Introduction
Surface epithelial carcinomas are the most common
type of ovarian cancer and the most lethal gynecological
malignancy. Epithelial ovarian cancer (EOC) comprises the majority
of malignant ovarian tumors in adult women, and its high mortality
is mainly due to late diagnosis (1). Serous epithelial carcinoma, the
primary type of ovarian cancer, usually presents at advanced stage.
Endometrioid, mucinous and clear-cell carcinomas, accounting for
non-serous carcinomas, more often appear as low-stage disease
(1,2). In advanced stages, the response rate
to first-line chemotherapy with a platinum combination after
surgical resection is approximately 80%, with 40–60% complete
response. However, the median progression-free survival is only 18
months in these patients, as most relapse. The overall response
rate in platinum-refractory or drug-resistant tumors is only 10–25%
with subsequent relapse, resulting in a 5-year survival of only 25%
(3). In addition, clinicians have
long known that different subtypes of ovarian cancer respond
differently to treatment and have different prognoses. High-grade
serous ovarian cancers typically harbor mutations in p53. These
cancers also have mutations of BRCA1 or BRCA2, as well as defective
homologous recombination, the preferred mechanism of the DNA
double-strand break repair pathway (3,4).
EOC, a morphologically and biologically heterogeneous disease, is
affected by various gene activations and alterations (2–5).
Current studies suggest that the different
histological types of EOC represent distinct disease entities and
exhibit varied gene expression patterns (6,7).
Thus, a better understanding of the molecular basis of EOC subtypes
and identification of a reliable gene expression profile for each
type is necessary to uncover the fundamental mechanisms of
carcinogenesis and to predict prognosis, and may provide
therapeutic guidance in these diseases (8).
DNA microarray research is widely applied in
determining gene expression profiles and has opened the field for
analysis of the expression levels of thousands of genes (9). However, a more accurate technique is
needed for a better-tailored approach to identify special targets
of diagnostic and prognostic markers that predict patient response
to chemotherapy and survival outcomes. We used real-time
relative-quantity (RQ)-PCR assay to illustrate a gene expression
signature and to distinguish expression patterns in EOC subtypes in
a large patient sample panel. Based on the function of targeted
genes and their expression patterns, we identified ILF3 and
UBE2I as potential biomarkers for this disease.
Materials and methods
Human tissue specimens
Forty-eight pairs of ovarian specimens (tumor and
adjacent normal tissues), plus an additional 35 tumors from a total
of 83 patients with advanced ovarian cancer were obtained from the
Cooperative Human Tissue Network (CHTN), Pediatric Division,
Children's Hospital, Columbus, OH, USA. Tumor and normal samples
were collected at primary surgery prior to chemotherapy, flash
frozen in liquid nitrogen, and stored at −80°C until RNA/DNA
extraction. All samples were evaluated by pathologists, and the 83
tumors were classified as serous carcinoma (n=51), endometrioid
carcinoma (n=13), mucinous carcinoma (n=11) and clear cell
carcinoma (n=8).
RNA extraction
Total RNA from each specimen and the normal control
of the ovarian cancer patients was extracted and purified by the
method of hot phenol/chloroform extraction as previously reported
(10). Isolated RNA was purified
and dissolved in DEPC water and stored at −80°C.
Oligo synthesis
Following a search of the gene database, we selected
a total of 50 genes based on their functions and the expression
literature in ovarian, breast and lung cancers for this
investigation. Primary gene functions included transcription
factor, DNA/RNA and protein binding activity, gene activation and
regulation. Forward and reverse primers for each of the 50 genes
were synthesized by Gene Probe Technologies, Inc. (Gaithersburg,
MD, USA). Primer sequences of the 4 targeted genes used in
real-time quantitative PCR assay are listed in Table I.
 | Table I.Primer sequences of the 4 targeted
genes used in real-time quantitative PCR. |
Table I.
Primer sequences of the 4 targeted
genes used in real-time quantitative PCR.
| Gene symbol | Gene ID | Forward primer | Reverse primer |
|---|
| TAL2 | 6887 |
5′-tcaccctccagacaaaaagc-3′ |
5′-ccaggtgaaggaacctggta-3′ |
| EGF | 1950 |
5′-aggtggctggaagcctttat-3′ |
5′-tgtggacagaacctccatca-3′ |
| ILF3 | 3609 |
5′-ctggtgctgctgtgtaagga-3′ |
5′-agggacaatggaggctcttt-3′ |
| UBE2I | 7329 |
5′-caggagaggaaagcatggag-3′ |
5′-tcgggtgaaataatggtggt-3′ |
Reverse transcription-PCR
Through reverse transcription (RT), using the Super
Script Preamplification System (Life Technologies, Inc.), cDNA was
generated with oligo-dT primers from 5 μg of total RNA of 83
ovarian tumor tissues and 48 adjacent normal samples. RT-PCR was
performed using AmpliTaq DNA polymerase, FS cells and gene-specific
primers of the 50 genes in all cDNA samples. PCR amplicons were
separated by 1% agarose gel electrophoresis, and visible density
bands of the gene amplification in these samples were indicative of
gene expression. Genes expressed in RT-PCR detection were selected
for subsequent real-time quantitative PCR analysis.
Real-time quantitative PCR
Real-time RQ-PCR was performed using
SYBR® Green reagent kit (ABI Cat. 4367659) according to
manufacturer's recommendations. This assay was used for 20 selected
genes in 30 EOC specimens for the gene expression signature
profile. Amplifications were carried out on ABI PRISM 7000
Detection System and analyzed by ABI 7500 software (PE Applied
Biosystems, Foster City, CA, USA).
In brief, all reactions were optimized to obtain the
best amplification kinetics under the same cycling conditions (10
min at 95°C, 40 cycles of 15 sec at 95°C, and 1-min at 60°C). The
composition of the reaction mixture in a final volume of 20 μl
contained Power SYBR Green Master Mix 10 μl, gene-specific primers
(10 μM) 1 μl and cDNA 2 μl. Negative controls
containing all PCR components without template DNA (denoted NTC)
were used to ensure that the reagent mix was free of contamination.
Each reaction was run in triplicate. The average threshold cycle
(Ct) and the comparative ΔΔCt method were automatically calculated
for the expression of the gene and normalized to the mean Ct-value
of 18S ribosomal. The RQ-value was calculated using the ΔΔCt
method. Fold change in gene expression was calculated as
2−ΔΔCt. For each gene in this study, a
Ct-value <32 was considered as positive expression and vice
versa.
Statistical analysis
In real-time RQ-PCR assays, the average Ct, ΔCt,
ΔΔCt and RQ were calculated by Applied Biosystems Sequence
detection software (7500 Fast System SDS Software version 1.4). RQ
(ΔΔCt value) was used to compare gene expression between samples.
Since RQ equals 2−ΔΔCt, a higher negative
ΔΔCt value, represents higher expression. SPSS 15.0 software was
used to determine statistical significance. A P-value <0.05 was
considered statistically significant between test means.
Results
Patient characteristics
Forty-eight pairs of ovarian specimens (tumor and
adjacent normal tissues) plus an additional 35 tumors (from a total
of 83 ovarian cancer patients) were assessed in this investigation.
The clinicopathological characteristics of the 83 EOC patients in
this study are shown in Table II.
The median age of the patients at diagnosis was 59 years (range,
37–83 years). Approximately 65.1% of the patients (54 out of 83)
were diagnosed with advanced stage tumors (FIGO stages III/IV), and
84.3% (70 out of 83) had moderately poorly differentiated tumors
(grades 2 and 3).
 | Table II.Clinicopathological characteristics
of the EOC cases. |
Table II.
Clinicopathological characteristics
of the EOC cases.
| Patient no. | Age range
(years) | Histological
type | FIGO stage at
diagnosis
| Tumor grade
|
|---|
| I | II | III | IV | G1 | G2 | G3 |
|---|
| 51 | 40–83 | Serious | 3 (5.88%) | 2 (3.92%) | 42 (82.35%) | 4 (7.84%) | 3 (5.88%) | 9 (17.65%) | 39 (76.47%) |
| 11 | 40–74 | Mucinous | 7 (63.64%) | 3 (27.27%) | 1 (45.45%) | 0 | 5 (36.36%) | 4 (18.18%) | 2 (9.09%) |
| 13 | 37–76 | Endometrioid | 4 (30.77%) | 3 (7.69%) | 6 (46.15%) | 0 | 2 (15.38%) | 4 (30.77%) | 7 (53.85%) |
| 8 | 48–77 | Clear cell | 6 (75%) | 1 (12.50%) | 1 (12.50%) | 0 | 3 (37.50%) | 1 (12.50%) | 4 (50%) |
Reproducible gene expression identified
in the studied samples
Initially, we selected 50 genes based on their
functions and the expression literature. Using reverse
transcription-polymerase chain reaction assay (RT-PCR), 39 of the
50 genes were expressed by showing gel electrophoresis density; the
remaining 11 genes were not expressed (data not shown). Of the 39
expressed genes, 20 demonstrated dominant and reproducible
expression among the samples. These 20 genes were further evaluated
in 30 EOC specimens by real-time quantitative PCR. The functions of
these 20 genes are summarized in Table
III.
 | Table III.Function of 20 dominant and
reproducible genes expressed in the studied ovarian specimens. |
Table III.
Function of 20 dominant and
reproducible genes expressed in the studied ovarian specimens.
| Gene symbol | Gene name | Function |
|---|
| RAD52 | RAD52 homolog
(S. cerevisiae) | DNA double-strand
break repair and homologous recombination |
| RPUSD2 | RNA pseudouridylate
synthase domain containing 2 | Pseudouridine
synthase activity |
| SEH1L | SEH1-like (S.
cerevisiae) | Intracellular
protein transport across a membrane |
| SLC25A5 | Solute carrier
familly 25 member 5 | Adenine
transmembrane transporter |
| PLSCR1 | Phospholipid
scramblase 1 | Phospholipid
scramblase activity |
| INPPL1 | Inositol
polyphosphate phosphatase-like 1 | Inositol or
phosphatidylinositol phosphatase activity |
| TXNRD1 | Thioredoxin
reductase 1 | Protein disulfide
oxidoreductase activity, thioredoxin-disulfide reductase
activity |
| SSBP1 | Single-stranded DNA
binding protein 1 | Housekeeping gene
involved in mitochondrial biogenesis |
| FAT | Fat tumor
suppressor homolog 1 (Drosophila) | Calcium ion
binding, protein binding |
| SMARCD2 | SWI/SNF-related,
matrix-associated, actin-dependent regulator of chromatin,
subfamily d, member 2 | Transcription
coactivator activity protein binding |
| MAP2K2 | Mitogen-activated
protein kinase 2 | Protein
serine/threonine kinase activity, protein tyrosine kinase activity,
transferase activity |
| HNRNPA3 | Heterogeneous
nuclear ribonucleoprotein A3 | Nuclear mRNA
splicing, via spliceosome |
| MSX2 | msh homeobox 2 | Transcription
factor activity |
| TAL2 | T-cell acute
lymphocytic leukemia 2 | Transcription
regulator activity |
| EGF | Epidermal growth
factor | Epidermal growth
factor receptor activating ligand activity, calcium ion
binding |
| ZNF71 | Zinc finger protein
71 | Metal ion binding,
zinc ion binding |
| ILF3 | Interleukin
enhancer binding factor 3 | Double-stranded RNA
binding, transcription regulation |
| UBE2I |
Ubiquitin-conjugating enzyme E2I (UBC9
homolog, yeast) | Post-translational
protein modification, ubiquitin-dependent protein catabolic
process |
| INSR | Insulin
receptor | Insulin receptor
activity, phosphoinositide 3-kinase binding receptor signaling
protein tyrosine kinase activity |
| NP220 | Zinc finger protein
638 | Double-stranded DNA
binding, metal ion binding, zinc ion binding |
Expression patterns of TAL2, EGF, ILF3
and UBE2I in normal and EOC specimens
Using real-time RQ-PCR we further identified four
genes (TAL2, EGF, ILF3 and UBE2I) among the 20
selected genes for extensive study. These genes demonstrated
distinct expression patterns among the EOC subtypes. In brief,
TAL2 was expressed in 80 of the 83 EOC and all 48 normal
samples. EGF was expressed in 69 of the 83 EOC and 45 of the
48 normal samples. Seventy-one of the 83 EOC samples exhibited
ILF3 expression, and 54 of the 83 EOC exhibited UBE2I
expression. In comparison, ILF3 was expressed in 28 of the
48 and UBE2I was expressed in 12 of the 48 normal samples.
The differences in ILF3 and UBE2I expression between
the tumor and normal tissues were statistically significant
(P<0.05) (Table IV). As shown
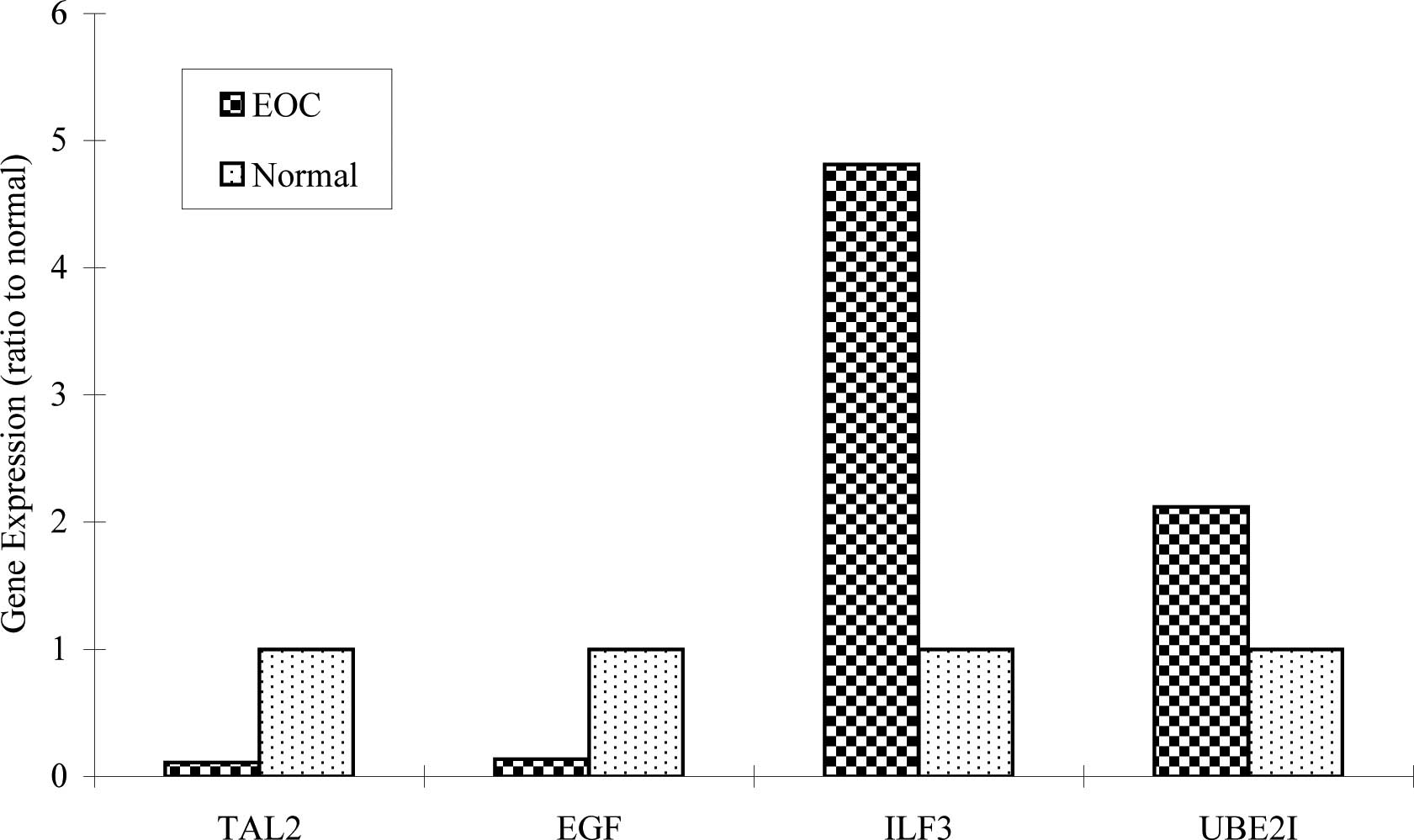
in Fig. 1, 4 genes were expressed
in all types of EOC and normal tissues. However, ILF3
expression in EOC was 4.8-fold higher than in the normal samples;
UBE2I expression in EOC was >2-fold higher compared to
that of the normal samples.
 | Table IV.Comparison of the rate of expression
between tumor and normal samples (Chi-square test). |
Table IV.
Comparison of the rate of expression
between tumor and normal samples (Chi-square test).
| TAL2 | EGF | ILF3 | UBE2I |
|---|
| EOC tumors
(83) | 96.4% (80/83) | 83.1% (69/83) | 85.5% (71/83) | 65.1% (54/83) |
| Normal samples
(48) | 100.0% (48/48) | 93.8% (45/48) | 58.3% (28/48) | 25.0% (12/48) |
| P-value | 0.468 | 0.081 |
<0.05 |
<0.05 |
The expression patterns of TAL2, EGF,
ILF3 and UBE2I in the histological types of EOC
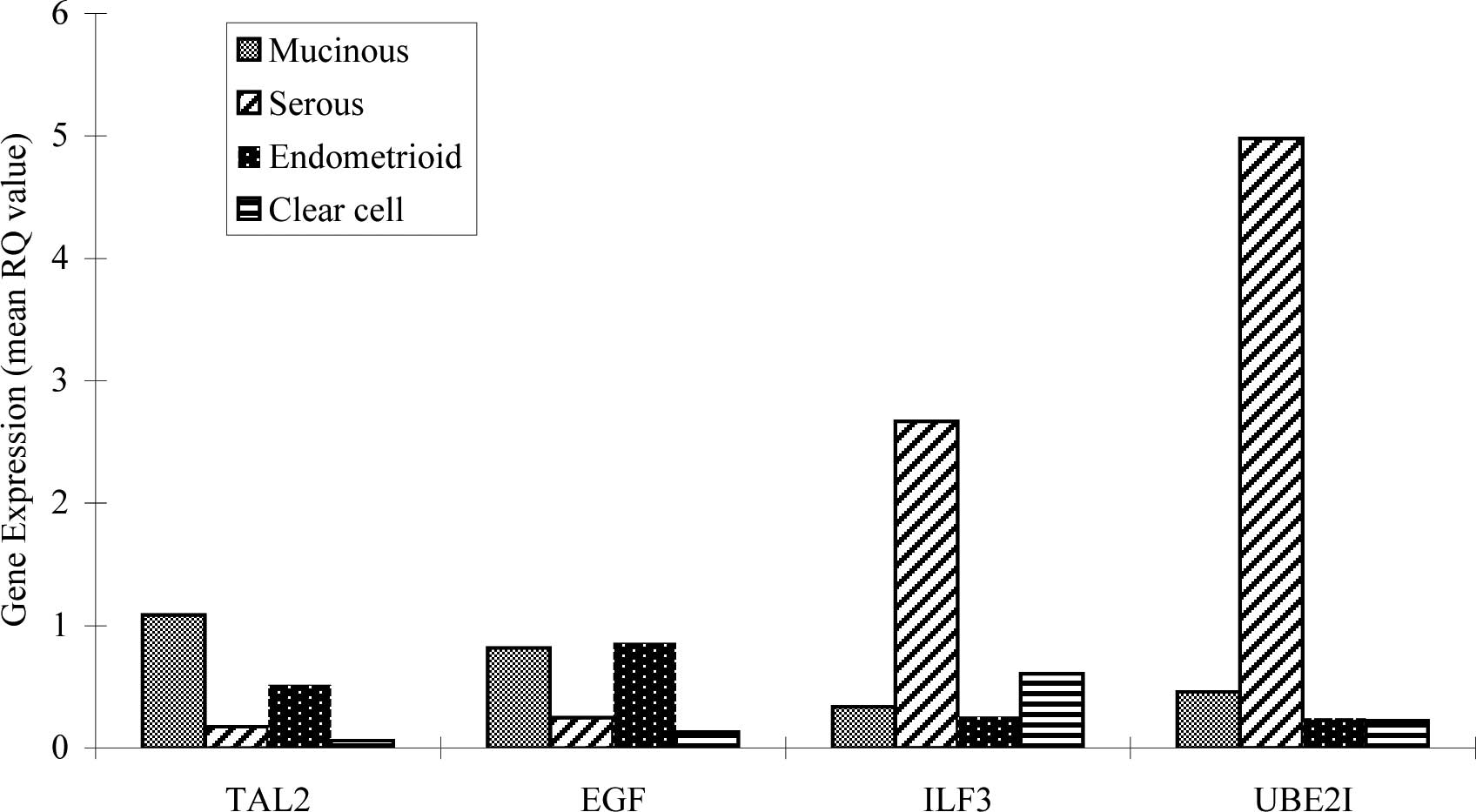
Fig. 2 shows the
mean RQ-value of TAL2, EGF, ILF3 and UBE2I in
histological types of EOC. TAL2, EGF, ILF3 and UBE2I
were expressed in all EOC subtypes. Interestingly, the mean RQ
value of ILF3 in the serous patients was 2.671 compared to
0.6 in clear cell, 0.256 in endometrioid and 0.336 in mucinous
carcinomas. The mean RQ value of UBE21 in serous patients
was 4.979 compared to 0.224 in clear cell, 0.243 in endometrioid
and 0.46 in mucinous carcinomas. In other words, ILF3 and
UBE2I showed extremely high expression in serous
carcinomas.
Association of expression patterns with
FIGO stage and tumor grade
The expression patterns of TAL2, EGF, ILF3
and UBE2I were correlated with FIGO stage and tumor grade
among 83 EOC tissues using real-time quantitative PCR analysis
(Table V). The mean RQ-values of
TAL2 and EGF in early stage or low grade and in
advanced disease were overall low. In contrast, the mean RQ for
ILF3 in advanced stage vs. early stage was 3.8; in poorly
differentiated (G2/G3) vs. well-differentiated (G1) this value was
9.34; for UBE2I, the RQ-value was 5.76 and 3.85. The
overexpression of ILF3 and UBE2I in advanced stage
and advanced grade indicates that these two genes may play an
important role in tumor progression and pathological
differentiation of this disease.
 | Table V.Association of expression patterns
with FIGO stage and tumor grade. |
Table V.
Association of expression patterns
with FIGO stage and tumor grade.
| Mean RQ-value | TAL2 | EGF | ILF3 | UBE2I |
|---|
| Early stage
(I–II) | 0.424 | 0.514 | 0.671 | 0.864 |
| Advanced stage
(III–IV) | 0.301 | 0.402 | 2.550 | 4.975 |
| Advanced stage vs.
early stage (fold) | 0.710 | 0.782 | 3.800 | 5.758 |
| G1 | 0.517 | 0.582 | 0.241 | 0.885 |
| G2–G3 | 0.310 | 0.400 | 2.251 | 3.406 |
| G2–G3 vs. G1
(fold) | 0.600 | 0.687 | 9.340 | 3.849 |
Discussion
TAL2, one of three transcription factors of
the basic helix-loop-helix (bHLH) family, was found at junctions of
chromosomal translocations associated with T-cell acute
lymphoblastic leukaemia (T-ALL). This gene was activated in the
chromosomal translocation t(7;9) (q35;q34) in a subset of T-ALL
patients causing overexpression in the T-cell lineage (11,12).
Thus, TAL2 is identified as an oncogenic transcription
factor of T-ALL (13). TAL2
expression in adult testes represents the gene's role in the
developing midbrain, diencephalon and anterior pons (11). Bucher et al reported that
TAL2 normally plays a pivotal role in brain development, and
that without this gene, mice cannot survive to maturity (12). In the present study, we report, for
the first time, TAL2 expression in normal human ovarian and
ovarian tumor tissues.
Epidermal growth factor (EGF) has a profound
effect on the differentiation of ovarian surface epithelial cells
by enhancing motility and inducing secretion of pro-MMP-2 and MMP-9
resulting in localized stimulation (14). EGF likely contributes to
ovarian surface epithelium (OSE) rapid post-ovulatory proliferation
and to the epithelio-mesenchymal conversion trapped in the ruptured
follicle. Failure of such function may lead to the formation of
epithelial inclusion cysts, which are known to be the preferential
sites of malignant transformation by generating a microenvironment
enriched of growth factors, cytokines and hormones to the entrapped
OSE (15). There are a few reports
regarding EGF expression in tumors. Stromberg and colleagues
reported that 10 of 10 (100%) borderline tumors and 10 of 14 (71%)
epithelial ovarian tumors expressed EGF (16). Niikura et al also observed
EGF expression in 18 of 25 (72%) studied epithelial ovarian
tumors (17). Our study of a large
sample panel (83 ovarian patients) demonstrated that EGF was
expressed in both normal and the four histological tumor subtypes,
which is consistent with other reports.
ILF3 (a protein known as NFAR or NF90), not
previously found in ovarian cancer, was originally identified as a
component of a dimeric transcription regulator and has a regulatory
function in vitro. More recent studies suggest that
ILF3 plays a role in transcriptional and
post-transcriptional regulation (18). NFAR or NF90 are ubiquitously
expressed in the nucleus of many cell types and tissues. They
interact with PKR and PKR-mediated signaling, and may be involved
in the mRNA processing in cells (19). Vumbaca et al have
demonstrated that the DRBP76/NF90 isoform facilitates the
expression of vascular endothelial growth factor (VEGF) by
promoting VEGF mRNA loading onto polysomes and translation under
hypoxic conditions, thus promoting breast cancer growth and
angiogenesis in vivo (20).
VEGF, the key angiogenic factor expressed under restricted nutrient
and oxygen conditions in most solid tumors, is up-regulated in
ovarian tumors and promotes tumor cell growth, migration and
survival (21,22). Thus, ILF3 may play a role in
tumorigenesis of EOC via regulation of VEGF expression. In our
study, we observed that ILF3 was overexpressed in serous
carcinoma. Serous carcinoma has the highest percentage of advanced
stage and poorly differentiated cases among these four subtypes. We
also found that ILF3 exhibited a higher expression trend in
advanced stage or poorly differentiated EOC, compared to early
stage or well-differentiated EOC.
UBE2I (Ubc9) is important for genome
integrity, particularly during mitosis and overall cell survival
(23). Recently, sumoylation,
small ubiquitin-related modifier (SUMO) conjugation, has been
identified as another type of protein modification. Ubc9, an
essential E2-conjugating enzyme for sumoylation, seems to play a
central role in sumoylation-mediated cellular pathways (24). In addition, several important DNA
repair enzymes are subject to sumoylation, which appears to be
involved in DNA damage/repair. Moreover, Ubc9/SUMO are recently
reported to have a fundamental effect in tumorigenesis and tumor
progression (23,25). Many oncoproteins and tumor
supressors including PML, MDM2, c-MYB, c-JUN and TP53 are involved
in SUMO (23–25). Our findings demonstrated that
UBE2I mRNA was up-regulated in serous carcinoma of the ovary
which is consistent with a report by Mo et al (24). The molecular mechanism by which
Ubc9 promotes tumor growth and interacts with other factors is as
yet unclear.
In conclusion, we provide the first evidence of
TAL2 and ILF3 expression in normal human ovary and
epithelial ovarian cancer. We demonstrated that UBE2I and
ILF3 expression was higher in advanced stage or poorly
differentiated tumors, compared to early stage or
well-differentiated EOC. Our results indicate that ILF3 and
UBE2I may play an important role in tumorigenesis of EOC.
Further investigations to elucidate the molecular mechanisms
involved in ILF3 and UBE2I activiation in EOC
development are warranted.
Acknowledgements
This study was supported by NIH Grant
RO3 CA107979-01 and the WVU Mary Babb Randolph Cancer Center,
Molecular Medicine Core Facility, with editorial assistance by
Michael D. Mueller.
References
|
1.
|
Shih IM and Kurman RJ: Ovarian
tumorigenesis. Am J Pathol. 164:1511–1518. 2004. View Article : Google Scholar
|
|
2.
|
Baranova A, Gowder S, Naouar S, et al:
Expression profile of ovarian tumors distinct signature of
Sertoli-Leydig cell tumor. Int J Gynecol Cancer. 16:1963–1972.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Yu JJ: Unlocking the molecular mechanisms
of DNA repair and platinum drug resistance in cancer chemotherapy.
Curr Drug Ther. 4:19–28. 2009. View Article : Google Scholar
|
|
4.
|
Bell DA: Origins and molecular pathology
of ovarian cancer. Mod Pathol. 18:19–32. 2005. View Article : Google Scholar
|
|
5.
|
Cecco LD, Marchionni L, Gariboldi M, et
al: Gene expression profiling of advanced ovarian cancer
characterization of a molecular signature involving fibroblast
growth factor 2. Oncogene. 23:8171–8183. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Tothill RW, Tinker AV, Joshy G, et al:
Novel molecular subtypes of serous and endometrioid ovarian cancer
linked to clinical outcome. Clin Cancer Res. 14:5198–5208. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Reed E, Yu JJ, Davies A, Gannon J and
Armentrout SL: Clear cell tumors have higher mRNA levels of ERCC1
and XPB than other histological types of epithelial ovarian cancer.
Clin Cancer Res. 9:5299–5305. 2003.PubMed/NCBI
|
|
8.
|
Schwartz DR, Kardia SLR, Shedden KA, et
al: Gene expression in ovarian cancer reflects both morphology and
biological behavior, distinguishing clear cell from other
poor-prognosis ovarian carcinomas. Cancer Res. 62:4722–4729.
2002.
|
|
9.
|
Fehrmann RS, Li XY, van Der Zee AG, de
Jong S, Te Meerman GJ, de Vries EG and Crijns AP: Profiling studies
in ovarian cancer: a review. The Oncologist. 12:960–966. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Yu JJ, Dabholkar M, Bennett WP, Welsh JA,
Mu CJ, Bostick-Bruton F and Reed E: Platinum-sensitive and
platinum-resistant ovarian cancer tissues show differences in the
relationships between mRNA levels of p53, ERCC1 and XPA. Int J
Oncol. 8:313–317. 1996.PubMed/NCBI
|
|
11.
|
Pinheiro P, Gering M and Patient R: The
basic helix-loop-helix transcription factor, TAL2, marks the
lateral floor plate of the spinal cord in zebrafish. Gene Expr
Patterns. 4:85–92. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Bucher K, Sofroniew MV, Pannell R, et al:
The T cell oncogene Tal2 is necessary for normal development of the
mouse brain. Dev Biol. 227:533–544. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ferrando AA, Neuberg DS, Staunton J, et
al: Gene expression signatures define novel oncogenic pathways in T
cell acute lymphoblastic leukemia. Cancer Cell. 1:75–87. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Wong AST and Leung PCK: Role of endocrine
and growth factors on the ovarian surface epithelium. J Obstet
Gynaecol Res. 33:3–16. 2007. View Article : Google Scholar
|
|
15.
|
Ahmed N, Maines-Bandiera S, Quinn MA,
Unger WG, Dedhar S and Auersperg N: Molecular pathways regulating
EGF-induced epithelio-mesenchymal transition in human ovarian
surface epithelium. Am J Physiol Cell Physiol. 290:1532–1542. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Stromberg K, Johnson GR, O'Connor DM,
Sorensen CM, Gullick WJ and Kannan B: Frequent immunohistochemical
detection of EGF supergene family members in ovarian
carcinogenesis. Int J Gynecol Pathol. 13:342–347. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Niikura H, Sasano H, Sato Sh and Yajima A:
Expression of epidermal growth factor-related proteins and
epidermal growth factor receptor in common epithelial ovarian
tumors. Int J Gynecol Pathol. 16:60–68. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Cazanove O, Batut J, Scarlett G, et al:
Methylation of XILF3 by Xprmt1b alters its DNA, but not RNA,
binding activity. Biochemistry. 47:8350–8357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Saunders LR and Barber GN: The dsRNA
binding protein family: critical roles, diverse cellular functions.
FASEB J. 17:961–983. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Vumbaca F, Phoenix KN, Rodriguez-Pinto D,
Han DK and Claffey KP: Double-stranded RNA-binding protein
regulates vascular endothelial growth factor mRNA stability,
translation and breast cancer angiogenesis. Mol Cell Biol.
28:772–783. 2008. View Article : Google Scholar
|
|
21.
|
Schumacher JJ, Dings RPM, Cosin J,
Subramanian IV, Auersperg N and Ramakrishnan S: Modulation of
angiogenic phenotype alters tumorigenicity in rat ovarian
epithelial cells. Cancer Res. 67:3683–3690. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Bermudez Y, Yang H, Saunders BO, Cheng JQ,
Nicosia SV and Kruk PA: VEGF- and LPA-induced telomerase in human
ovarian cancer cells is Sp1-dependent. Gynecol Oncol. 106:526–537.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Moschos SJ and Mo YY: Role of SUMO/Ubc9 in
DNA damage repair and tumorigenesis. J Mol Hist. 37:309–319. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Mo YY, Yu Y, Theodosiou E, Ee PL and Beck
WT: A role for Ubc9 in tumorigenesis. Oncogene. 24:2677–2683. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Dűnnebier T, Bermejo JL, Haas S, et al:
Common variants in the UBC9 gene encoding the SUMO-conjugating
enzyme are associated with breast tumor grade. Int J Cancer.
125:596–602. 2009.
|