the
immune response can escape equilibrium and progress (1,2).
Functional antitumor immunity requires a response
initiated by potent antigen-presenting cells, such as dendritic
cells, and the engagement of a variety of effector cells, not only
cytotoxic T cells, but also T-helper cells and B cells, which
initiate antibody immunity (3).
CD4+ and CD8+ T cells are the adaptive
components of cell-mediated immunity. These cells express a
comprehensive repertoire of antigen-specific receptors (cell
surface immunoglobulin receptors for B cells and cell surface T
cell receptors for T cells) that can recognize over one million
distinct antigens (4). To treat
patients with advanced disease and larger tumor burdens, it might,
therefore, be necessary to achieve and maintain T cell levels that
are greater than the 1–2% that is currently attainable (5). Therefore, T cell number is a crucial
parameter in experimental and clinical models of adoptive cell
therapy.
Esophageal cancer is a highly aggressive malignancy
with a very poor prognosis. Surgery, radiation and chemotherapy are
the mainstay of esophageal squamous cell carcinoma (ESCC)
management (6). Different cancer
adjuvant therapies confer selective influence on immune recovery
(7). The combination of
chemotherapy, fluorouracil and cisplatin, and radiation has
improved the outcome for patients with esophageal cancer (8). Postoperative alterations in host
immune functions after major surgical interventions have been
extensively described and investigated for carcinoma of the
esophagus (9). Either
radiotherapy- or chemotherapy-induced apoptosis of tumor cells has
been shown to carry concurrent effects on the immune
microenvironment of the tumor (10). Cytotoxic chemotherapy exerts a
systemic effect and is used to cytoreduce established tumors and
eradicate micrometastatic disease. Chemotherapy can be used to
groom the local tumor microenvironment to optimally support a
productive immune response. Cytotoxic chemotherapeutic agents
might, in some cases, directly modulate the immune response.
Chemotherapy has been shown to potentially reduce the number of
T-regulatory cells in the environment, promote the expansion of
tumor-specific memory T cells and enhance the expression of
immune-recognition molecules on the surface of the tumor cell
(11). Antimetabolite drugs such
as 5-fluorouracil (5-FU), which is a fluorinated derivative of
pyrimidines, and cisplatin, a platinum-based DNA damaging
anticancer drug, are widely used in the treatment of ESCC patients.
Platinum compounds have immune-suppressive effects when tested at a
low dose in preclinical models, impairing T cell function, whereas
other chemotherapy agents might be devoid of such effects and can
even be harnessed to enhance an immune response (12). Preoperative combination
radiochemotherapy of patients with ESCC results in the suppression
of T lymphocyte functions. The proliferative defects of T cells
after radiochemotherapy may be linked to an impaired immune
surveillance of cancer and to a higher risk of surgical
complications associated with esophagectomy (13). Research found no significant
changes in the absolute numbers of T cells and monocytes or in
their phenotyping, but a decreasing ratio of CD4+ to
CD8+ T cells with advancement of tumor stage in
adenocarcinoma of the esophagus was observed (14). A previous study suggested that
cellular immunity, especially cytotoxicity, shortly after
esophagectomy may be greatly impaired by the surgical stress of
esophagectomy and the added effect of chemotherapy (15), and cooperation of CD4+
and CD8+ T cells appears to drastically improve the
prognosis of patients with ESCC (16).
Much has been learned about the potential of the
immune system to control cancer, and adjuvant therapy is known to
alter immune responses significantly (17). Yet, little has been investigated
about how the type of cancer therapy and interval after sugery
interact with altered immune responses in ESCC patients after
surgery. The interaction among the tumor, the stroma and the immune
system may play a critical role in patient outcome. Flow cytometric
evaluation of the proportions and absolute numbers of lymphocytes
and their subsets in the peripheral circulation of cancer patients
is important and might provide insights into the redistribution of
those lymphocyte subsets that may mediate the antitumor defense
(18). Nevertheless, different
treatments might have diverse impacts on the immune system, and
improving the understanding of circulating lymphocytes could
benefit the choice of intervention (19). This study was conducted to clarify
the cellular immune response to postoperative adjuvant therapy and
interval in patients with ESCC. Cellular immunity was assessed as
the counts of leukocytes, lymphocytes and subsets of circulating
lymphocytes immediately in response to adjuvant therapy by flow
cytometry before adjuvant treatment at 1–3 days of the next cycle
for ESCC patient.
Materials and methods
Patients
Between January 2000 and January 2009, 73
consecutive patients with histologically proven ESCC who were
scheduled for surgical resection with curative intent consented to
participate in the present study. The patients were all treated in
the same surgery and oncology unit of ChangZhou Tumor Hospital,
according to the National Treatment Guidelines which was used to
categorize the patients in the different treatment groups in
relation to tumor size, lymph node involvement and relevant
clinical data. All patients had locally resectable disease without
distant metastases on preoperative investigations, and none had
received neoadjuvant chemotherapy or irradiation. Routine
preoperative evaluation included upper gastrointestinal endoscopy
with biopsy, endoscopic ultrasonography and external
ultrasonography of the neck and upper abdomen, indirect
laryngoscopy and chest radiography. All specimens of surgical
resection were evaluated by an experienced gastrointestinal
pathologist, in accordance with the criteria of the International
Union Against Cancer, including stage, classification and grade
(20). After discharge, patients
were seen regularly in the outpatient clinic (every 3 months for 2
years and every 6 months thereafter). Additional diagnostic
procedures were performed only when indicated. Approximately 60–70
Gy total radiation, fractioned in 1.8–2.0 Gy doses (6 MeV photons)
was delivered to the ESCC patients after surgery. Patients with
lymph node involvement had additional radiation delivered to the
adjacent lymph nodes. Thirteen patients received chemotherapy (CT)
alone (CT group), 43 patients received radiotherapy (RT) alone (RT
group) and 17 received a combination of radiation and chemotherapy
(RT+CT group) with 5-fluorouracil and cisplain. None of the
patients received other adjuvant immunotherapy.
Patients were treated in accordance with the
Helsinki Declaration on the participation of human subjects in
medical research. The study was approved by the institutional
medical ethics committee, and all study participants gave written
informed consent.
Blood sampling
On admission to the hospital (1–3 days prior to
adjuvant therapy), peripheral venous blood samples were obtained in
ethylenediamine tetra-acetic acid (EDTA) tubes for white blood
cells (WBCs), differential counts and flow cytometric analysis.
Adjuvant therapy was carried out until haematological recovery,
defined as total WBCs >500/μl and platelets >20,000/μl. To
minimize diurnal influences, all blood samples were drawn between 8
and 9 a.m. There were 139 blood samples in total obtained at
different time points relative to surgery from 73 patients with
ESCC.
Haematological analysis
The Beckman Coulter ACT DiV Analyser (Global Medical
Instrumentation, Inc., Ramsey, MN, USA) was used for determining
the absolute number of WBCs and lymphocytes in peripheral venous
blood samples, collected in EDTA tubes. 4C Plus Cell Control
(Beckman Coulter, Miami, FL, USA) was used to monitor the
performance of the Coulter machine. To determine the absolute
number for each cell subset reported here as specific cells/ml of
blood, the percentage fraction among lymphocytes was multiplied by
the number of lymphocytes/l as determined by the lymphocyte count
on the same sample.
Flow cytometric analysis
Analysis of the peripheral blood lymphocyte
phenotypes was performed on an FACS Calibur flow cytometer
(FACScan; Becton Dickinson, San Jose, CA, USA) using the Multiset
software package according to the instructions of the manufacturer.
A two-color direct immunofluorescence flow cytometric assay was
carried out using a Simultest IMK-Lymphocyte kit (Becton-Dickinson)
for enumeration of mature human T (CD3+), helper/inducer
T (CD3+CD4+), suppressor/cytotoxic T
(CD3+CD8+) and natural killer (NK) cells and
(CD3−CD16+ and/or CD56+)
lymphocytes. Samples were stained and analyzed within 6 h of
collection following the manufacturer’s instructions. Fresh cells
were used whenever possible, failing which cryopreserved cells,
ficolled to remove dead cells and debris, were used. For each
antibody panel, 5 μl was added to the blood cell samples. The
samples were incubated in ice for 30 min in the dark. The red blood
cells were lysed with ammonium chloride (10%). After two washing
steps, the cell pellets were resuspended in 130 μl PBS with 10%
fetal calf serum. A minimum of 2,000 and 5,000 lymphocytes were
measured for T cell and for NK, respectively. An isotype-negative
control optimized the settings of the fluorescence detectors for
each subject. For analyses, a gate was set for lymphocytes using
forward vs. the side light scattering property of the cells.
CD4+ and CD8+ cells were gated on side
scatter and PerCP fluorescence in order to obtain a minimum of
1,500 CD4+ or CD8+ T lymphocytes from the
lymphocyte gate. The analysis gate for CD4 or CD8 subsets was set
on CD4+ or CD8+ bright events to avoid
contaminating CD3−CD4+ monocytes and
CD3−CD8+ NK cells. The FACScan was calibrated
with CaliBrite fluorescent beads and FACScomp software weekly.
Statistical analysis
Data were entered and analyzed by Excel database and
SPSS statistical software (ver. 17.0). Median and reference ranges
(percentile 25.0–75.0) were calculated for each marker. Comparisons
between groups were made using the Mann-Whitney U test (two group
differences) or the Kruskal-Wallis test (three or more group
differences) for continuous variables. The cell subset percentage
or counts between the types of adjuvant therapy and intervals after
surgery was compared using univariate analysis. Differences were
considered significant when the P-value was <0.05.
Results
Patient characteristics
The characteristics of all the patients included in
this study are shown in Table I.
The cohort of 73 patients included 51 men and 22 women with a
median age of 61 years (range, 38–86 years) at the time of
diagnosis. Height ranged from 145 to 178 cm, with a median height
of 165 cm. Weight ranged from 35 to 85 kg, with a median weight of
55 kg. Histologically, 15 (20.6%) tumors were well-differentiated,
45 (61.6%) were moderately differentiated and 13 (17.8%) were
poorly differentiated. Thirty-eight cases were in stage II, 20 were
in stage III and 15 were in stage IV.
 | Table I.Clinicopathological parameters in
patients with esophageal squamous cell carcinoma. |
Table I.
Clinicopathological parameters in
patients with esophageal squamous cell carcinoma.
| Variablea | Total | CT (n=13) | RT (n=43) | CT+RT (n=17) |
|---|
| Gender | | | | |
| Male | 51 | 10 | 28 | 13 |
| Female | 22 | 3 | 15 | 4 |
| Location | | | | |
| Upper
esophagus | 10 | 0 | 6 | 4 |
| Middle
esophagus | 49 | 9 | 31 | 9 |
| Lower
esophagus | 14 | 4 | 6 | 4 |
|
Differentiationb | | | | |
| Poorly | 13 | 3 | 6 | 4 |
| Moderately | 45 | 6 | 30 | 9 |
| Well | 15 | 4 | 7 | 4 |
| Nodal
metastasis | | | | |
| Absent | 38 | 5 | 25 | 8 |
| Present | 35 | 8 | 18 | 9 |
| Smoking | | | | |
| Yes | 37 | 6 | 23 | 8 |
| No | 36 | 7 | 20 | 9 |
| Alcohol | | | | |
| Yes | 51 | 8 | 31 | 12 |
| No | 22 | 5 | 12 | 5 |
| T stage | | | | |
| II | 38 | 3 | 28 | 7 |
| III | 20 | 3 | 10 | 7 |
| IV | 15 | 7 | 5 | 3 |
| Median height
(range), in cm | | 165 (158–174) | 165 (145–175) | 168 (154–178) |
| Median weight
(range), in kg | | 54 (44–62) | 56 (35–85) | 56 (47–73) |
| Median age (range),
in years | | 56 (47–63) | 67 (46–86) | 59 (38–79) |
In 14 patients (19.2%), cancer was localized within
the lower third of the esophagus; 49 cases (67.1%) were localized
within the middle of the esophagus; 10 (13.7%) cases were localized
within the upper esophagus. Lymph node metastasis, as determined by
histological examination, was noted in 38 (52.1%) cases. All
patients received continuous postoperative adjuvant therapy (CT
alone, RT alone, both CT+RT) in accordance with our management
protocol. In the CT group (n=13, 17.8%), the median patient age was
56 years (range, 44–62 years) and 10 patients were male. In the RT
group (n=43, 58.9%), the median patient age was 67 years (range,
46–86 years) and 28 patients were male. In the CT+RT group (n=17,
23.3%), the median patient age was 59 years (range, 38–79 years)
and 13 patients were male. The median interval between the last
dose of adjuvant therapy and surgical resection was 6 months
(range, 0–53 months).
Analysis of the percentages of each
lymphocyte subset in ESCC patients with postoperative adjuvant
therapy
Using the single-platform method, we initially
compared the percentages of each lymphocyte subset in ESCC patients
with postoperative adjuvant therapy. Table II shows the distribution of
lymphocyte subsets in the peripheral blood of ESCC patients with
postoperative chemotherapy and/or radiotherapy. We found that the
percentages of T lymphocytes were associated with the types of
postoperative adjuvant therapy. There were significant differences
between the percentages of each lymphocyte subset (CD3+,
CD4+, CD8+ and NK) and the types of adjuvant
therapy (P=0.001, P=0.01, P=0.006 and P=0.037, respectively). The
percentages of CD3+ and CD8+ were relatively
lower in the RT group than those in the CT and CT+RT groups. The
percentage of NK cells and the CD4/CD8 ratio were relatively higher
in the RT group than the percentage of NK cells and the CD4/CD8
ratio in the CT and CT+RT groups. But the percentage of
CD4+ was relatively higher in the CT group than the
percentage in the RT and CT+RT groups. Comparing the CT and RT
groups, differences in the percentages of CD3+,
CD4+, CD8+ and NK cells were observed.
Comparing the CT and CT+RT groups, differences in the percentage of
CD4+ and the CD4/CD8 ratio were also observed.
Meanwhile, there were significant differences in the percentages of
CD3+, CD8+ and the CD4/CD8 ratio between the
RT and CT+RT groups.
 | Table II.The percentage of lymphocyte subsets
in different treatment groups after surgery in ESCC. |
Table II.
The percentage of lymphocyte subsets
in different treatment groups after surgery in ESCC.
| Patients
(n=139) | CT (n=41) | RT (n=66) | CT+RT (n=32) | P-valuea |
|---|
| CD3+
(%) | 57.8
(46.9–69.6) | 66.3b (53.4–82.0) | 51.7c (44.6–62.2) | 62.1
(50.1–69.5) | 0.001 |
| CD4+
(%) | 27.7
(19.8–37.2) | 32.0b,d (23.8–44.4) | 27.7
(19.2–33.9) | 23.9
(17.6–32.7) | 0.010 |
| CD8+
(%) | 24.7
(18.6–33.4) | 27.3b (21.5–32.5) | 21.5c (16.0–30.9) | 30.2
(22.3–40.7) | 0.006 |
| CD4/CD8
(ratio) | 1.1 (0.7–1.7) | 1.1d (0.8–2.0) | 1.3c (0.8–1.9) | 0.8 (0.5–1.3) | 0.022 |
| NK (%) | 18.5
(11.1–27.4) | 16.4b (8.0–20.8) | 20.2
(12.4–32.3) | 19.9
(10.3–30.1) | 0.037 |
Analysis of the absolute counts of each
lymphocyte subset and WBCs in the ESCC patients with postoperative
adjuvant therapy
To examine the correlation between cell immune
reactions and postoperative adjuvant therapy, we compared the
absolute counts of each lymphocyte subset in different adjuvant
therapy groups (Table III). We
found no significant differences in the absolute counts of NK and
lymphocyte blood cells (LBCs) among the adjuvant therapy groups
(P=0.291 and P=0.130, respectively). Significant differences were
noted in the absolute counts of CD3+, CD4+,
CD8+ and WBCs among the adjuvant therapy groups
(P=0.001, P=0.012, P=0.003 and P=0.006, respectively).
Surprisingly, we found that the absolute count of WBCs was
increased in the RT group as compared to that in the CT and CT+RT
groups, while the absolute counts of CD3+,
CD4+ and CD8+ were relatively lower. This
implies that radiotherapy has an important impaired response on the
absolute counts of the lymphocyte subsets.
 | Table III.The lymphocyte subsets and WBC counts
in different treatment groups after surgery in ESCC |
Table III.
The lymphocyte subsets and WBC counts
in different treatment groups after surgery in ESCC
| Total (n=139) | CT (n=41) | RT (n=66) | CT+RT (n=32) | P-valuea |
|---|
| CD3+
(cells/μl) | 760.6
(505.2–1063.4) | 932.8b (699.6–1203.9) | 661.0c (440.8–900.3) | 828.1
(526.7–1304.6) | 0.001 |
| CD4+
(cells/μl) | 355.6
(242.5–537.0) | 501.7b (287.3–715.4) | 328.0
(252.0–426.4) | 387.7
(151.0–581.0) | 0.012 |
| CD8+
(cells/μl) | 329.3
(213.9–511.7) | 352.0b (269.4–504.0) | 273.6c (165.7–438.4) | 377.8
(293.3–684.7) | 0.003 |
| NK (cells/μl) | 218.9
(116.6–365.6) | 184.5
(108.1–304.8) | 234.1
(120.3–394.2) | 227.6
(120.9–535.8) | 0.291 |
| WBCs
(×109/l) | 5.6 (4.2–8.0) | 4.3b,d (3.7–6.7) | 6.1 (4.7–8.0) | 5.3 (4.4–8.6) | 0.006 |
| LBCs
(×109/l) | 1.4 (0.9–1.7) | 1.5b (1.2–1.8) | 1.2 (0.9–1.6) | 1.4 (0.8–2.1) | 0.130 |
Analysis of the lymphocyte subsets and
WBCs in ESCC patients during the interval after surgery
To examine the correlation between cell immune
reactions during the interval following postoperative adjuvant
therapy, we compared the percentages and absolute counts of each
lymphocyte subset and WBCs in the different interval groups
(Tables IV and V). The time duration between surgery and
blood collection for this study was variable, ranging from 0 to 53
months, classified by five groups (I, ≤3 months; II, 4–6 months;
III, 7–12 months; IV, 13–24 months; V, >24 months). We found
that the percentages of CD3+, CD4+ and
CD8+ were significant different among the interval
groups (P=0.013, P=0.042 and P=0.040, respectively). However, there
were no significant differences in the percentages and absolute
counts of NK cells among the interval groups (P=0.492 and P=0.360,
respectively). Notably, that the CD4/CD8 ratio exhibited a
decreasing trend with prolonged interval (P=0.010). We found
significant differences in the absolute counts of CD4+,
CD8+ and WBCs among the interval groups (P=0.015,
P=0.030 and P=0.005 respectively). The absolute counts of all the
lymphocyte subsets (CD3+, CD4+,
CD8+ and NK) and LBCs, except for WBCs, were relatively
lower in the >24 month group than the counts in the other
groups. This implies that the absolute counts of the lymphocyte
subsets decreased with interval during postoperative adjuvant
therapy.
 | Table IV.The percentage of lymphocyte subsets
during various periods after surgery in ESCC patients. |
Table IV.
The percentage of lymphocyte subsets
during various periods after surgery in ESCC patients.
| I (n=52)
≤3 months | II (n=23)
4–6 months | III
(n=24)
7–12 months | IV (n=18)
13–24 months | V (n=22)
>24 months | P-valuea |
|---|
| CD3+
(%) | 56.3b (44.0–68.8) | 63.5e (55.0–73.2) | 62.2f (52.2–78.9) | 60.1g (51.5–67.9) | 49.7
(38.7–59.9) | 0.013 |
| CD4+
(%) | 29.2d (23.6–36.9) | 29.5e (22.0–42.3) | 27.5
(19.3–42.3) | 24.0
(20.7–31.0) | 19.8
(14.8–37.2) | 0.042 |
| CD8+
(%) | 20.1b,c (14.6–30.4) | 24.8
(20.0–39.1) | 28.2
(23.3–34.7) | 31.6
(24.3–38.8) | 26.1
(17.8–30.9) | 0.040 |
| CD4/CD8
(ratio) | 1.4b,c,d (0.9–2.1) | 1.3 (0.7–1.9) | 1.0 (0.6–1.6) | 0.9 (0.5–1.0) | 0.9 (0.7–1.2) | 0.010 |
| NK (%) | 18.5
(10.2–26.5) | 14.3
(8.8–22.5) | 16.9
(8.4–28.4) | 21.9
(14.2–30.4) | 19.9
(14.0–25.2) | 0.492 |
 | Table V.The total WBC and lymphocyte subset
counts during various times after surgery in ESCC. |
Table V.
The total WBC and lymphocyte subset
counts during various times after surgery in ESCC.
| I (n=52)
≤ 3 months | II (n=23)
4–6 months | III
(n=24)
7–12 months | IV (n=18)
13–24 months | V (n=22)
>24 months | P-valuea |
|---|
| CD3+
(cells/μl) | 747.0e (495.0–1048.7) | 793.2g (546.3–998.6) | 912.9h (557.1–1209.0) | 1039.4i (554.0–1357.0) | 543.6
(301.3–804.4) | 0.070 |
| CD4+
(cells/μl) | 430.4e (286.5–509.3) | 351.7g (281.3–644.3) | 406.3h (194.2–721.0) | 347.3i (230.5–633.8) | 246.3
(160.2–335.1) | 0.015 |
| CD8+
(cells/μl) | 261.2c,d (172.4–465.9) | 336.3
(270.0–443.7) | 376.1h (316.7–524.9) | 493.8i (297.8–757.9) | 254.3
(137.1–426.5) | 0.030 |
| NK (cells/μl) | 225.2
(106.4–391.3) | 214.8
(114.8–301.5) | 228.8
(82.1–462.7) | 323.3i (198.8–515.9) | 197.8
(111.8–317.4) | 0.360 |
| WBCs
(×109/l) | 5.4b,e (4.2–7.1) | 4.2f,g (3.2–6.0) | 5.5h (4.2–8.2) | 6.3 (4.7–9.6) | 7.3 (5.6–10.7) | 0.005 |
| LBCs
(×109/l) | 1.4e (1.0–1.7) | 1.3 (0.9–1.8) | 1.5h (0.9–1.9) | 1.6i (1.0–2.1) | 0.9 (0.8–1.5) | 0.070 |
Combined influence of adjuvant therapy
and interval after surgery on lymphocyte subsets and WBCs in ESCC
patients
We examined whether the percentages and counts of T
lymphocyte subsets changed during the postoperative interval when
following adjuvant chemotherapy and/or radiotherapy. For this
reason, a more detailed analysis of the relationship between cell
subsets and interval after surgery in different adjuvant therapy
groups was undertaken (Figs. 1 and
2). Univariate analysis revealed
that the interaction between the type of adjuvant therapy and
interval correlated only with the percentage of CD4+
(Fig. 1B, P<0.001). Meanwhile,
we found that interval was one of the factors contributing to the
percentages of CD3+, CD8+ and the CD4/CD8
ratio (P=0.018, P=0.017 and P=0.011, respectively), while the type
of adjuvant therapy was one of the factors contributing to the
percentages of CD3+ and NK cells (P=0.042 and P=0.027).
The absolute counts of NK cells and LBCs were unrelated to interval
in both adjuvant groups. Nevertheless, the interval was one of the
factors contributing to the absolute counts of CD8+ and
WBCs (P=0.017 and P=0.027); the type of adjuvant therapy was one of
the factors contributing to the absolute counts of CD3+
and CD4+ (P=0.006 and P=0.006). Overall, it should be
noted that the combination of the interval and adjuvant therapy was
the main factor contributing to the percentage of CD4+
in postoperative ESCC patients. Notably, there was a similar trend
for the percentage of CD4+ during 13–24 months after
surgery in ESCC patients. During the postoperative adjuvant
therapy, an interval (month)-dependent decrease in the percentage
of CD4+ T cells was found in the CT and CT+RT groups
throughout the five-year interval after surgery (Fig. 1F, R=−0.525 and R=−0.387), except
for the RT group. The percentage of CD4+ in the RT group
was negatively correlated with the follow-up interval (months)
after surgery. This indicates a long-term effect on CD4+
T cells during RT after surgery in ESCC patients; a minimum of at
least more than two years. The percentage of CD4+ T
cells appeared to be steadily regulated during this interval in the
RT group.
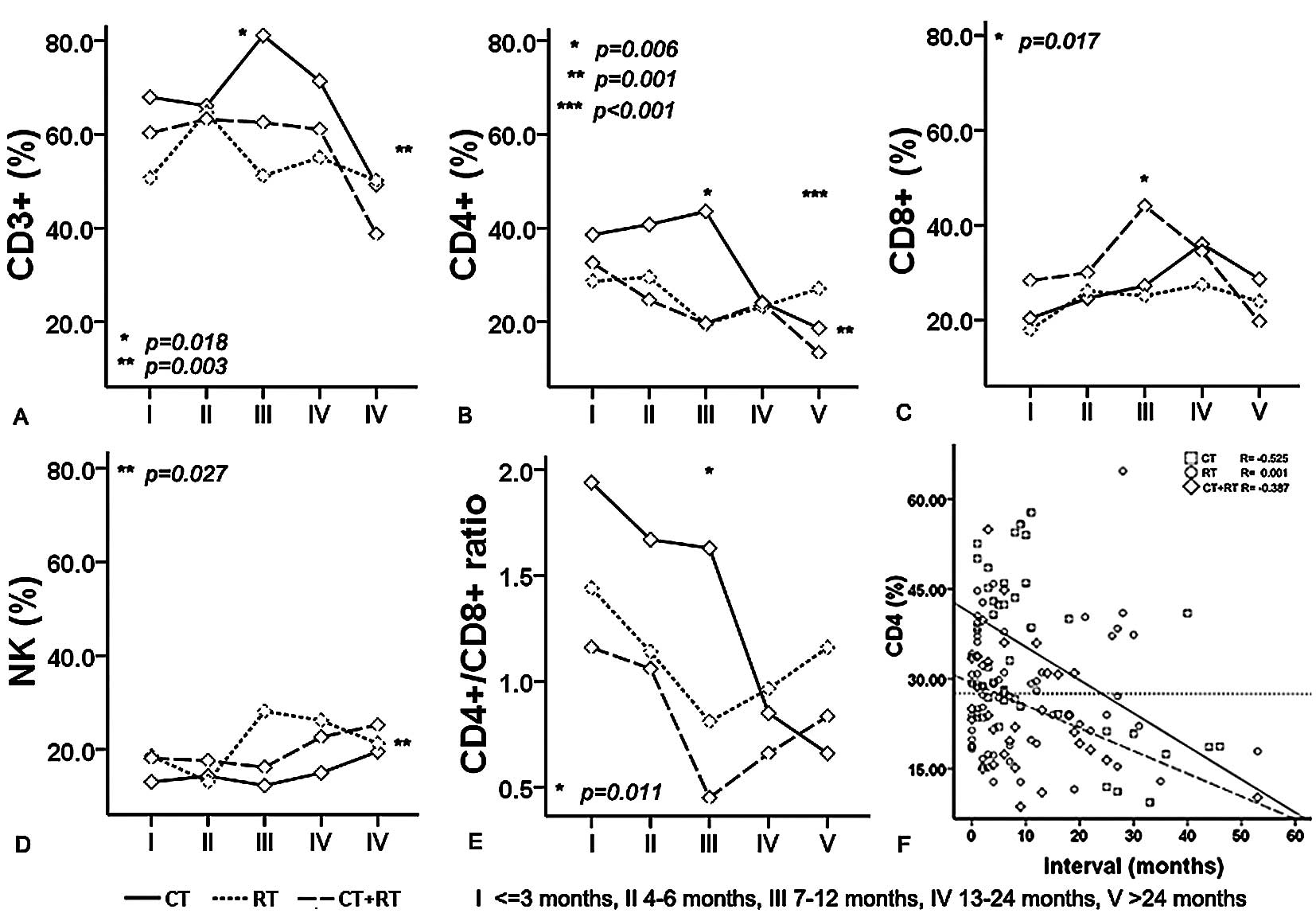 | Figure 1.Combined influence of adjuvant
therapy and interval after sugery on the percentages of
CD3+ (A), CD4+ (B), CD8+ (C) and
NK (D) lymphocyte subsets and the CD4/CD8 ratio (E) in the
peripheral circulation of ESCC patients. Univariate analysis
revealed that the interaction of the type of adjuvant therapy and
interval was correlated only with the percentage of CD4+
(P<0.001). The time duration between surgery and a blood
collection for this study was variable, ranging from 0 to 53
months, classified by five groups (I, ≤3 months; II, 4–6 months;
III, 7–12 months; IV, 13–24 months; V, >24 months). The
percentage of CD4+ T cells in an individual sample at
different time points relative to surgery is shown (F). As
indicated by the regression lines, the percentage of
CD4+ T cells decreased with interval in the CT and CT+RT
groups throughout the five years after surgery (R=−0.525 and
−0.387, respectively). However, the percentage of CD4+
in the RT group was negatively correlated with interval after
surgery (R=0.001). CT, chemotherapy; RT, radiotherapy; NK, natural
killer cells; *Significant between interval groups on
the percentages of CD3+, CD4+ and
CD8+ T cells and CD4/CD8 ratio; **Significant
between adjuvant therapy groups on the percentages of
CD3+, CD4+ and NK cells;
***Interaction of adjuvant therapy and interval on the
percentages of CD4+ T cells. P, by univariate
analysis. |
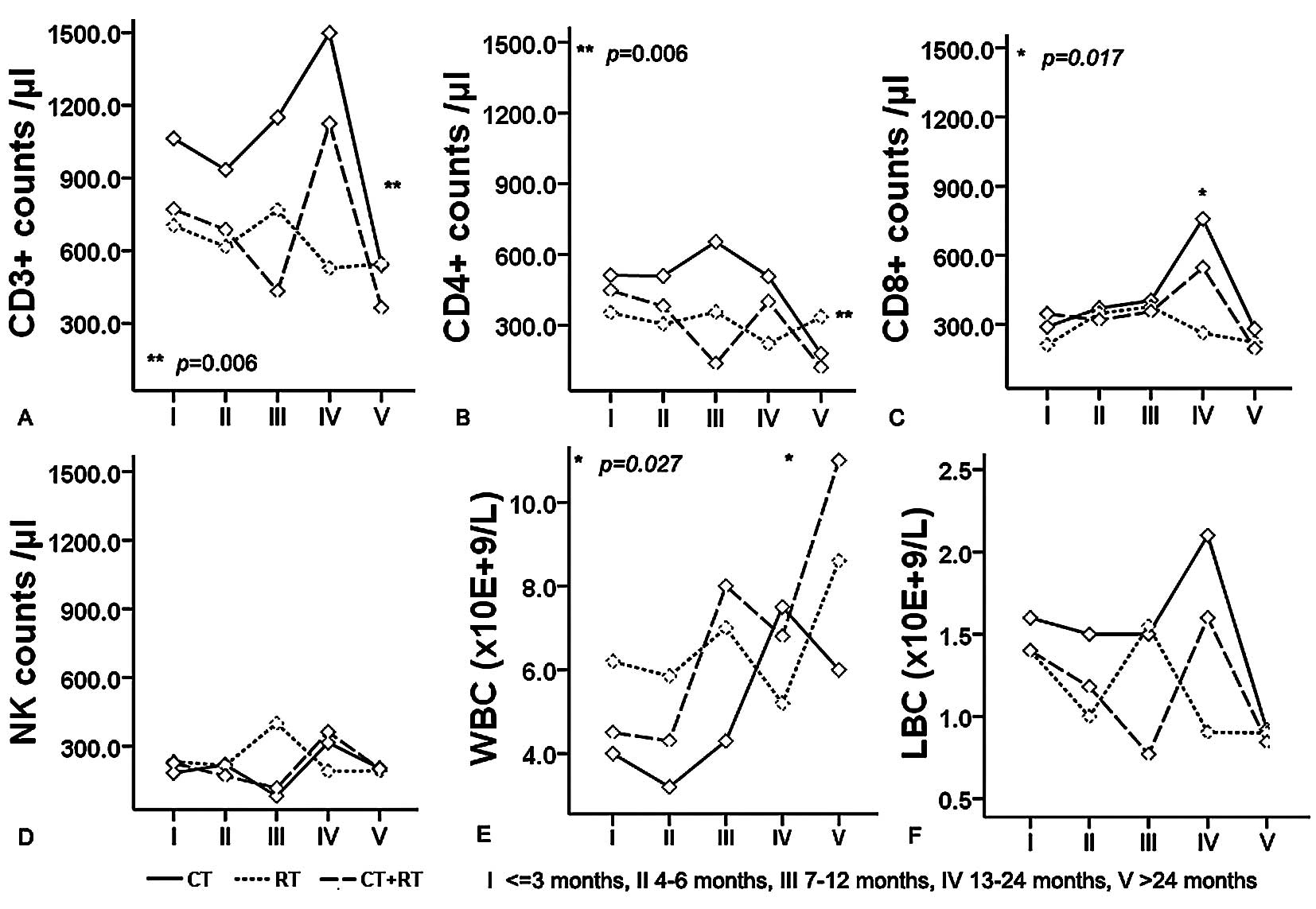 | Figure 2.Combined influence of adjuvant
therapy and interval after sugery on the absolute counts of
CD3+ (A), CD4+ (B), CD8+ (C) and
NK (D) lymphocyte subsets and WBCs (E), LBCs (F) in the peripheral
circulation of ESCC patients. *Significant between
interval groups on the absolute counts of CD8+ and WBCs;
*Significant between adjuvant therapy groups on the absolute counts
of CD3+ and CD4+. I, ≤3 months; II, 4–6
months; III, 7–12 months; IV, 13–24 months; V, 24 months. CT,
chemotherapy; RT, radiotherapy; NK, natural killer cells; LBC,
lymphocyte blood cells; WBC, white blood cells. P, by univariate
analysis. |
Discussion
The loss of lymphocytes contributing to
immunosuppression by decreasing the number of available immune
cells is a well-known phenomenon. Neoadjuvant therapy for
esophageal cancer causes significant disturbances in host cellular
immunity with reduced T, NK and B cell counts (9). Although the role of the immune
response in controlling tumor growth and cancer recurrence is
controversial, there is evidence in esophageal cancer suggesting
that T cells have a beneficial prognostic impact (14).
We assessed the cellular immune response of 73
patients with ESCC who were treated with postoperative adjuvant
therapy. The implications of the changes in the absolute and
relative proportions of lymphocyte phenotypes may be significant.
Patients undergoing dose-intensive chemotherapy and/or radiotherapy
may have both qualitative and quantitative deficiencies in cellular
immunity. In our study, we found that the percentages of each T
lymphocyte subset (CD3+, CD4+,
CD8+ and NK) were associated with the types of
postoperative adjuvant therapy. Significant differences were noted
in the absolute counts of CD3+, CD4+,
CD8+ and WBCs among adjuvant therapy groups. In
addition, the absolute count of WBCs was increased in the RT group
when compared with that in the CT and CT+RT groups, while the
absolute counts of CD3+, CD4+ and
CD8+ were relatively lower. This implies that RT alone
has a profound effect on the absolute counts of CD3+,
CD4+ and CD8+, compared with the CT and CT+RT
groups.
Most patients with solid malignancies are exempt
from diseases primarily affecting the lymphoid organs possibly
interfering with immune reconstitution. Therefore, both short- and
long-term alterations of the lymphocyte subsets may have relevant
clinical impact on the immune competence of patients with adjuvant
therapy (21). As most peripheral
lymphocyte subsets shared a significant difference in the rate of
recovery, we found that the percentages of CD3+,
CD4+ and CD8+ were significantly different
among the interval groups. Notably, the CD4/CD8 ratio exhibited a
decreasing trend as the interval prolonged. Decreased CD4/CD8
ratios have been found to be associated with a high tumor load,
which was correlated with poor survival (14). We found significant differences in
the absolute counts of CD4+, CD8+ and WBCs
among the interval groups. All of the absolute counts of each
lymphocyte subset (CD3+, CD4+,
CD8+ and NK) and LBCs, except for WBCs, were relatively
lower in the >24 month group than those of the other groups.
These findings provide an overall image of lymphocyte subset
homeostasis in ESCC patients with adjuvant therapy and suggest a
new scheme for the interpretation of immunodeficiency derived from
chemo- and/or radiotherapy after surgery. In general, a significant
time effect for ESCC patients following adjuvant therapy is
reflected.
While most of the focus in cancer immunology is on
CD8+ cytotoxic T lymphocyte responses, recent evidence
indicates that CD4+ T lymphocyte cells play an important
role in the modulation of immune responses by enhancement and
suppression of CD8+ CTL responses (22,23).
Successful immunity to cancer will therefore require activation of
tumor-specific CD4+ T cells (24,25).
In our study, we found that the impaired immunity was profound;
particularly CD4+ T helper cells exhibited a decreasing
trend during a long interval, leading to a prolonged T-cell
imbalance after surgery. Univariate analysis revealed that the
interaction of the type of adjuvant therapy and interval was
correlated only with the percentage of CD4+ T cells.
This relationship appeared to result mainly from an impaired
response in the percentage of CD4+ T cells with a long
interval after surgery in the CT or CT+RT patients, rather a steady
regulation throughout interval in the RT alone patients. Variations
in circulating CD4+ cell subsets may account for the
alterations in CD4+ activity of peripheral blood
lymphocytes. CD4+ recovery was delayed for more than one
year following dose-intensive chemotherapy in an adult population
(26). An unfavorable prognosis
can be expected when CD4+ T helper cell functions
progressively fail to mediate protective antitumor immunity
(14). Therefore, the percentages
of CD4+ can be used as an indicator of patient cellular
immunity after surgery in ESCC.
NK cells are effector lymphocytes of the innate
immune system that control several types of tumors and microbial
infections by limiting their spread and subsequent tissue damage.
NK cells are components of the innate immune system capable of
lysing target cells without prior sensitization (27). We found no significant differences
in the absolute counts of the NK subset among adjuvant therapy
groups, and no significant difference in the percentages and
absolute counts of NK cells among interval groups. Meanwhile, the
interaction of adjuvant therapy and interval displayed no
correlation with the percentages and absolute counts of NK.
However, contrary to previous reports (28), the number of NK cells did not
impair a response in our study. This implies that NK cells
tranquilize and participate in antitumor immunity in patients with
ESCC.
In conclusion, it may be important to analyze
significant disturbances of host cellular immunity induced by
postoperative adjuvant therapy in esophageal cancer patients. This
adjuvant therapy caused impaired T and NK cells, reduced
CD4+ T cells, leading to prolonged T-cell imbalance
after surgery. These findings suggest that an alteration in the
immune function in patients with adjuvant therapy may account for
these differences. An adjuvant therapy-induced lymphocyte imbalance
may play a significant role in ESCC after surgery. The main
limitation of this study is the absence of sequential data for
detailed analysis of trends in such a long interval considered
after surgery. Nevertheless, improving the understanding of the
relations between treatment, immune system and course of cancer may
be relevant in order to develop more effective treatment options
for patients with ESCC.
Abbreviations:
|
ESCC
|
esophageal squamous cell
carcinoma;
|
|
CT
|
chemotherapy;
|
|
RT
|
radiotherapy;
|
|
NK
|
natural killer cells;
|
|
LBC
|
lymphocyte blood cell;
|
|
WBC
|
white blood cell
|
Acknowledgements
Financial support was provided by the
Science and Technology Planning Project of Changzhou Municipality,
P.R. China (grant no. CS2008214).
References
|
1.
|
Koebel CM, Vermi W, Swann JB, et al:
Adaptive immunity maintains occult cancer in an equilibrium state.
Nature. 450:903–907. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Kim R, Emi M and Tanabe K: Cancer
immunoediting from immune surveillance to immune escape.
Immunology. 121:1–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Disis ML and Lyerly HK: Global role of the
immune system in identifying cancer initiation and limiting disease
progression. J Clin Oncol. 23:8923–8925. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Knutson KL and Disis ML: Tumor
antigen-specific T helper cells in cancer immunity and
immunotherapy. Cancer Immunol Immunother. 54:721–728. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ohlen C, Kalos M, Hong DJ, Shur AC and
Greenberg PD: Expression of a tolerizing tumor antigen in
peripheral tissue does not preclude recovery of high-affinity CD8+
T cells or CTL immunotherapy of tumors expressing the antigen. J
Immunol. 166:2863–2870. 2001.PubMed/NCBI
|
|
6.
|
Lin DC, Du XL and Wang MR: Protein
alterations in ESCC and clinical implications: a review. Dis
Esophagus. 22:9–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kang DH, Weaver MT, Park NJ, Smith B,
McArdle T and Carpenter J: Significant impairment in immune
recovery after cancer treatment. Nurs Res. 58:105–114. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kleinberg L and Forastiere AA:
Chemoradiation in the management of esophageal cancer. J Clin
Oncol. 25:4110–4117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Westerterp M, Boermeester MA, Omloo JM, et
al: Differential responses of cellular immunity in patients
undergoing neoadjuvant therapy followed by surgery for carcinoma of
the oesophagus. Cancer Immunol Immunother. 57:1837–1847. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Formenti SC and Demaria S: Effects of
chemoradiation on tumor-host interactions: the immunologic side. J
Clin Oncol. 26:1562–1563. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Emens LA and Jaffee EM: Leveraging the
activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res.
65:8059–8064. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Heidecke CD, Weighardt H, Feith M, et al:
Neoadjuvant treatment of esophageal cancer: immunosuppression
following combined radiochemotherapy. Surgery. 132:495–501. 2002.
View Article : Google Scholar
|
|
14.
|
Van Sandick JW, Boermeester MA, Gisbertz
SS, et al: Lymphocyte subsets and T(h)1/T(h)2 immune responses in
patients with adenocarcinoma of the oesophagus or oesophagogastric
junction: relation to pTNM stage and clinical outcome. Cancer
Immunol Immunother. 52:617–624. 2003.PubMed/NCBI
|
|
15.
|
Mafune K and Tanaka Y: Influence of
multimodality therapy on the cellular immunity of patients with
esophageal cancer. Ann Surg Oncol. 7:609–616. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Cho Y, Miyamoto M, Kato K, et al: CD4+ and
CD8+ T cells cooperate to improve prognosis of patients with
esophageal squamous cell carcinoma. Cancer Res. 63:1555–1559.
2003.
|
|
17.
|
Van der Most RG, Currie A, Robinson BW and
Lake RA: Cranking the immunologic engine with chemotherapy: using
context to drive tumor antigen cross-presentation towards useful
antitumor immunity. Cancer Res. 66:601–604. 2006.PubMed/NCBI
|
|
18.
|
Appay V, van Lier RA, Sallusto F and
Roederer M: Phenotype and function of human T lymphocyte subsets:
consensus and issues. Cytometry. A73:975–983. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Lindemalm C, Mozaffari F, Choudhury A, et
al: Immune response, depression and fatigue in relation to support
intervention in mammary cancer patients. Support Care Cancer.
16:57–65. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Union Internationale Contre le Cancer: TNM
Classification of Malignant Tumours. 6th edition. Wiley-Liss; New
York: 2002
|
|
21.
|
Fagnoni FF, Lozza L, Zibera C, et al:
T-cell dynamics after high-dose chemotherapy in adults: elucidation
of the elusive CD8+ subset reveals multiple homeostatic T-cell
compartments with distinct implications for immune competence.
Immunology. 106:27–37. 2002.PubMed/NCBI
|
|
22.
|
Shiku H: Importance of CD4+ helper T-cells
in antitumor immunity. Int J Hematol. 77:435–438. 2003.
|
|
23.
|
Ostrand-Rosenberg S: CD4+ T lymphocytes: a
critical component of antitumor immunity. Cancer Invest.
23:413–419. 2005.
|
|
24.
|
Pardoll DM and Topalian SL: The role of
CD4+ T cell responses in antitumor immunity. Curr Opin Immunol.
10:588–594. 1998.
|
|
25.
|
Velders MP, Markiewicz MA, Eiben GL and
Kast WM: CD4+ T cell matters in tumor immunity. Int Rev Immunol.
22:113–140. 2003.
|
|
26.
|
Hakim FT, Cepeda R, Kaimei S, et al:
Constraints on CD4 recovery postchemotherapy in adults: thymic
insufficiency and apoptotic decline of expanded peripheral CD4
cells. Blood. 90:3789–3798. 1997.PubMed/NCBI
|
|
27.
|
Vivier E, Tomasello E, Baratin M, Walzer T
and Ugolini S: Functions of natural killer cells. Nat Immunol.
9:503–510. 2008. View
Article : Google Scholar
|
|
28.
|
Sinkovics JG and Horvath JC: Human natural
killer cells: A comprehensive review. Int J Oncol. 27:5–47.
2005.PubMed/NCBI
|
















