Introduction
The fibroblast growth factor (FGF) signaling system
controls a variety of cellular functions, including cell
proliferation, differentiation, migration and apoptosis. In humans
and mice, this system consists of 22 FGF ligands and 4 FGF
receptors (FGFRs) (1–3). The FGFs are monomeric proteins that
interact with heparan sulfate and bind, together with this
glycosaminoglycan, to one or more of the FGFRs. Upon ligand
binding, the FGFRs dimerize and trans-phosphorylate specific
tyrosine residues in the cytoplasmic domain of the receptor. The
signal is then passed on by various pathways involving Ras/MAP
kinase, phospholipase Cγ, PI3-kinase and STAT.
All the FGFRs are expressed in the musculoskeletal
system. FGFR1 and FGFR2 are particularly prevalent in bone. FGFR3
is found preferentially in cartilage and FGFR4 in muscle. It is
therefore not surprising that germline mutations in the FGFR genes
can cause a number of skeletal disorders, including
craniosynostosis syndromes and chondrodysplasias (3–5).
Somatic mutations in FGFRs can lead to unrestricted cellular growth
and cancer. In fact, one third of all bladder carcinomas display
nucleotide substitutions in FGFR3. Some hematological disorders
such as chronic myeloproliferative diseases exhibit chromosomal
translocations involving the FGFR1 gene at chromosome 8p11.
Patients with multiple myelomas often exhibit translocations
involving the FGFR3 locus at 4p16.
Ten years ago we described a fifth FGFR that we
termed FGFRL1 (FGFR-like 1) (6).
Independently, the same protein was discovered by two other
research groups and termed FGFR5 (7,8).
Similar to the classical FGFRs, the novel receptor contains an
extracellular domain with three immunoglobulin(Ig)-like repeats and
a single transmembrane domain. However, in contrast to the
classical receptors, FGFRL1 lacks the protein tyrosine kinase
domain but instead contains a short intracellular tail of 100
residues with a peculiar histidine-rich sequence. By Northern
blotting and in situ hybridization experiments, FGFRL1
expression is detected at low levels in virtually all mesenchymal
tissues and at higher levels in cartilage, bone and some muscles
(9,10).
When produced in HEK293 cells or in Sf9 insect
cells, the novel receptor binds FGF2 (7,9).
Furthermore, recombinant FGFRL1 interacts strongly with heparin and
heparan sulfate (9,11). Based on the interaction of the
novel receptor with FGF ligands and heparin and on the absence of
the tyrosine kinase domain, we speculated that FGFRL1 might
function as a decoy receptor that modulates or inhibits FGF
signaling (6,9). In fact, when over-expressed in MG63
osteosarcoma cells, it inhibits cell proliferation (9). In a luciferase system, it is able to
reduce the activity of the FGF inducible responsive promoter
element FIRE (12). Moreover,
FGFRL1 expression is markedly increased during differentiation of
myoblasts to myotubes, while it is barely expressed in
undifferentiated cells (13).
Taken together, these results suggest that FGFRL1 has a negative
effect on cell proliferation and a positive effect on cell
differentiation. Although the hypothesis of the decoy receptor is
plausible and straightforward, it does not explain the existence of
the relatively long intracellular domain with the peculiar
histidine-rich sequence and several tyrosine motifs.
More information about the potential functions of
FGFRL1 can be learned from experiments, in which its expression is
specifically suppressed. Knock-down experiments with morpholino
constructs in a Zebrafish model indicate that FGFRL1 is involved in
gill cartilage development (14).
Animals that have been injected with such morpholino constructs
fail to properly form the pharyngeal arches. Our group recently
demonstrated that mice with a targeted disruption of the FGFRL1
gene develop normally to term, but die immediately after birth due
to severe respiratory distress (13). The respiratory problems are
explained by the malformation of the diaphragm, which is not strong
enough to inflate the lungs after birth. The knock-out animals also
exhibit subtle bone alterations such as a dome-shaped head with a
high front reminiscent of many human craniosynostosis syndromes
(12). Another research group has
generated similar FGFRL1 deficient mice and found alterations in
the heart, especially in the ventricular valves, in addition to
alterations in the diaphragm and the skull (15). The involvement of FGFRL1 in the
formation of the skull was recently confirmed by the identification
of the first human FGFRL1 mutation in a craniosynostosis patient
(12). This patient displayed a 4
bp insertion in the last exon of the FGFRL1 gene that disrupted the
reading frame of the intracellular domain. In contrast to wild-type
protein, which was rapidly removed from the cell membrane and
sorted to lysosomes, the mutant protein appeared to stay for a
prolonged time at the plasma membrane where it interacted with FGF
ligands (12).
The overall structure of FGFRL1 with its three
Ig-like domains and the transmembrane segment is not unique. It is
needless to say that Ig-like domains occur in all immunoglobulins
but they are also found in a variety of other molecules, including
cell adhesion proteins, cell surface receptors and muscle proteins
(16,17). All Ig-like domains share a common
core β-sandwich structure. According to sequence pattern and
overall length, the Ig-like domains can be grouped into four sets:
V (variable), C1 (constant-1), C2 (constant-2) and I
(intermediate).
Here we have used a comprehensive bioinformatics
approach to compare the domain structure of FGFRL1 with all
proteins of the UniProt Databank. We found that the human genome
encodes at least 42 molecules that exhibit a related domain
structure with three extracellular Ig-like domains and a single
transmembrane domain. Five of these molecules are involved in FGF
signaling, 9 are involved in cell-cell contact at adherens
junctions and 25 are involved in the control and modulation of the
immune system. A detailed analysis of these proteins may yield
valuable clues about a putative signaling mechanism utilized by
FGFRL1.
Methods
Sequence searches were performed on the Vital-IT
platform of the Swiss Institute of Bioinformatics (http://www.vital-it.ch) using the HitKeeper query
language (18) and the MyHits
interface (19). Pre-calculated
hit lists on the human entries in the UniProtKB database (20) were queried for the presence of
various Ig-domain descriptors originating from Pfam (21) (PF00047, PF07686, PF08205), SMART
(22) (SM00409, SM00408), PROSITE
(23) (PS50835) and Swiss-Prot
features (20). Only proteins
displaying the presence of exactly 3 Ig-like domains were kept for
further analysis. The presence of transmembrane regions within
these proteins was assessed using the transmembrane predictor
software Phobius (24). The
extracellular domains of the extracted proteins were compared
pairwise with the extracellular domain of FGFRL1 using the program
Gap and the scoring matrix Blosum62 (GCG, Accelrys Software Inc,
Cambridge, UK). To enforce alignment over the entire length of the
two sequences, the gap shift limits were set to 20.
Results and Discussion
Seven protein families with a related
domain structure
To identify proteins with a domain structure similar
to that of FGFRL1, we screened the UniProt databank for entries
with three Ig-like domains and a single transmembrane domain. The
major problem of this approach was the fact that no well defined
domain descriptor for ‘Ig-like’ existed. In the first round of
databank screening we therefore included all human proteins with
domains conforming to at least one of the following descriptors,
prf:IG_LIKE; iprsmart:SM00409.IG; iprpfam: PF00047.ig;
iprpfam:PF07686.V-set; iprpfam:PF08205.C2-set_2;
iprsmart:SM00408.IGc2; ft:DOMAIN_Ig-like_V;
ft:DOMAIN_Ig-like_C2-type. Proteins with four or more Ig-like
domains were then eliminated. According to this approach UniProt
comprised a total of 3350 human proteins with 3 or less Ig-like
domains. Of these, 52 had exactly 3 Ig-like domains and at least 1
transmembrane domain, 219 had exactly 3 Ig-like domains but no
annotated transmembrane domain. The latter proteins were passed
through a transmembrane predictor program (Phobius), which divided
them into 140 proteins with a potential transmembrane domain and 79
proteins without. The remaining 192 proteins with 3 Ig-like domains
and at least 1 transmembrane domain were manually inspected. Double
hits, fragmented proteins as well as proteins with extracellular
domains in addition to the 3 Ig-like domains were eliminated. This
procedure yielded a list of 42 proteins that possessed exactly 3
Ig-like domains, a single transmembrane helix and an intracellular
domain (Table I).
 | Table I.Proteins encoded by the human genome
that display a domain structure related to FGFRL1. |
Table I.
Proteins encoded by the human genome
that display a domain structure related to FGFRL1.
| Gene symbol | Common name | Accession number | Order of domains | Extracellular
residues (Total amino acids) | Sequence
identity |
|---|
| FGFRL1 | FGF receptor -like 1
(FGFR5) | Q8N441 | C2, C2, C2, TM,
His | 25–378 (504) | 100 |
| FGFR1 | FGF receptor 1 | P11362 | C2, C2, C2, TM,
kinase | 22–376 (822) | 32 |
| FGFR2 | FGF receptor 2 | P21802 | C2, C2, C2, TM,
kinase | 22–377 (821) | 33 |
| FGFR3 | FGF receptor 3 | P22607 | C2, C2, C2, TM,
kinase | 23–375 (806) | 35 |
| FGFR4 | FGF receptor 4 | P22455 | C2, C2, C2, TM,
kinase | 22–369 (802) | 34 |
| FCGR1A | Fcγ receptor 1 | P12314 | C2, C2, C2, TM | 16–292 (374) | 18 |
| FCRL1 | Fc receptor -like
1 | Q96LA6 | C2, C2, C2, TM,
ITAM | 17–307 (429) | 24 |
| FCRL6 | Fc receptor -like
6 | Q6DN72 | C2, C2, C2, TM,
ITIM | 20–307 (434) | 22 |
| IL-1R1 | IL-1 receptor
1 | P14778 | C2, C2, C2, TM,
TIR | 18–336 (569) | 22 |
| IL-1R2 | IL-1 receptor
2 | P27930 | C2, C2, C2, TM | 14–343 (398) | 20 |
| IL-18R1 | IL-18 receptor | Q13478 | C2, C2, C2, TM,
TIR | 19–329 (541) | 18 |
| IL-1RL1 | IL-1 receptor -like
1 | Q01638 | C2, C2, C2, TM,
TIR | 19–328 (556) | 19 |
| IL-1RL2 | IL-1 receptor -like
2 | Q9HB29 | C2, C2, C2, TM,
TIR | 20–335 (575) | 19 |
| IL-1RAP | IL-1 receptor
accessory protein | Q9NPH3 | C2, C2, C2, TM,
TIR | 21–367 (570) | 23 |
| IL-18RAP | IL-18 receptor
accessory protein | O95256 | C2, C2, C2, TM,
TIR | 20–356 (599) | 21 |
| IL-1RAPL1 | IL-1 receptor
accessory protein-like 1 | Q9NZN1 | C2, C2, C2, TM,
TIR | 19–357 (696) | 21 |
| IL-1RAPL2 | IL-1 receptor
accessory protein-like 2 | Q9NP60 | C2, C2, C2, TM,
TIR | 17–354 (686) | 20 |
| KIR3DL1 | Killer cell Ig-like
receptor 3DL1 | P43629 | C2, C2, C2, TM,
ITIM | 22–340 (444) | 20 |
| KIR3DL2 | Killer cell Ig-like
receptor 3DL2 | P43630 | C2, C2, C2, TM,
ITIM | 22–340 (455) | 21 |
| KIR3DL3 | Killer cell Ig-like
receptor 3DL3 | Q8N743 | C2, C2, C2, TM,
ITIM | 26–322 (410) | 22 |
| KIR3DS1 | Killer cell Ig-like
receptor 3DS1 | Q14943 | C2, C2, C2, TM | 22–340 (387) | 21 |
| PVR | Poliovirus receptor
(nectin-like 5) | P15151 | V, C2, C2, TM | 21–343 (417) | 25 |
| PVRL1 | Poliovirus
receptor-related P1 (nectin 1) | Q15223 | V, C2, C2, TM | 31–355 (517) | 19 |
| PVRL2 | Poliovirus
receptor-related P2 (nectin 2) | Q92692 | V, C2, C2, TM | 32–360 (538) | 20 |
| PVRL3 | Poliovirus
receptor-related P3 (nectin 3) | Q9NQS3 | V, C2, C2, TM | 58–404 (549) | 26 |
| PVRL4 | Poliovirus
receptor-related P4 (nectin 4) | Q96NY8 | V, C2, C2, TM | 32–349 (510) | 22 |
| CADM1 | Cell adhesion
molecule 1 (nectin-like 2) | Q9BY67 | V, C2, C2, TM | 45–374 (442) | 21 |
| CADM2 | Cell adhesion
molecule 2 (nectin-like 3) | Q8N3J6 | V, C2, C2, TM | 25–367 (435) | 21 |
| CADM3 | Cell adhesion
molecule 3 (nectin-like 1) | Q8N126 | V, C2, C2, TM | 25–330 (398) | 24 |
| CADM4 | Cell adhesion
molecule 4 (nectin-like 4) | Q8NFZ8 | V, C2, C2, TM | 21–324 (388) | 21 |
| SIGLEC6 | Sialic acid-binding
Ig-like lectin 6 | O43699 | V, C2, C2, TM,
ITIM | 27–347 (453) | 20 |
| SIGLEC7 | Sialic acid-binding
Ig-like lectin 7 | Q9Y286 | V, C2, C2, TM,
ITIM | 19–353 (467) | 23 |
| SIGLEC8 | Sialic acid-binding
Ig-like lectin 8 | Q9NYZ4 | V, C2, C2, TM,
ITIM | 17–363 (499) | 24 |
| SIGLEC9 | Sialic acid-binding
Ig-like lectin 9 | Q9Y336 | V, C2, C2, TM,
ITIM | 18–348 (463) | 22 |
| SIGLEC14 | Sialic acid-binding
Ig-like lectin 14 | Q08ET2 | V, C2, C2, TM | 17–358 (396) | 20 |
| SIRPA | Tyrosine
phosphatase substrate 1 | P78324 | V, C1, C1, TM,
ITIM | 27–372 (503) | 20 |
| SIRPB1 | Signal-regulatory
protein β1 | O00241 | V, C1, C1, TM | 27–371 (398) | 20 |
| SIRPG | Signal-regulatory
protein γ | Q9P1W8 | V, C1, C1, TM | 29–360 (387) | 22 |
| AGER | Advanced
glycosylation end product receptor | Q15109 | V, C2, C2, TM | 23–342 (404) | 24 |
| BSG | Basigin | P35613 | (C2), C2, V,
TM | 22–323 (385) | 21 |
| CD96 | T-cell surface
protein tactile | P40200 | V, V, C2, TM | 22–519 (585) | 21 |
| HHLA2 | HERV-H
LTR-associating protein 2 | Q9UM44 | V, C1, V, TM | 23–344 (414) | 24 |
It is obvious that this list might still not be
complete. There are several entries in UniProt that are
ill-defined, mainly entries that were automatically derived from
TREMBL. Moreover, there are proteins with four or more Ig-like
domains that may also exist in an alternatively spliced form with
three Ig-like domains. Furthermore, some generally accepted Ig-like
domains might not be picked up correctly by the Ig-domain
descriptors mentioned above.
The 42 proteins from Table I fall into 7 protein families: FGF
receptors, Fc receptor-like proteins, IL-1 receptor-like proteins,
natural killer cell Ig-like receptors, nectin-like proteins, sialic
acid binding Ig-like lectins and signal-regulatory proteins. By
definition, all these proteins contain a cleavable signal peptide,
followed by three Ig-like domains and a transmembrane domain
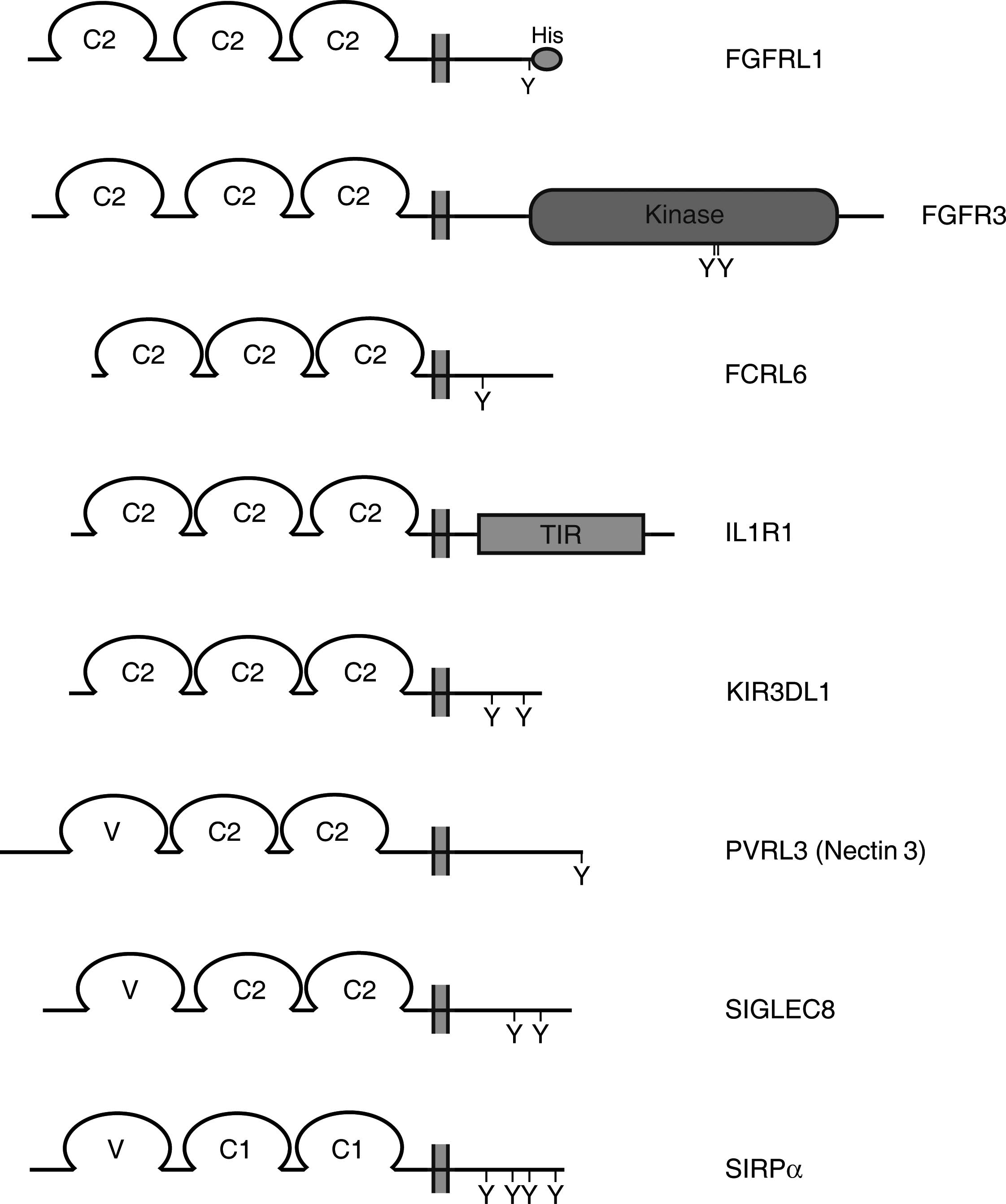
(Fig. 1). Consequently, the size
of the extracellular domain of these proteins varies between
300–350 amino acid residues. When compared with the extracellular
domain of FGFRL1, this region shares 18–26% sequence identity with
the exception of the extracellular domains from FGFRs, which
display 32–35% sequence identity (Table I). In the latter case, the
similarity increases to 39–42% if conservative amino acid
replacements are included, underlining the previous conclusion that
FGFRL1 is most closely related to the FGFRs. The size of the
intracellular domain varies between 4 (SIRPγ) and 425 (FGFR1) amino
acid residues. For down-stream signaling, a protein must utilize
this domain in order to trigger a certain pathway. This domain may
therefore provide valuable clues about a putative signaling
mechanism utilized by FGFRL1.
In the following, we will briefly summarize the
major properties and signaling mechanisms of the different protein
families. The FGFR family has already been described in the
introduction in some detail. For a comprehensive survey of an
individual protein, the reader is referred to more detailed reviews
of a particular family.
Fc Receptor-like molecules
Fc receptors are molecules found on the surface of
various cells from the immune system, including macrophages,
neutrophils, mast cells and natural killer cells (25). They bind to the Fc region of
antibodies that are attached to invading pathogens or infected
cells. There are Fc receptors for all types of antibodies but only
the Fcγ receptor I resembles FGFRL1 with its 3 Ig-like domains.
Besides the classical Fc receptors, there are six Fc receptor-like
molecules that occur preferentially on the surface of B cells. Two
of these proteins possess 3 Ig-like domains (FCRL1 and FCRL6) and
are therefore included in Table I.
So far there is no direct evidence to support a role for the Fc
receptor-like molecules as Ig-binding receptors. However, these
molecules contain cytoplasmic tails that harbor immunoreceptor
tyrosine-based activation (ITAM) and inhibition (ITIM) motifs.
Through these motifs they deliver activating or inhibiting signals
to the interior of the cells (see below), suggesting that these
receptors play a role in regulating activation and differentiation
of B cells and other cells. Fcγ receptor I does not have an ITAM
but it still transmits activating signals to the interior of the
cells by interacting with another protein that does possess an
ITAM.
The interleukin-1 receptor family
Inflammatory cytokines such as IL-1 and IL-18
mediate their effects through specific transmembrane receptors
present on the surface of target cells (26,27).
These receptors possess three Ig-like domains in their
extracellular part and a signaling domain termed TIR (Toll/IL-1
receptor) in their intracellular part. Altogether, there are nine
related IL-1 receptor-like molecules. The receptor for IL-1 is a
dimer consisting of IL-1R1 and IL-1RAP (IL-1 receptor accessory
protein), the receptor for IL-18 a dimer of IL-18R and IL-18RAP.
Other cytokines such as IL-33 and IL-1F6 bind to dimeric receptors
consisting of various combinations of the other chains listed in
Table I. Upon ligand binding
cytosolic adaptor proteins such as MyD88 are recruited to the
intracellular TIR domain of the receptors and these adaptor
proteins trigger down-stream signaling by phosphorylation. IL-1
receptor II (IL1R2) is a negatively acting receptor that lacks the
intracellular TIR domain. It still forms dimers with IL1R1 but does
not take part in signaling. Since it can bind IL-1 it appears to
act as a decoy receptor.
Killer cell Ig-like receptors (KIRs)
KIRs are transmembrane proteins expressed by natural
killer cells and some T cells (28,29).
The genes for the KIRs are found in a cluster on human chromosome
19q13.4 within the leukocyte receptor complex. The KIR proteins are
classified by the number of Ig-like domains (2D or 3D) and by the
presence of a long (L) or a short (S) cytoplasmic domain. KIR
proteins with the longer cytoplasmic domain elicit inhibitory
signals upon ligand binding via the ITIM motif introduced above.
KIR proteins with the shorter cytoplasmic domain lack the ITIM
motif. Instead they associate with the tyrosine kinase binding
protein TYRO to transduce activating signals to the cells. The
ligands for KIR proteins are members of the HLA class I antigens.
KIR proteins are therefore thought to play an important role in the
regulation of the immune response. Besides the genes for three
KIR3DL proteins and one KIR3DS protein, there are two KIR3D genes
(KIR3DP1, KIR3DX1) not listed here that may represent
pseudogenes.
Nectins and nectin-like proteins
Nectins (nectin 1–4) and nectin-like proteins
(nectin-like 1–5) constitute a family of 9 cell surface proteins
that are involved in cell adhesion, migration and proliferation
(30,31). The nectins form homodimers on the
plasma membrane of various cell types and interact in trans with
nectins and nectin-like proteins expressed on neighboring cells.
These interactions result in calcium-independent cell adhesion at
cell-cell adherens junctions and lead to the reorganization of the
actin cytoskeleton. For the interaction with the cytoskeleton, the
nectins bind with their intracellular, C-terminal end to afadin.
Afadin in turn interacts with filamentous actin. Nectin-like
protein 5 has a unique additional function at the leading edge of
migrating cells where it interacts with integrin αvβ3 to facilitate
cell movement. Some nectins are important ways of entry for viruses
into cells. Nectin-1 is the receptor for herpes virus, nectin-like
5 is the receptor for poliovirus.
Sialic acid binding Ig-like lectins
(SIGLECs)
SIGLECs are cell surface receptors that bind
carbohydrates, especially sialic acid from glycoproteins of other
cells, but also from viruses and bacteria (32,33).
The SIGLECs are primarily, but not exclusively, expressed by
hematopoietic cells. They contain one amino-terminal V-set Ig-like
domain that interacts with the carbohydrates and a variable number
of C2-set Ig-like domains. The human genome contains 14 different
SIGLEC genes (SIGLEC1-15, SIGLEC13 is found in chimpanzee and
baboon, but not in humans) and a similar number of SIGLEC
pseudogenes. Only 5 SIGLECs are listed in Table I because only these proteins
contain exactly 3 Ig-like domains. Most SIGLECs possess one or more
copies of the ITIM element in their cytoplasmic domain. This motif
is phosphorylated by cytoplasmic tyrosine kinases and plays a key
role in cell signaling (see below). Consequently, the SIGLECs are
believed to have a function in the activation of cells from the
immune system.
Signal-regulatory proteins (SIRPs)
The SIRPs form a family of related proteins that are
mainly expressed on the surface of myeloid cells (34). There are three family members,
SIRPα, SIRPβ and SIRPγ. In the human population, the genes for the
SIRPs appear to be highly polymorph. The cytoplasmic domain of
SIRPα contains four tyrosine residues that conform loosely to the
ITIM motif and can become phosphorylated. The phosphorylated motifs
associate with tyrosine phosphatases and induce inhibitory
functions in cell signaling. SIRPβ has a very short cytoplasmic
region of 6 amino acids and lacks the ITIM motif. Instead it
contains a lysine residue that interacts with a protein called
DAP12 which in turn transmits activating signals. Because the two
proteins elicit opposing functions, they are also referred to as
paired receptors. SIRPγ has only 4 amino acid residues in its
cytoplasmic domain and does not appear to transmit any signals.
Other proteins
The remaining four proteins do not appear to belong
to any of the above mentioned protein families. AGER is a receptor
for advanced glycosylation end products, which may occur by
non-enzymatic glucosidation of α- and ε-amino groups with blood
sugars (35). Owing to its
extracellular Ig-like domains (V, C2, C2) and its sugar binding
properties, it resembles the SIGLECs. Basigin (CD147) is thought to
have a function in intercellular recognition (36). It interacts with many different
ligands, including certain cyclophilins and integrins. CD96
(tactile) plays a role in the adhesive interactions of T-cells and
natural killer cells during the late phase of the immune response
(37). It may also be involved in
antigen presentation. Finally, HHLA2 has been identified by virtue
of several EST and cDNA clones (38). Its gene is associated with the
human endogenous retrovirus H family long terminal repeat (HERV-H
LTR).
ITIM/ITAM containing receptors
When the functions and signaling mechanisms of the
seven protein families are compared it becomes evident that at
least some members of each family are equipped with intracellular
domains or motifs that are directly or indirectly involved in
down-stream signaling (Fig. 1).
The classical FGFRs harbor a well-defined tyrosine kinase domain of
approximately 300 amino acid residues that phosphorylates strategic
tyrosine residues in the C-terminal domain of an adjacent receptor.
FGFRL1 is clearly lacking such a tyrosine kinase domain. On the
other hand, the IL-1 receptor I contains an intracellular TIR
domain of approximately 200 amino acid residues that interacts with
a multifunctional adaptor protein (MyD88), which in turn recruits
signaling molecules including tyrosine kinases to the plasma
membrane. The intracellular domain of FGFRL1 is shorter than that
of IL-1 receptor I and does not display any conserved, well-defined
domain like TIR. One will therefore have to focus on conserved,
short sequence motifs rather than extended domains. In fact, such
motifs are found in many of the seven protein families, namely in
the Fc receptor-like proteins, the KIRs, the SIGLECs and the SIRPs.
Members of these families harbor specific sequence motifs termed
ITAM (immunoreceptor tyrosine-based activation motifs) and ITIM
(immunoreceptor tyrosine-based inhibition motifs) that are involved
in down-stream signaling.
The ITAM is a tandem motif that conforms to the
amino acid sequence YxxL/I(x)6–12YxxL/I, whereas the
ITIM is a single motif that conforms to the sequence
S/I/V/LxYxxI/V/L (39–41). After ligand binding, ITAM-bearing
receptors become phosphorylated by Src family kinases. The
phosphorylated tyrosines serve subsequently as docking sites for
the recruitment of other tyrosine kinases such as Syk or ZAP-70,
resulting in the activation of several down-stream signaling
pathways. ITIM-bearing receptors can also become phosphorylated
after ligand binding by Src family kinases but they elicit opposing
effects. The phosphorylated ITIMs bind SH2-containg phosphatases
such as SHP-1 (phosphotyrosine phosphatase-1), SHP-2 and SHIP
(inositol phosphatase). These enzymes can dephosphorylate the
cytoplasmic domains of adjacent receptors, thereby inhibiting
activation of the cells.
The ITAM/ITIM signaling motifs reveal some
similarity to a tandem tyrosin-based motif occurring in the FGFRL1
receptor, PKLYPKLYTDI. We have demonstrated that this tyrosine
based motif is responsible for intracellular sorting of FGFRL1
(12). When the tyrosines were
changed to alanine by in vitro mutagenesis, the mutated
protein stayed for a prolonged time at the cell membrane, whereas
the wild-type protein was rapidly removed from the cell membrane
and sorted to lysosomes. It is intriguing to speculate that the
same tandem tyrosine-based motif could also become phosphorylated
by an intracellular kinase. The phosphorylated motif would then
bind SH2 containing phosphatases, which in turn could down-regulate
the activity of adjacent FGFRs.
We have tried to check this possibility.
Phosphorylation of FGFRL1 was investigated in cells, before and
after stimulation by FGF2 or EGF, with pan-phosphotyrosine specific
antibodies (Steinberg and Trueb, unpublished observation). However,
we have not been able to detect any phosphorylation of FGFRL1. It
is therefore unlikely that FGFRL1 is involved in a signaling
pathway with SH2 domain containing adaptor proteins that would bind
to its C-terminal end. Nevertheless, this possibility should be
kept in mind when the signaling mechanism of the novel receptor is
investigated.
Reorganization of the cytoskeleton
If FGFRL1 has no tyrosine kinase domain similar to
the FGFRs, no TIR domain similar to the IL-1 receptor and no
ITAM/ITIM signaling motif similar to many other receptors of the
immune system, how might it participate in down-stream signaling?
One group of proteins that still remains for comparison are the
nectin and nectin-like proteins. In fact, with 26% sequence
identity (33% sequence similarity if conservative amino acid
substitutions are included), nectin-3 displays, after the classical
FGFRs, the best similarity to FGFRL1 of the 42 proteins listed in
Table I. Like the nectins, FGFRL1
forms homodimers on the cell membrane as demonstrated by FRET
measurements and by immunoprecipitation experiments (11). Furthermore, FGFRL1 occurs
preferentially at cell-cell contact sites similar to the
nectins.
Nectins are involved in the initial steps of the
formation of intercellular adherens junctions (30,31).
For this purpose, they interact with a variety of peripheral
membrane proteins including the actin filament-binding protein
afadin and the cell polarity protein Par-3. These interactions are
achieved via binding of the carboxy-terminal end of the nectins
(amino acid residues E/A-X-Y-V) with the PDZ domains of afadin and
Par-3. Afadin in turn can bind to F-actin and link the nectins in
this way to the cytoskeleton. In addition to its PDZ and
actin-binding domains, afadin has other interaction domains, such
as two Ras association domains, a forkhead-associated domain, a DIL
domain and three proline-rich domains. Likewise, Par-3 possesses
three PDZ domains and two CR domains. By virtue of interaction with
such versatile adaptor proteins, the nectins can indirectly induce
the activation of several intracellular signaling molecules,
including Rap1, Cdc42 and Rac, and trigger the reorganization of
the cytoskeleton.
It is of interest to note that FGFRL1 exhibits a
C-terminal sequence with a tyrosine residue (H-Y-Q-C) that is
somewhat related to that of the nectins. Together with the
histidine-rich sequence, this motif is the only element of the
intracellular domain that is conserved among different species,
from mammals to fish and lancelet. We do not know whether this
C-terminal sequence can indeed be recognized by a specific PDZ
domain. On the other hand, PDZ recognition motifs that interact
with similar C-terminal sequences have been described in the
literature, such as the PDZ domain of the adaptor protein Mint1
that recognizes the C-terminal sequence D-H-W-C of a calcium
channel (42,43). It remains to be determined whether
cells express an adaptor protein with a PDZ domain that
specifically interacts with the C-terminus of FGFRL1. Such a
protein might anchor FGFRL1 to the cytoskeleton or to another
intracellular protein complex.
Conclusions
The domain structure of FGFRL1 with its 3 Ig-like
domains and the transmembrane segment is not unique but occurs in
more than 40 human proteins, which can be grouped in 7 families.
Most of these proteins play a role in the immune system, but there
are also some that serve an important function in cell-cell
adhesion at adherens junctions. A great deal of these proteins
forms homodimers on cell surfaces and acts as some kind of
receptors. The ligands for these receptors are either cytokines,
antibodies, carbohydrate chains or cell surface molecules. A
structure with three Ig-like domains appears to be particularly
well suited for recognition and interaction. In the adaptive immune
system, this structure is employed to recognize a nearly unlimited
number of antigens.
FGFRL1 shares many similarities with the prototype
of such a 3 Ig-domain receptor. It also forms constitutive dimers
on the cell surface and it has the ability to bind heparin chains
and FGF ligands. During evolution, the other Ig-domain proteins
have adapted various possibilities to signal their ligand binding
state to the interior of the cells. For this purpose, some make use
of strategic tyrosine motifs that become phosphorylated and provide
binding sites for SH2 domain containing adaptor proteins, or
alternatively some interact through their C-terminal end with a PDZ
domain protein, which in turn interacts with the cytoskeleton.
FGFRL1 displays some parallels in this respect. It possesses
several tyrosine motifs in its C-terminal domain that could
theoretically become phosphorylated upon ligand binding or that
might interact with a PDZ domain protein which links it to the
cytoskeleton. To date there is no evidence for the first
possibility. We therefore favor the second possibility of an
interaction with a PDZ domain protein. In this way, FGFRL1 might be
able to signal information from the outside of the cell to the
interior although it is lacking the tyrosine kinase domain
typically associated with the classical FGFRs. Thus, the
genome-wide comparison of FGFRL1 with structurally related proteins
from the UniProt databank has opened new avenues for research that
can now be tackled experimentally.
Acknowledgements
This study was supported by grants
from the Swiss National Science Foundation (3100A0-113806), the
Swiss Foundation for Research on Muscular Diseases and the Olga
Mayenfisch Foundation.
References
|
1.
|
Itoh N: The Fgf families in humans, mice,
and zebrafish: their evolutional processes and roles in
development, metabolism, and disease. Biol Pharm Bull.
30:1819–1825. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Itoh N and Ornitz DM: Evolution of the Fgf
and Fgfr gene families. Trends Genet. 20:563–569. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Eswarakumar VP, Lax I and Schlessinger J:
Cellular signaling by fibroblast growth factor receptors. Cytokine
Growth Factor Rev. 16:139–149. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Wilkie AO: Bad bones, absent smell,
selfish testes: the pleiotropic consequences of human FGF receptor
mutations. Cytokine Growth Factor Rev. 16:187–203. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Coumoul X and Deng CX: Roles of FGF
receptors in mammalian development and congenital diseases. Birth
Defects Res C Embryo Today. 69:286–304. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Wiedemann M and Trueb B: Characterization
of a novel protein (FGFRL1) from human cartilage related to FGF
receptors. Genomics. 69:275–279. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Sleeman M, Fraser J, McDonald M, Yuan S,
White D, Grandison P, Kumble K, Watson JD and Murison JG:
Identification of a new fibroblast growth factor receptor, FGFR5.
Gene. 271:171–182. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kim I, Moon S-O, Yu K-H, Kim U-H and Koh
GY: A novel fibroblast growth factor receptor-5 preferentially
expressed in the pancreas. Biochim Biophys Acta. 1518:152–156.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Trueb B, Zhuang L, Taeschler S and
Wiedemann M: Characterization of FGFRL1, a novel FGF receptor
preferentially expressed in cartilage. J Biol Chem.
278:33857–33865. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Trueb B and Taeschler S: Expression of
FGFRL1, a novel fibroblast growth factor receptor, during embryonic
development. Int J Mol Med. 17:617–620. 2006.PubMed/NCBI
|
|
11.
|
Rieckmann T, Kotevic I and Trueb B: The
cell surface receptor FGFRL1 forms constitutive dimers that promote
cell adhesion. Exp Cell Res. 314:1071–1081. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Rieckmann T, Zhuang L, Flück CE and Trueb
B: Characterization of the first FGFRL1 mutation identified in a
craniosynostosis patient. Biochim Biophys Acta. 1792:112–121. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Baertschi S, Zhuang L and Trueb B: Mice
with a targeted disruption of the Fgfrl1 gene die at birth due to
alterations in the diaphragm. FEBS J. 274:6241–6253. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Hall C, Flores MV, Murison G, Crosier K
and Crosier P: An essential role for zebrafish Fgfrl1 during gill
cartilage development. Mech Dev. 123:925–940. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Catela C, Bilbao-Cortes D, Slonimsky E,
Kratsios P, Rosenthal N and Te Welscher P: Multiple congenital
malformations of Wolf-Hirschhorn syndrome are recapitulated in
Fgfrl1 null mice. Dis Model Mech. 2:283–294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Barclay AN: Ig-like domains: evolution
from simple interaction molecules to sophisticated antigen
recognition. Proc Natl Acad Sci USA. 96:14672–14674. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Smith DK and Xue H: Sequence profiles of
immunoglobulin and immunoglobulin-like domains. J Mol Biol.
12:530–545. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Hau J, Muller M and Pagni M: HitKeeper, a
generic software package for hit list management. Source Code Biol
Med. 28:2:22007.PubMed/NCBI
|
|
19.
|
Pagni M, Ioannidis V, Cerutti L,
Zahn-Zabal M, Jongeneel CV, Hau J, Martin O, Kuznetsov D and
Falquet L: MyHits: improvements to an interactive resource for
analyzing protein sequences. Nucleic Acids Res. 35:W433–W437.
2007.PubMed/NCBI
|
|
20.
|
UniProt Consortium: The universal protein
resource (UniProt). Nucleic Acids Res. 36:D190–D195. 2008.
|
|
21.
|
Finn RD, Tate J, Mistry J, Coggill PC,
Sammut JS, Hotz HR, Ceric G, Forslund K, Eddy SR, Sonnhammer EL and
Bateman A: The Pfam protein families database. Nucleic Acids Res.
36:D281–D288. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Letunic I, Copley RR, Pils B, Pinkert S,
Schultz J and Bork P: SMART 5: domains in the context of genomes
and networks. Nucleic Acids Res. 34:D257–D260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Hulo N, Bairoch A, Bulliard V, Cerutti L,
De Castro E, Langendijk-Genevaux PS, Pagni M and Sigrist CJA: The
PROSITE database. Nucleic Acids Res. 34:D227–D230. 2006. View Article : Google Scholar
|
|
24.
|
Käll L, Krogh A and Sonnhammer EL: A
combined transmembrane topology and signal peptide prediction
method. J Mol Biol. 338:1027–1036. 2004.PubMed/NCBI
|
|
25.
|
Davis RS: Fc receptor-like molecules. Annu
Rev Immunol. 25:525–560. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
O’Neill LA: The interleukin-1
receptor/Toll-like receptor super-family: 10 years of progress.
Immunol Rev. 226:10–18. 2008.PubMed/NCBI
|
|
27.
|
Boraschi D and Tagliabue A: The
interleukin-1 receptor family. Vitam Horm. 74:229–254. 2006.
View Article : Google Scholar
|
|
28.
|
Gardiner CM: Killer cell
immunoglobulin-like receptors on NK cells: the how, where and why.
Int J Immunogenet. 35:1–8. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Long EO: Negative signaling by inhibitory
receptors: the NK cell paradigm. Immunol Rev. 224:70–84. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Miyoshi J and Takai Y: Nectin and
nectin-like molecules: biology and pathology. Am J Nephrol.
27:590–604. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Rikitake Y and Takai Y: Interactions of
the cell adhesion molecule nectin with transmembrane and peripheral
membrane proteins for pleiotropic functions. Cell Mol Life Sci.
65:253–263. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Crocker PR and Redelinghuys P: Siglecs as
positive and negative regulators of the immune system. Biochem Soc
Trans. 36:1467–1471. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Von Gunten S and Bochner BS: Basic and
clinical immunology of Siglecs. Ann N Y Acad Sci. 1143:61–82.
2008.
|
|
34.
|
Barclay AN and Brown MH: The SIRP family
of receptors and immune regulation. Nat Rev Immunol. 6:457–464.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Neeper M, Schmidt AM, Brett J, Yan SD,
Wang F, Pan YC, Elliston K, Stern D and Shaw A: Cloning and
expression of a cell surface receptor for advanced glycosylation
end products of proteins. J Biol Chem. 267:14998–15004.
1992.PubMed/NCBI
|
|
36.
|
Yurchenko V, Constant S and Bukrinsky M:
Dealing with the family: CD147 interactions with cyclophilins.
Immunology. 117:301–309. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Fuchs A, Cella M, Giurisato E, Shaw AS and
Colonna M: CD96 (tactile) promotes NK cell-target cell adhesion by
interacting with the poliovirus receptor (CD155). J Immunol.
172:3994–3998. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Mager DL, Hunter DG, Schertzer M and
Freeman JD: Endogenous retroviruses provide the primary
polyadenylation signal for two new human genes (HHLA2 and HHLA3).
Genomics. 59:255–263. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Barrow AD and Trowsdale J: You say ITAM
and I say ITIM, let’s call the whole thing off: the ambiguity of
immunoreceptor signalling. Eur J Immunol. 36:1646–1653.
2006.PubMed/NCBI
|
|
40.
|
Daeron M, Jaeger S, Du Pasquier L and
Vivier E: Immunoreceptor tyrosine-based inhibition motifs: a quest
in the past and future. Immunol Rev. 224:11–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Pinheiro da Silva F, Aloulou M, Benhamou M
and Monteiro RC: Inhibitory ITAMs: a matter of life and death.
Trends Immunol. 29:366–373. 2008.PubMed/NCBI
|
|
42.
|
Bezprozvanny I and Maximov A:
Classification of PDZ domains. FEBS Lett. 509:457–462. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Maximov A, Sudhof TC and Bezprozvanny I:
Association of neuronal calcium channels with modular adaptor
proteins. J Biol Chem. 274:24453–24456. 1999. View Article : Google Scholar : PubMed/NCBI
|