Introduction
Hyperthermia is a heat treatment that directly
targets cancer cells or the environment surrounding them (1). According to the tenets of
hyperthermic oncology, significant tumor cell killing could
theoretically be achieved if cells or tissues were heated to over
42°C for 1 h or more (2). It is
speculated that such heat treatment would induce radio- and
chemosensitization, in part by inhibiting DNA damage repair
(1). However, clinical experience
over the past 25 years has shown that it is not possible to
routinely achieve thermal doses of over 42°C for 1 h or more. It is
now known that, during typical hyperthermia treatments with
currently available heating technologies (except for thermal
ablation), cytotoxic temperatures are only achieved in small
sub-volumes of tumors (3).
Meanwhile, until recently the effects of mild temperature
hyperthermia (MTH) (39–42°C for 1–2 h) on tissue have largely been
ignored. However, MTH has subtle effects, including heat-mediated
tumor reoxygenation (4) and the
inhibition of sublethal and potentially lethal damage repair
(5), which provide a very strong
rationale for using MTH in combination with radiotherapy. In
addition, the physiological and cellular effects of MTH can improve
the delivery of drug vehicles (6),
activate promoters for heat-mediated gene therapy (7) and increase immune response to tumors
through a variety of mechanisms (8). Indeed, MTH has been reported to
increase the response of tumors, in particular of quiescent
(Q)-cell populations, to radiation or chemotherapeutic agents, in
part by improving oxygenation through an increase in tumor blood
flow (9).
Tumor hypoxia results from limited oxygen diffusion
(chronic hypoxia) or limited perfusion (acute hypoxia, transient
hypoxia or ischemic hypoxia) (10). Chronically hypoxic tumor cells
existing at the rim of the oxygen diffusion distance are killed by
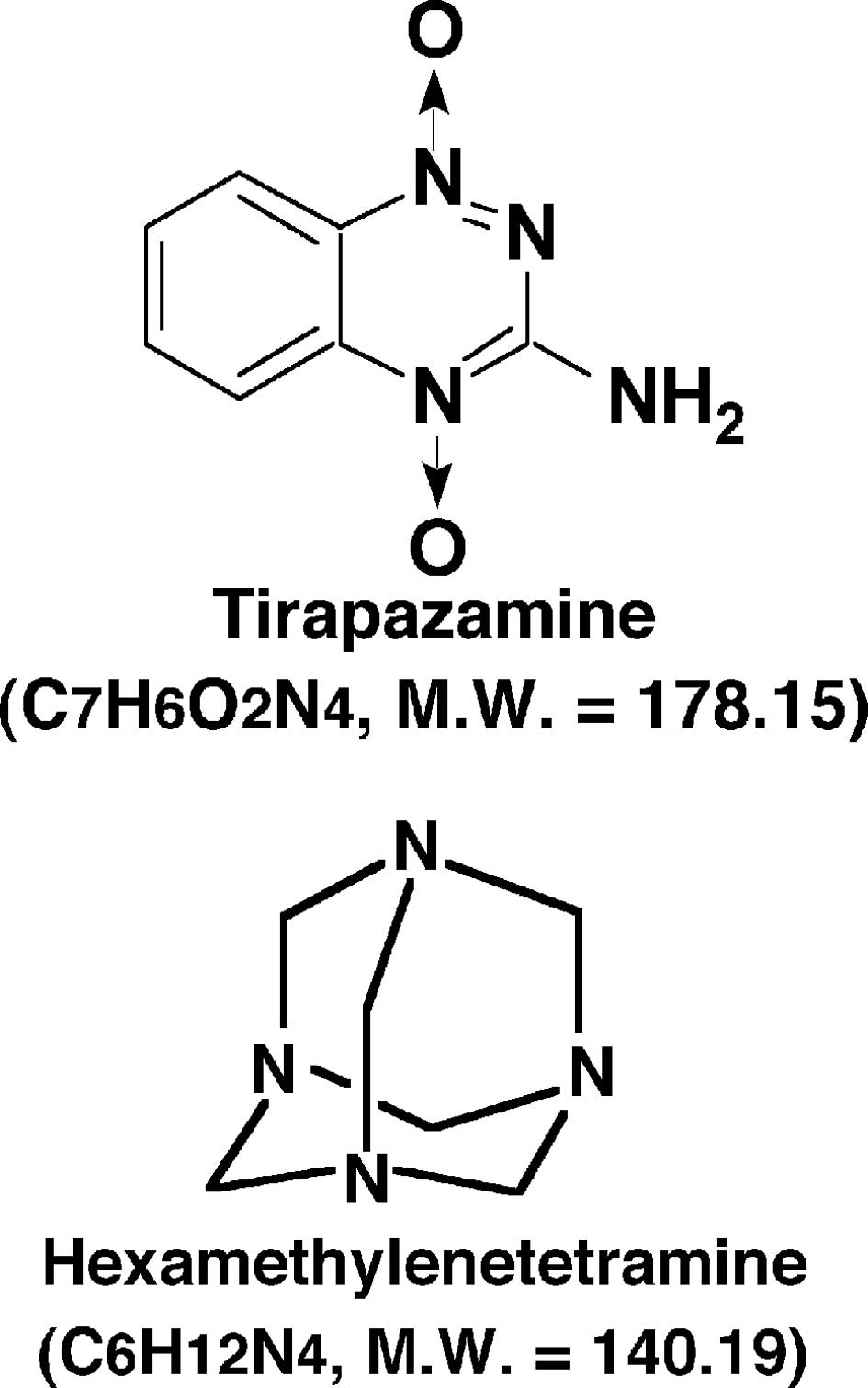
just a single administration of tirapazamine (TPZ), a lead compound
identified in the development of a bioreductive hypoxic cytotoxin
(Fig. 1), while acutely hypoxic
tumor cells occurring sporadically throughout solid tumors are
killed by long-term continuous administration of TPZ. Therefore,
the long-term continuous administration of TPZ is capable of
killing both chronically and acutely hypoxic tumor cells (9).
Although TPZ has enhanced some standard clinical
regimens, the results are variable and, with certain combinations,
toxicity is enhanced (11).
Neutropenia is commonly reported, but usually within a tolerable
range. The most frequent non-hematological toxicities reported are
nausea, vomiting, diarrhea and skin rash. Two additional unusual
toxicities are reversible deafness and muscle cramping (11). According to a recently reported
in vivo animal study, the addition of TPZ to
cisplatin/radiotherapy treatment caused a significant increase in
toxicity, in particular to the heart, liver, kidney and stomach,
and had only moderate effects on the tumor (12). As a result, TPZ has exhibited no
clear beneficial effects compared to conventional drugs in clinical
trials conducted to date.
Formaldehyde preserves or fixes tissues or cells by
irreversibly crosslinking primary amine groups in proteins with
other nearby nitrogen atoms in protein or DNA through a -CH2-
linkage (13).
Hexamethylenetetramine (HMTA) (Fig.
1), an acid-dependent formaldehyde donor, has been used as an
antiseptic for urinary tract infections and is characterized as
non-carcinogenic in animals (13).
Under hypoxic conditions in solid tumors, pyruvate generated by
glycolysis creates a low pH environment that produces formaldehyde
through the dissociation of HMTA (14).
Excepting surgical resection, chemotherapy using
cis-diamminedichloroplatinum (cisplatin) and radiotherapy are the
most frequently employed anticancer therapeutic modalities applied
in the clinical setting. Based on clinical experience and using a
previously developed method for selectively detecting the response
of Q cell populations within solid tumors (15), the combined effect of MTH with
γ-ray irradiation or cisplatin treatment in combination with or
without HMTA or TPZ administration was evaluated. In addition, the
efficacy of continuous administration of HMTA or TPZ versus single
injection was examined.
Materials and methods
Mice and tumors
SCC VII squamous cell carcinomas (Department of
Radiotherapy, Kyoto University) derived from C3H/He mice were
maintained in vitro in Eagle's minimum essential medium
supplemented with 12.5% fetal bovine serum. The cells
(1.0×105) were inoculated subcutaneously into the left
hind legs of 8- to 11-week-old syngeneic female C3H/He mice (Japan
Animal Co., Ltd., Osaka, Japan). After 14 days, tumors with a
diameter of ∼1 cm were selected for use in the experiments. The
body weight of the tumor-bearing mice was 22.1±2.3 g (mean ± SD).
Mice were handled according to the Recommendations for Handling of
Laboratory Animals for Biomedical Research, compiled by the
Committee on Safety and Ethical Handling Regulations for Laboratory
Animal Experiments of Kyoto University. The p53 of the SCC VII
tumor cells was wild-type (15).
Labeling with
5-bromo-2′-deoxyuridine
Nine days after inoculation, mini-osmotic pumps
(Durect Corporation, Cupertino, CA, USA) containing
5-bromo-2′-deoxyuridine (BrdU) dissolved in physiological saline
(250 mg/ml) were implanted subcutaneously for 5 days to label all
proliferating (P)-cells. The percentage of labeled cells in the SCC
VII tumors after continuous labeling with BrdU reached a plateau at
55.3±4.5%. Intratumor cells not incorporating BrdU after continuous
labeling were determined to be Q cells.
Treatment
After labeling with BrdU, TPZ and HMTA dissolved in
physiological saline were administered at doses of 0.224 mmol/kg or
28.5 mmol/kg, respectively, by single intraperitoneal injection or
by continuous administration for 24 h by means of subcutaneously
implanted mini-osmotic pumps. MTH (40°C, 60 min) was initiated
immediately after single intraperitoneal injection or was performed
during the continuous administration, immediately before γ-ray
irradiation or cisplatin treatment. Not all tumors received MTH
prior to γ-ray irradiation or cisplatin treatment.
To detect the radiosensitizing effects of TPZ or
HMTA, whole body irradiation with γ-rays was performed at a dose of
4 or 20 Gy using a cobalt-60 γ-ray irradiator at a dose rate of
∼2.5 Gy/min. Cisplatin was intraperitoneally administered at a dose
of 0.5 LD50 (the dose required to kill 50% of cells,
LD50=17.7 mg/kg) to detect the cisplatin
sensitivity-enhancing effects of TPZ or HMTA. Cytotoxicity was
assessed in terms of cell survival and induced micronucleus (MN)
frequency following the intraperitoneal or continuous
administration of TPZ or HMTA alone at the above doses (16).
For MTH, the tumors grown in the left hind legs of
mice were heated at 40°C for 60 min by immersion of the
tumor-bearing foot in a water bath as follows: the mouse was held
in a specially constructed device with the tail and right leg
firmly fixed with adhesive tape. The left tumor-bearing leg was
pulled down by a special sinker (∼45 g) affixed to the skin of the
toe with Superglue (Arone-arufa, Konishi Co., Osaka, Japan). The
mouse was placed in a circulating water bath maintained at the
desired temperature, and was air-cooled during the heat treatment
(17). Temperatures at the tumor
center equilibrated within 3–4 min of immersion in the water bath
and remained 0.2–0.3°C below bath temperature. The water bath
temperature was therefore maintained at 0.3°C above the desired
tumor temperature.
In mice receiving HMTA or TPZ treatment alone, the
implanted tumors were excised 1 h after the single intraperitoneal
injection or after the 24-h continuous subcutaneous infusion. In
mice receiving the combination of each drug with γ-ray irradiation,
implanted tumors were excised immediately after irradiation. For
the combination with cisplatin treatment, implanted tumors were
excised 1 h after the administration of cisplatin.
The above-mentioned sequences and the timing of each
treatment were based on data obtained from preliminary experiments.
Procedures were appropriate and were carried out successfully
(9,16).
Immunofluorescence staining of
BrdU-labeled cells and micronucleus assay
Tumors excised from the mice administered BrdU were
minced and trypsinized with 0.05% trypsin and 0.02%
ethylenediamine-tetraacetic acid (EDTA) in phosphate-buffered
saline (PBS) at 37°C for 15 min. Tumor cell suspensions were
incubated for 72 h in tissue culture dishes containing complete
medium and 1.0 μg/ml of cytochalasin-B in order to inhibit
cytokinesis while allowing nuclear division. The cultures were
subsequently trypsinized and the cell suspensions were fixed. After
the centrifugation of the fixed cell suspensions, the cell pellet
was resuspended with cold Carnoy's fixative (ethanol:acetic acid,
3:1 volume). The suspension was then placed on a glass microscope
slide and the sample was dried at room temperature. The slides were
treated with 2 M hydrochloric acid for 60 min at room temperature
to dissociate the histones and to partially denature the DNA, and
were then immersed in borax-borate buffer (pH 8.5) to neutralize
the acid. BrdU-labeled tumor cells were detected by indirect
immunofluorescence staining using monoclonal anti-BrdU antibody
(Becton Dickinson, San Jose, CA, USA) and fluorescein
isothiocyanate (FITC)-conjugated antimouse IgG antibody (Sigma, St.
Louis, MO, USA). To observe the double staining of tumor cells with
green-emitting FITC and red-emitting propidium iodide (PI), cells
on the slides were treated with PI (2 μg/ml in PBS) and
monitored under a fluorescence microscope.
Since cytochalasin-B inhibits cytokinesis while
allowing nuclear division, cultured tumor cells were transformed
into binuclear cells after the first cell division. When cell
division is disrupted or chromosomes have been broken or damaged by
chemicals or radiation, the distribution of genetic material
between the two-daughter nuclei during cell division is affected,
and pieces or entire chromosomes fail to be included in either of
the two-daughter nuclei. Genetic material that is not incorporated
into a new nucleus forms its own ‘micronucleus’. Micronucleus (MN)
frequency in cells not labeled with BrdU was examined by counting
the micronuclei in the binuclear cells that showed only red
fluorescence. The MN frequency was defined as the ratio of the
number of micronuclei in the binuclear cells to the total number of
binuclear cells observed (15). MN
frequency may also be used to detect sensitivity to
chemotherapeutic agents (15).
The ratios obtained in tumors not pre-treated with
BrdU revealed the MN frequency at all phases in the total (P + Q)
tumor cell population. More than 400 (444±31) binuclear cells were
counted to determine MN frequency. The MN frequencies in control
cells receiving absolutely no treatment were 0.056±0.005 and
0.081±0.011, with respect to the total tumor and Q cell populations
in the SCC VII tumors. For baseline correction, the induced MN
frequency (the MN frequency of the treated tumors minus that of the
non-treated control tumors) was used to exclude the MN frequency in
control cells receiving absolutely no treatment.
Clonogenic cell survival assay
The clonogenic cell survival assay was also
performed in the mice not pre-treated with BrdU using an in
vivo-in vitro assay method. Tumors were excised from the mice,
minced and disaggregated by stirring for 20 min at 37°C in PBS
containing 0.05% trypsin and 0.02% EDTA. Through these procedures,
single tumor cell suspensions were obtained from the whole tumor.
The cell yield was (4.5±1.1)×107/g tumor weight.
Appropriate numbers of viable tumor cells from the single cell
suspension were plated on 60 or 100-mm tissue culture dishes. After
12 days, the colonies were fixed with ethanol, stained with Giemsa
and counted. Plating efficiency under the no treatment control
condition was 52.0±4.5%.
The sensitivity of Q cells was assessed in terms of
the MN frequency using immunofluorescence staining for BrdU, while
the sensitivity of the total (P + Q) tumor cells was determined
based on a comparison with the non-BrdU-treated tumors based on MN
frequency and clonogenic cell survival.
Four mice were used to assess each set of
conditions, and each experiment was repeated at least twice. To
examine the differences between pairs of values, the Student's
t-test was used when variances between the two groups were assumed
to be equal; otherwise, Welch's t-test was used. P-values were
determined by two-sided tests.
Results
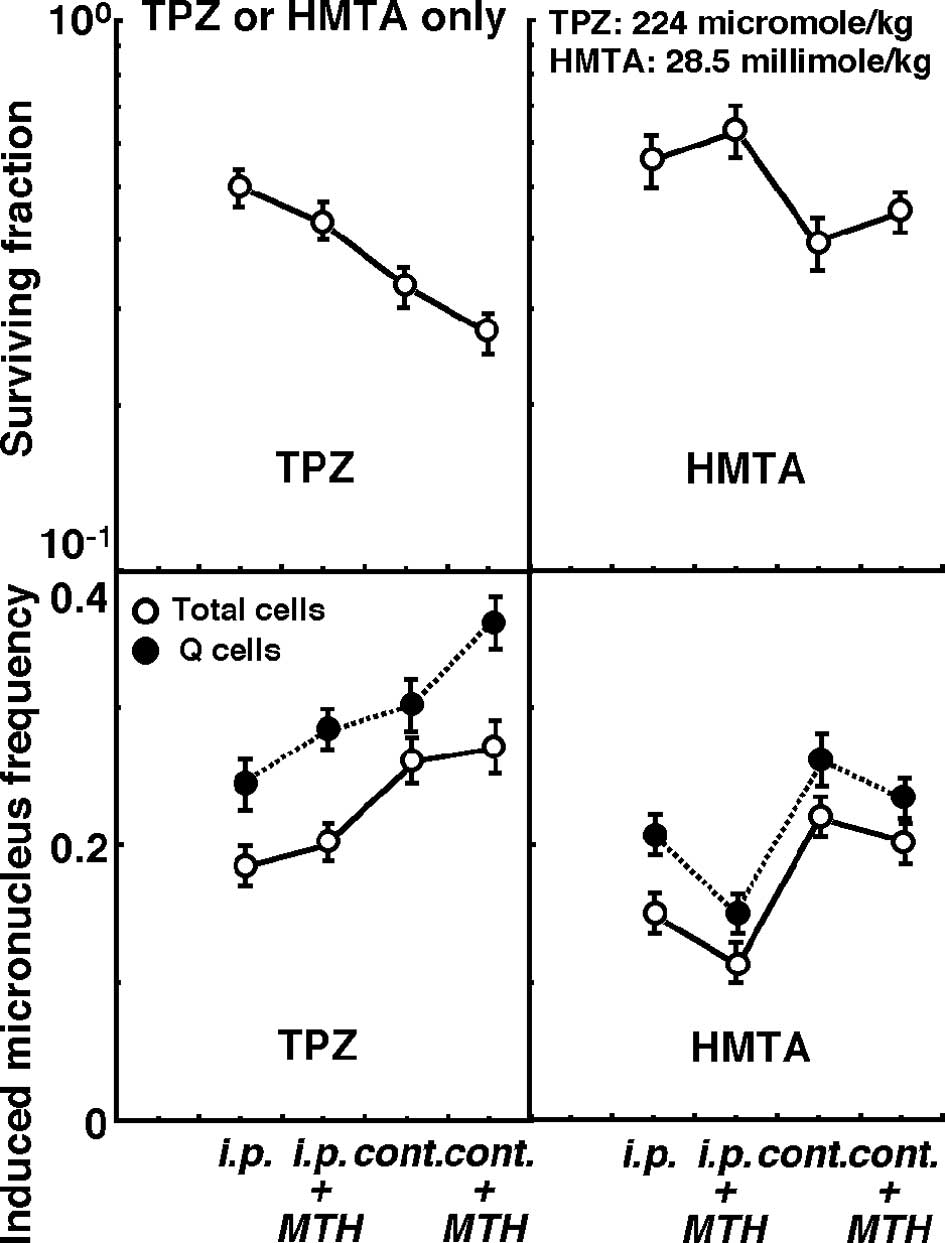
Changes in the clonogenic cell surviving fraction of
the total tumor cell population (upper panels), as well as changes
in the induced MN frequencies of the total and Q cell populations
(lower panels) following intraperitoneal single or subcutaneous
24-h continuous administration of TPZ (left panels) or HMTA (right
panels) in combination with or without MTH (40°C, 60 min) are shown
in Fig. 2.
Under all conditions, the sensitivity of the Q cells
was significantly higher than that of the total cells, regardless
of whether TPZ or HMTA was used (P<0.05). Concerning both TPZ
and HMTA, sensitivity was significantly higher with continuous
administration than with intraperitoneal single administration
(P<0.05). Sensitivity to TPZ was enhanced by the combination
with MTH, whether TPZ was administered by single or continuous
administration. In contrast, sensitivity to HMTA was decreased in
combination with MTH.
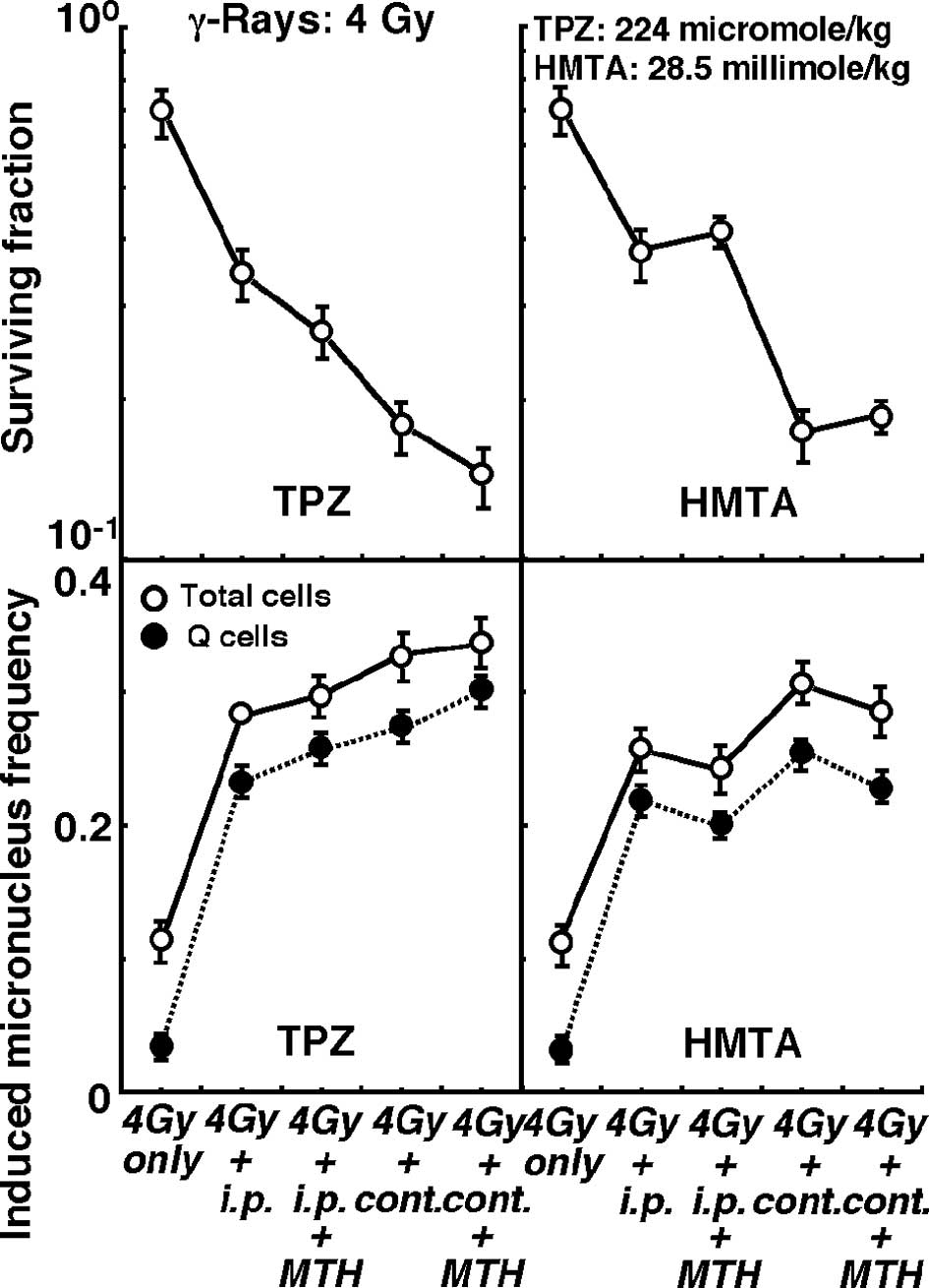
Changes in the clonogenic cell surviving fraction of
the total tumor cell population (upper panels) and the induced MN
frequencies of the total or Q cell populations (lower panels)
immediately after γ-ray irradiation in combination with or without
pre-irradiation intraperitoneal single or subcutaneous continuous
administration of TPZ (left panels) or HMTA (right panels) in
further combination with or without MTH, are shown in Figs. 3 (4 Gy irradiation dose) and 4 (20
Gy irradiation dose).
Concerning both TPZ and HMTA, subcutaneous
continuous administration compared with intraperitoneal single
administration induced significantly higher sensitivity to γ-rays
at doses of both 4 and 20 Gy in the total and the Q cells
(P<0.05). Regardless of whether TPZ was administered by single
or continuous administration, with γ-ray irradiation at doses of 4
and 20 Gy, the combination with MTH enhanced γ-ray sensitivity,
especially in the Q cells. In contrast, γ-ray sensitivity was
reduced by HMTA in combination with MTH, especially in the Q
cells.
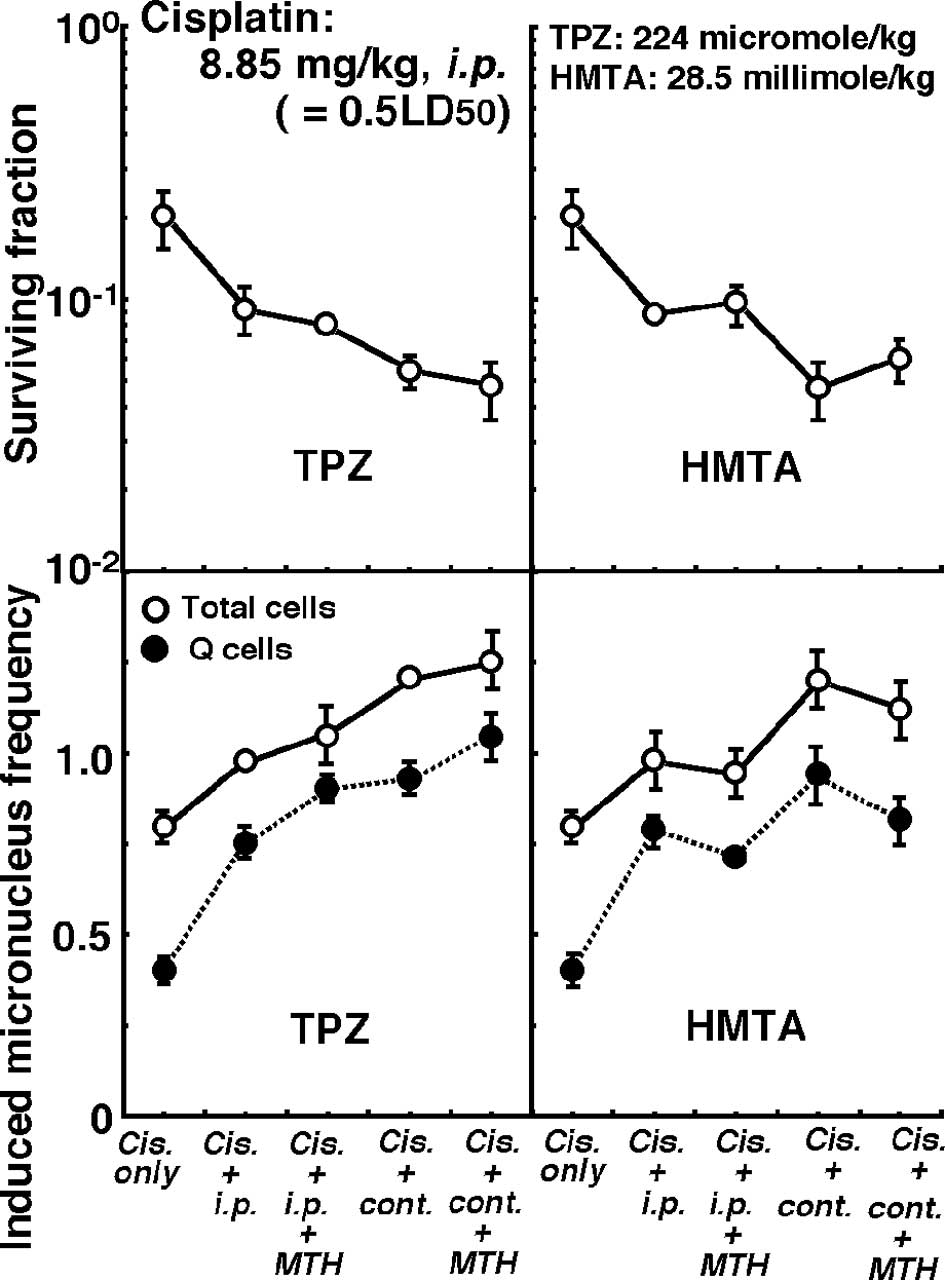
Changes in the clonogenic cell surviving fraction of
the total tumor cell populations (upper panels) and the induced MN
frequencies of the total or Q cell populations (lower panels) 1 h
after intraperitoneal single administration of cisplatin in
combination with or without pre-treatment intraperitoneal single or
subcutaneous continuous administration of TPZ (left panels) or HMTA
(right panels) in further combination with or without MTH are shown
in Fig. 5.
Regardless of whether TPZ or HMTA was used,
subcutaneous continuous administration induced significantly higher
sensitivity to cisplatin in both the total and Q cells compared to
intraperitoneal single administration (P<0.05). Concerning both
single or continuous administration of TPZ, the combination with
MTH enhanced cisplatin sensitivity, especially in the Q cells. In
contrast, cisplatin sensitivity in combination with HMTA was
reduced in further combination with MTH, especially in the Q
cells.
Without MTH, the enhancing effects of continuous
administration compared with intraperitoneal single administration
were more marked in total cells than in Q cells, regardless of
whether HMTA or TPZ was administered in combination with γ-ray
irradiation or cisplatin treatment. In cells treated with TPZ or
HMTA alone, both drugs had nearly the same cytotoxic effect in
terms of cell survival and induced MN frequency when administered
by intraperitoneal single administration.
Discussion
Formaldehyde is a naturally occurring biological
compound present in all tissues, cells and bodily fluids (13). It functions as a key intermediate
in the ‘one-carbon pool’ used for the biosynthesis of purines,
thymidine and some amino acids. It is usually rapidly metabolized
by reduction, oxidation and reduced glutathione-dependent pathways
(13). However, saturation in
formaldehyde metabolism leads to DNA damage. It has been shown that
cells exposed to formaldehyde exhibit, as a major form of DNA
damage, DNA-protein crosslinks (18). In a limited oxygen environment,
pyruvate generated by glycolysis in the cell cytoplasm is
preferentially converted into lactic acid by lactate dehydrogenase,
which creates a low pH environment (pH 6.4–6.8) (19). Furthermore, through the Warburg
effect, many cancer cells vigorously consume glucose and
preferentially produce lactic acid, even in the presence of
adequate oxygen (19). Thus, due
to the extracellular acidic conditions adjacent to solid tumors, an
acid-dependent formaldehyde donor, HMTA, dissociates to release
formaldehyde into nearby tissues.
HMTA has been employed as an antiseptic for the
treatment of urinary tract infections, and has been studied in
patients with maxillofacial phelegmons and for use as a
prophylactic agent against recurrent acute cystitis (13,14).
HMTA has been demonstrated to be well tolerated, even at doses of
up to 5 g/kg/day (14,20). Since HMTA is known to hydrolyze
under cellular conditions and to release 6 molecules of
formaldehyde in a pH-dependent manner (14), we examined HMTA for its potential
as a formaldehyde-releasing prodrug for damaging DNA within a tumor
cell. As shown in our previous report, γ-ray irradiation and
cisplatin treatment combined with continuous HMTA or TPZ
administration is promising in terms of the tumor cell-killing
effect as a whole (including Q cells) (16).
The MN frequencies of cells from tumors treated with
MTH only showed that MTH was not capable of inducing direct thermal
cytotoxicity. It has also been reported that this level of mild
heating cannot delay tumor growth or cause direct thermal
radiosensitization (17). In a
previous study, MTH mainly oxygenated the chronically hypoxic
fraction, though it had less of an impact than carbogen inhalation
(15). Moreover, it has been shown
that cells intermediate in oxygenation are able to influence tumor
response to radiation, and that such cells and hypoxic cell
cytotoxins potentially constitute a significant proportion of solid
tumors (21). Thus, it has been
suggested that MTH changes certain chronically hypoxic fractions to
a level intermediate between fully oxygenated and hypoxic through
an increase in tumor blood flow, and at the same time distributes
higher doses of TPZ and kills cells at these intermediate oxygen
tensions (21). Indeed, MTH has
been demonstrated to induce an increase in tumor pO2
(4), particularly in the
oxygenation of Q cell populations (15), resulting from an improvement in the
supply of oxygen via an increase in tumor blood flow. MTH has also
been shown to sensitize total cells and, in particular, Q cells to
the toxicity of TPZ when combined with or without γ-ray irradiation
and/or cisplatin (15). The
present study also demonstrated that the combination with MTH is
useful for enhancing TPZ toxicity in total cells and, in
particular, Q cells, when combined with or without γ-ray
irradiation or cisplatin treatment.
The Warburg effect refers to the observation that
most cancer cells predominantly produce energy by glycolysis
followed by lactic acid fermentation in the cytosol, rather than by
oxidation of pyruvate in mitochondria like most normal cells
(19). At present, the Warburg
effect is thought to be i) the result of damage to the mitochondria
in cancer, ii) an adaptation to low-oxygen environments within
tumors, or iii) the result of cancer genes shutting down the
mitochondria because they are involved in a cell apoptosis program
that would otherwise kill cancerous cells (22). Since the use of MTH can improve
intratumor oxygen supply via an increase in tumor blood flow, the
Warburg effect is suppressed with MTH treatment. Thereby, the
production of formaldehyde from HMTA under acidic conditions is
also suppressed, resulting in a decrease in the amount of
DNA-protein crosslink as a major form of DNA damage through
exposure to formaldehyde. Thus, whether combined with or without
γ-ray irradiation or cisplatin treatment, sensitivity to HMTA was
reduced in combination with MTH in total and Q cells. This was
particularly the case in Q cells, due to greater improvements in
the oxygen supply through MTH in the Q compared to total cell
population (15). Furthermore,
whether γ-ray irradiation was administered at a dose of 4 or 20 Gy,
further combination with MTH on the combined effect of radiation
with HMTA or TPZ had nearly the same effect. This means that the
effect of combination with MTH is almost interchangeable,
irrespective of radiation fraction size in fractionated
radiotherapy. The findings observed in this study can be applied to
fractionated radiotherapy with small fraction size, such as
practically performed radiotherapy.
It has previously been shown that total- and Q cell
populations in SCC VII tumors are rich in acutely and chronically
hypoxic fractions, respectively (15). Therefore, continuously administered
HMTA or TPZ was more cytotoxic to tumor cells in vivo than
single intraperitoneal administration, since the sensitizing effect
on the acutely hypoxia-rich total cell population was added to the
effect had on the chronically hypoxia-rich Q cell population
(9). This is why the enhancement
observed with continuous administration compared to single
administration was more marked in the total cell population than in
Q cells.
The presence of Q cells is thought to be due, in
part, to hypoxia and the depletion of nutrition in the tumor core.
This is another consequence of poor vascular supply (23), and might promote the formation of
micronuclei in Q tumor cells, even without any treatment.
Essentially, Q cells showed less sensitivity to γ-ray irradiation
and cisplatin treatment (15),
which means more Q cells than P cells survive after conventional
radiotherapy or chemotherapy. Thus, the control of Q cells has a
great impact on the outcome of anticancer treatment. As a result,
conventional radiotherapy or chemotherapy in combination with HMTA
or TPZ, which in and of themselves have significantly more toxicity
to Q cells than to the total cell population, is thought to be
useful. Taking into account that HMTA has a history of clinical use
as an antiseptic for urinary tract infections (13,14),
it may have more potential than TPZ to be employed with
conventional anticancer therapy. However, studies on the toxicity
of HMTA in normal tissue must still be carried out for safety
assurance. In terms of the tumor cell-killing effect as a whole,
including intratumor Q cell control, continuously administered HMTA
in combination with conventional radiotherapy and chemotherapy may
be a promising treatment modality for refractory tumors due to its
advantageous cytotoxic effects. However, HMTA combined with MTH
should not be employed, due to a reduction in the radiosensitizing
or chemotherapy sensitivity-enhancing effect observed when HMTA is
used as a combined agent in conventional radiotherapy and
chemotherapy.
Acknowledgements
This study was supported, in part, by
a grant-in-aid for Scientific Research (C) (20591493) from the
Japan Society for the Promotion of Science.
References
|
1.
|
Hall EJ and Roizin-Towle L: Biological
effects of heat. Cancer Res. 44:4708–4713. 1984.PubMed/NCBI
|
|
2.
|
Sapareto SA and Dewey WC: Thermal dose
determination in cancer therapy. Int J Radiat Oncol Biol Phys.
10:787–800. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Oleson JR, Samulski TV, Leopold KA, et al:
Sensitivity of hyperthermia trial outcomes to temperature and time:
Implications for thermal goals of treatment. Int J Radiat Oncol
Biol Phys. 25:289–297. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Song CW, Park HJ, Lee CK and Griffin R:
Implications of increased tumour blood flow and oxygenation caused
by mild temperature hyperthermia in tumor treatment. Int J
Hyperthermia. 21:761–767. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Armour EP and Raaphorst GP: Long duration
mild temperature hyperthermia and brachytherapy. Int J
Hyperthermia. 20:175–189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kong G, Braun RD and Dewhirst MW:
Hyperthermia enables tumor-specific nanoparticle delivery: effect
of particle size. Cancer Res. 60:4440–4445. 2000.PubMed/NCBI
|
|
7.
|
Li CY and Dewhirst MW:
Hyperthermia-regulated immunogene therapy. Int J Hyperthermia.
18:586–596. 2002.PubMed/NCBI
|
|
8.
|
Kinuya S, Yokoyama K, Michigishi T and
Tonami N: Optimization of radioimmunotherapy interactions with
hyperthermia. Int J Hyperthermia. 20:190–200. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Masunaga S, Nagasawa H, Uto Y, et al: The
usefulness of continuous administration of hypoxic cytotoxin
combined with mild temperature hyperthermia, with reference to
effects on quiescent tumour cell populations. Int J Hyperthermia.
21:305–318. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Brown JM: Evidence of acutely hypoxic
cells in mouse tumours and a possible mechanism of reoxygenation.
Br J Radiol. 52:650–656. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
McKeown SR, Cowen RL and Williams KJ:
Bioreductive drugs: from concept to clinic. Clin Oncol. 19:427–442.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Adam M, Bayer C, Henke J, Grosu A, Molls M
and Nieder C: Tirapazamine plus cisplatin and irradiation in a
mouse model: improved tumor control at the cost of increased
toxicity. J Cancer Res Clin Oncol. 134:137–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ridpath JR, Nakamura J, Tano K, et al:
Cells deficient in the Fanc/Brac pathway are hypersensitive to
plasma levels of formaldehyde. Cancer Res. 67:11117–11122. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Swift LP, Cutts SM, Rephaeli A, Nudelman A
and Phillips DR: Activation of adriamycin by the pH-dependent
formaldehyde-releasing prodrug hexamethylenetetramine. Mol Cancer
Therapeut. 2:189–198. 2003.PubMed/NCBI
|
|
15.
|
Masunaga S and Ono K: Significance of the
response of quiescent cell populations within solid tumors in
cancer therapy. J Radiat Res. 43:11–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Masunaga S, Tano K, Watanabe M, et al:
Evaluation of the potential of hexamethylenetetramine, compared
with tirapazamine, as a combined agent with γ-irradiation and
cisplatin treatment in vivo. Br J Radiol. 82:392–400.
2009.PubMed/NCBI
|
|
17.
|
Nishimura Y, Ono K, Hiraoka M, et al:
Treatment of murine SCC VII tumors with localized hyperthermia and
temperature-sensitive liposomes containing cisplatin. Radiat Res.
122:161–167. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Hubal EA, Schlosser PM, Conolly RB and
Kimbell JS: Comparison of inhaled formaldehyde dosimetry
predictions with DNA-protein cross-link measurements in the rat
nasal passages. Toxicol Appl Pharmacol. 143:47–55. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kim JW and Dang CV: Cancer's molecular
sweet tooth and the Warburg effect. Cancer Res. 66:8927–8930.
2006.
|
|
20.
|
Iskandarova GT: Hygienic rationale for
maximum permissible concentration of hexamethylenetetramine salt of
2-chloroethyl-phosphonic acid. Gig Sanit. 10:14–17. 1993.PubMed/NCBI
|
|
21.
|
Wouters BG and Brown JM: Cells at
intermediate oxygen levels can be more important than the ‘hypoxic
fraction’ in determining tumor response to fractionated
radiotherapy. Radiat Res. 147:541–550. 1997.
|
|
22.
|
Bertram JS: The molecular biology of
cancer. Mol Aspects Med. 21:167–223. 2000. View Article : Google Scholar
|
|
23.
|
Vaupel P: Tumor microenvironmental
physiology and its implications for radiation oncology. Semin
Radiat Oncol. 14:197–275. 2004. View Article : Google Scholar : PubMed/NCBI
|