Introduction
Perforation of gastric cancer is rare and it
accounts for less than 1% of the incidences of an acute abdomen
(1,2). It is difficult to identify the cause
of gastric perforation during emergency surgery, particularly when
a frozen section is unavailable. Even if the frozen section shows
malignant perforation, the surgeon should choose the optimal
surgical strategy, i.e., either a gastrectomy with lymphadenectomy
or local repair according to the severity of sepsis.
In this study, we reviewed cases of benign or
malignant gastric perforation in terms of the accuracy of diagnosis
and investigated the clinical outcome after emergency surgery for
patients with gastric cancer who had a free perforation.
Materials and methods
Patients
Between 1992 and 2007, 40 patients with perforation
caused by gastric ulcer or gastric cancer were admitted to the
National Defense Medical College Hospital. In all cases, the
presence of free perforation was confirmed by examination of chest
X-ray films and/or computed tomography (CT) scans. Patients with
perforation caused by endoscopic mucosal resection (EMR) or
endoscopic submucosal resection (ESD) were excluded from this
study. To compare the clinical outcomes in gastric cancer patients
with and without a free perforation, 196 patients with gastric
cancer who underwent gastrectomy between 1992 and 2007 and whose
tumors were classified as T3 of tumor depth without any evidence of
perforation were used as controls. Medical records were reviewed to
obtain information regarding patient demographics, surgical
procedure, postoperative morbidity and mortality, and long-term
survival. The pathological findings in patients with gastric cancer
were described on the basis of the Japanese Classification of
Gastric Carcinoma (3). For 2
patients with gastric cancer perforation who had not undergone a
gastrectomy, the clinical findings were described instead of the
pathological findings.
Statistical analysis
The data were expressed as the mean ± standard error
of the mean (SEM). Statistical analyses were performed using either
the Mann Whitney U test or the Chi-square test, and multivariate
analysis was performed using a Cox proportional hazards model. A
p-value of <0.05 was considered statistically significant. All
analyses were performed using the statistical software StatView
version 5.0 (SAS Institute, Inc., Cary, NC, USA).
Results
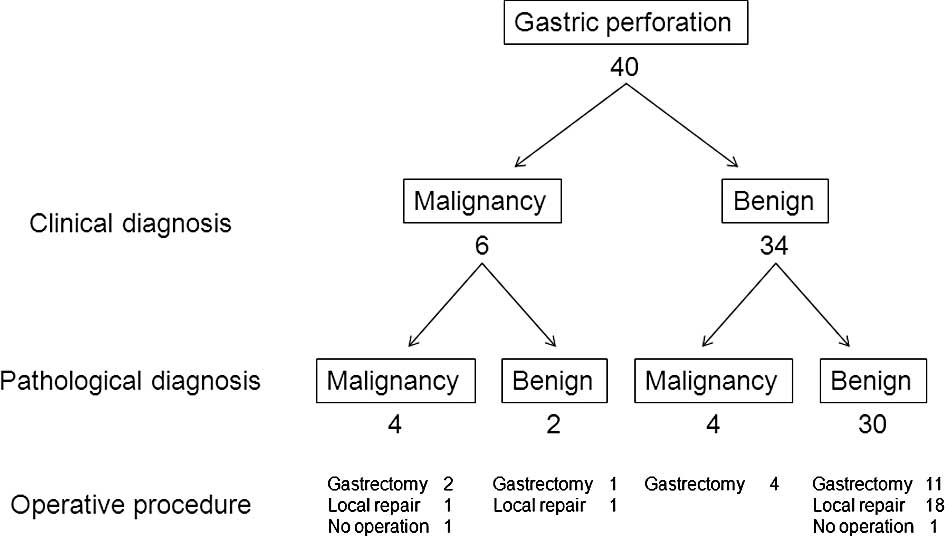
The clinical and pathological diagnoses for the 40
patients with free gastric ulcer or cancer perforation are shown in
Fig. 1. All patients were
diagnosed by pathological examination of the resected specimen or
biopsy specimen; none of them were diagnosed by examination of the
frozen section obtained during surgery. By pathological
examination, gastric cancer was diagnosed in 8 patients and benign
ulcer perforation in 32 patients. The sensitivity, specificity and
accuracy of intraoperative diagnosis by pathological examination
were 50, 93.8 and 85%, respectively. Patients with gastric ulcer
perforation (19 patients, 59.3%) underwent more frequent local
repair such as omental patch repair and omentopexy than those with
gastric cancer perforation (1 patient, 12.5%). No surgical
intervention was performed in the case of 2 patients: 1 patient
with gastric cancer perforation had general metastases and refused
surgery, and 1 patient with gastric ulcer perforation underwent
conservative therapy. The patients with gastric cancer perforation
were significantly older than those with gastric ulcer perforation
(Table I). There was no
significant difference between patients with gastric cancer and
benign ulcer perforation with regard to gender, location of
perforation, co-morbidity rate, white cell count on admission,
duration of postoperative stay in the hospital and postoperative
complications. Among the patients with gastric cancer perforation,
1 patient who underwent local repair died due to the development of
sepsis on postoperative day (POD) 16; 1 patient who underwent
subtotal gastrectomy died due to the rupture of an abdominal aortic
aneurysm on POD 5; 1 patient with benign ulcer who underwent local
repair died due to the development of sepsis on POD 27.
 | Table I.Clinicopathological characteristics of
patients with gastric perforation according to the cause of
perforation. |
Table I.
Clinicopathological characteristics of
patients with gastric perforation according to the cause of
perforation.
| Malignancy | Benign | P-value |
|---|
| No. of patients | 8 | 32 | |
| Age (years) | 65.6±4.8 | 55.1±2.3 | 0.04 |
| Gender (M/F) | 3/5 | 23/9 | 0.07 |
| Location of
perforation | | | |
| Upper third | 0 (0.0%) | 6 (18.9%) | |
| Middle third | 4 (50.0%) | 13 (40.6%) | 0.41 |
| Lower third | 4 (50.0%) | 13 (40.6%) | |
| Co-morbidity
(yes) | 2 (25.0%) | 15 (46.9%) | 0.25 |
| Peptic ulcer | 1 (12.5%) | 4 (12.5%) | >0.99 |
| Hypertension | 0 (0.0%) | 5 (15.6%) | 0.37 |
| Diabetes | 1 (12.5%) | 2 (6.3%) | 0.55 |
| Another
malignancy | 0 (0.0%) | 4 (12.5%) | 0.29 |
| Communication
problem | 0 (0.0%) | 5 (15.6%) | 0.23 |
| WBC (per μl) on
admission | 11,900±1,846 | 12,552±1,412 | 0.83 |
| Postoperative
hospital stay (days) | 18.7±3.5 | 21.6±2.2 | 0.56 |
| Postoperative
complication (yes) | 2 (25.0%) | 12 (37.5%) | 0.73 |
| ARDS | 0 (0.0%) | 5 (15.6%) | |
| Wound
infection | 2 (25.0%) | 6 (18.9%) | |
| Intraabdominal
abscess | 0 (0.0%) | 1 (3.1%) | |
| Mortality at 30
days | 2 (25.0%) | 1 (3.3%) | 0.04 |
| Sepsis (POD16) | Sepsis (POD27) | |
| Rupture of AAA
(POD5) | | |
The clinicopathological features of 8 patients with
gastric cancer who had a free perforation are listed in Table II. During the investigation period,
1,081 gastrectomies were performed in our hospital, and the
incidence of perforation in the case of gastric cancer was 0.74% of
all gastric cancer patients. All the patients were diagnosed by
pathological examination of the resected specimen or biopsy
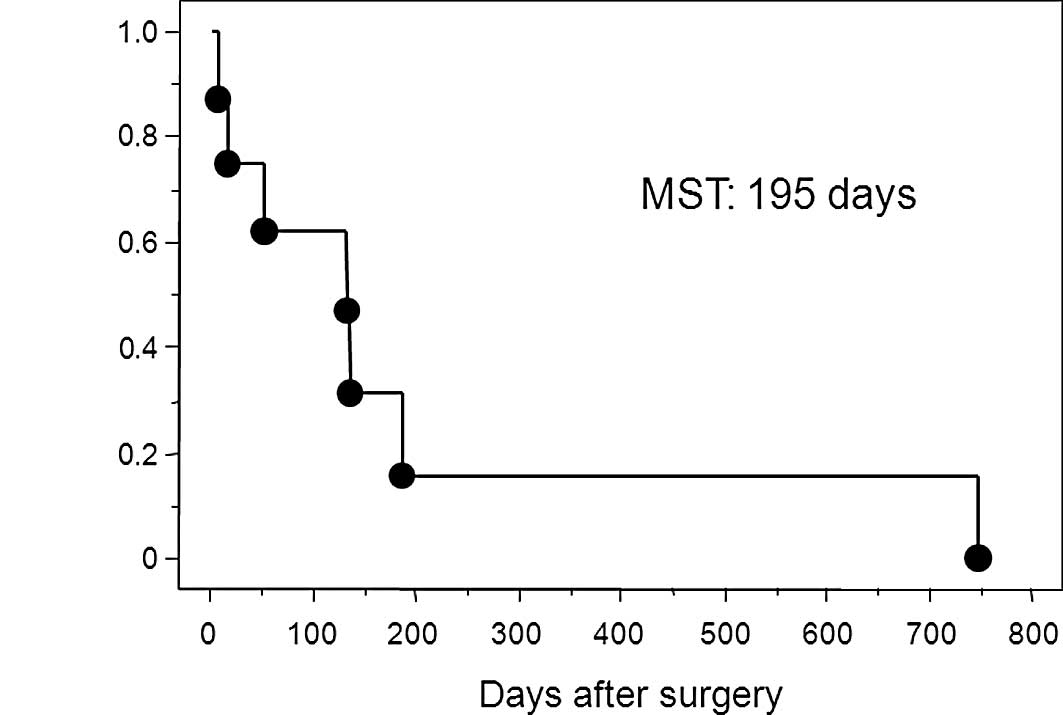
specimen. The Kaplan-Meier survival curve in patients with free
perforation due to gastric cancer is shown in Fig. 2. The median survival time of
patients with perforated gastric cancer was 195 days after surgery.
We compared the clinicopathologic characteristics between patients
with T3 tumors (classified according to the depth of tumor
invasion) with free perforation and those without free perforation
(Table III). There was no
difference between the groups in terms of age, gender, location of
the tumor, histology, nodal involvement, venous invasion and
lymphatic invasion. The maximal tumor size in patients with gastric
cancer perforation was significantly greater than that in patients
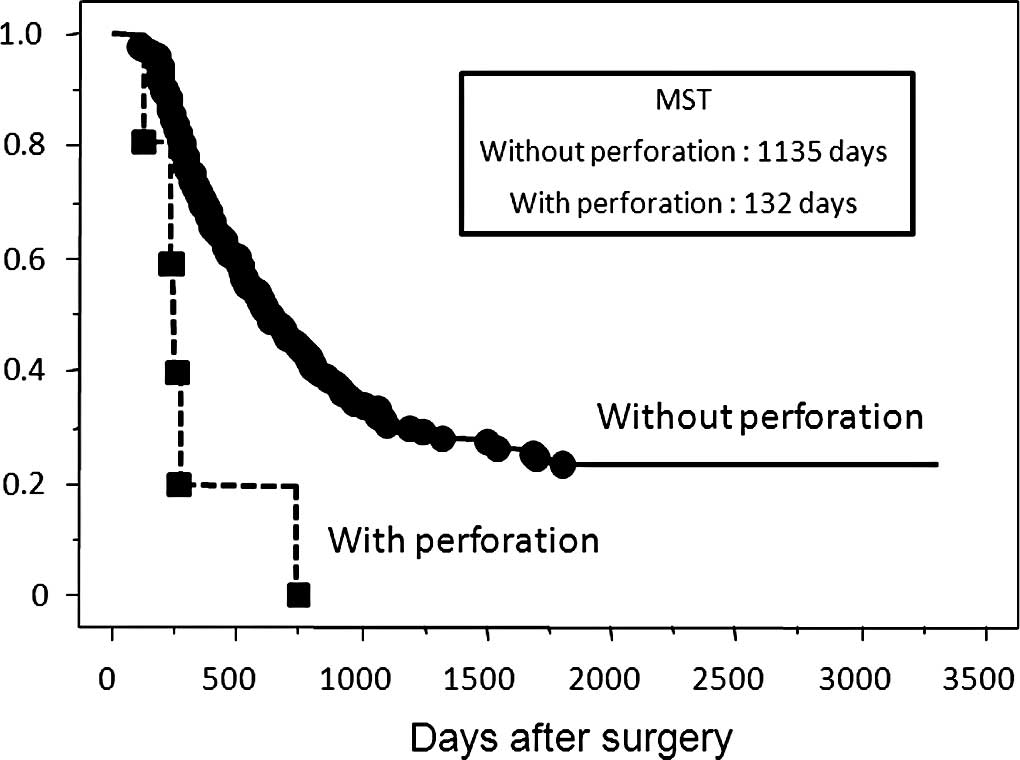
without perforation. Patients with perforation had a significantly
poorer overall survival rate than those who had T3 depth of tumor
without perforation (Fig. 3). In
addition, in patients with perforation, recurrence of peritoneum
occurred more frequently, but the data did not indicate statistical
significance (Table IV).
 | Table II.Clinicopathological features and
outcome of patients with perforated gastric cancer. |
Table II.
Clinicopathological features and
outcome of patients with perforated gastric cancer.
| Case no. | Age | Gender | Depth of tumor | Maximal tumor size
(cm) | Surgery | Lymph-adenectomy | Nodal
involvement | Hepatic
metastasis | Peritoneal
dissemination | Stage | Outcome (day) |
|---|
| 1 | 77 | F | T3 | 14.0 | STG | D1 | N1 | H0 | P0 | III | Died (133) |
| 2 | 52 | F | T3 | 8.0 | TG | D1 | N1 | H0 | P0 | III | Died (131) |
| 3 | 47 | M | T3 | 9.0 | TG | D1+α | N1 | H0 | P0 | III | Died (184) |
| 4 | 65 | M | T3 | 5.9 | STG | D1+α | N1 | H0 | P0 | III | Died (745) |
| 5 | 64 | F | T1 | 9.5 | STG | D1+α | N0 | H0 | P0 | IA | Alive (80) |
| 6 | 85 | F | T3 | 9.3 | STG | D1+α | N1 | H0 | P0 | III | Died (5) |
| 7 | 78 | M | T3 | – | Local repair | – | N2 | H0 | P0 | III | Died (16) |
| 8 | 57 | M | T4 | – | No operation | – | N2 | H1 | P0 | IV | Died (52) |
 | Table III.Clinicopathological characteristics of
patients with perforated gastric cancer and T3 tumor without a free
perforation. |
Table III.
Clinicopathological characteristics of
patients with perforated gastric cancer and T3 tumor without a free
perforation.
| With perforation
(n=5) | Without perforation
(n=196) | P-value |
|---|
| Age (years) | 65.2±7.2 | 61.8±0.9 | 0.55 |
| Gender (M/F) | 2/3 | 118/78 | 0.37 |
| Tumor location | | | |
| Upper third | 0 (0.0%) | 43 (21.9%) | |
| Middle third | 2 (40.0%) | 87 (44.4%) | 0.35 |
| Lower third | 3 (60.0%) | 66 (33.7%) | |
| Histology | | | |
| Diffuse | 3 (60.0%) | 134 (68.4%) | 0.68 |
| Intestinal | 2 (40.0%) | 62 (31.6%) | |
| Nodal
involvement | | | |
| pN0 | 0 (0.0%) | 21 (10.7%) | |
| pN1 | 5 (100.0%) | 90 (45.9%) | 0.22 |
| pN2, 3 | 0 (0.0%) | 85 (43.4%) | |
| Venous
invasion | | | |
| v0, v1 | 4 (80.0%) | 127 (64.8%) | 0.47 |
| v2, v3 | 1 (20.0%) | 69 (35.2%) | |
| Lymphatic
invasion | | | |
| ly0, ly1 | 0 (0.0%) | 51 (26.0%) | 0.19 |
| ly2, ly3 | 5 (100.0%) | 145 (74.0%) | |
| Maximal tumor size
(cm) | 92.4±13.3 | 62.6±2.4 | 0.03 |
 | Table IV.Recurrence of patients with
perforated gastric cancer and T3 tumor without a free
perforation. |
Table IV.
Recurrence of patients with
perforated gastric cancer and T3 tumor without a free
perforation.
| With perforation
(n=5) | Without perforation
(n=196) | P-value |
|---|
| Recurrence | | | |
| Yes | 4 (80.0%) | 75 (38.3%) | 0.50 |
| No | 1 (20.0%)a | 121 (67.7%) | |
| Site of
recurrence | | | |
| Peritoneum | 3 (75.0%) | 28 (37.3%) | 0.49 |
| Locoregional | 1 (25.0%) | 27 (36.0%) | |
| Liver | 0 (0.0%) | 10 (13.3%) | |
| Distant
organ | 0 (0.0%) | 6 (8.0%) | |
| Unknown | 0 (0.0%) | 4 (5.3%) | |
Discussion
In this study, we showed that intraoperative
findings could not be used to accurately diagnose the cause of
gastric perforation, since the sensitivity of these findings was
only 50%. In addition, patients with gastric cancer perforation had
a poorer overall survival rate than those who had T3 tumors without
perforation; this is consistent with the reports of previous
studies (2,4,6,7).
Perforation of gastric cancer results in an acute
abdominal syndrome due to leakage of gastric contents and the
consequent peritonitis. Although it has been reported that
approximately 10–16% of all gastric perforations are caused by
gastric cancer (7,8), malignancy is frequently diagnosed
only on the basis of postoperative pathological examination. It is
often difficult to recognize the type of lesion that caused gastric
perforation at the time of emergency surgery, particularly when
pathological evaluation of frozen sections cannot be performed due
to unavailability of the sections (9). Under such conditions, the surgeon
should diagnose the cause of perforation on the basis of rigidity
of the gastric wall and lymph nodes, size of ulceration and the
presence of metastasis in the liver and peritoneum. Moreover,
intraoperative endoscopic examination may be useful for identifying
the cause of gastric perforation (1,7,10).
In this study, the patients with gastric cancer perforation were
significantly older than those with gastric ulcer perforation and
had greater size of tumor than those with gastric cancer without
free perforation.
Lehnert et al proposed 2-stage radical
gastrectomy for the treatment of perforated gastric cancer
(8); however, this procedure is
associated with adhesion and the appropriate time point at which
the secondary radical gastrectomy should be conducted has not been
determined (5). Thus, the optimal
treatment for perforated gastric ulcer or cancer remains debatable.
Recently, many studies have reported the use of laparoscopic local
repair as the first step of surgery, followed by radical open
gastrectomy with appropriate lymphadenectomy 17–20 days after the
first-step surgery (11–13). All of the studies emphasized that
only slight adhesion was observed in the secondary radical surgery.
This surgical strategy may therefore be considered for the
treatment of perforated gastric cancer, even though this approach
was never chosen in our experience.
Numerous studies have shown that patients with
gastric cancer perforation have a poorer overall survival rate
after gastrectomy than those without perforation (2,4,6,7).
Besides the scattering of cancer cells due to perforation, this
difference in survival rates may also be due to inadequate
lymphadenectomy, inadequate examination for dissemination, lymph
node metastasis during emergency surgery and the potentially
advanced stage of the disease. In addition, preoperative
examination for metastasis in the lymph nodes and remote organs
could not be adequately performed, leading to underestimation of
the stage of the cancer.
In conclusion, to improve the survival rate of
patients with perforated gastric cancer and to improve the accuracy
of intraoperative diagnosis, endoscopic examination and/or
pathological examination of frozen sections should be performed,
whenever possible. In particular, malignant perforation should be
suspected when the patient is older and the tumor size is greater.
Next, a balanced surgical strategy must be chosen, i.e., either
radical gastrectomy or local treatment should be used according to
the severity of sepsis. Laparoscopic local repair as the first step
of surgery, followed by radical open gastrectomy with
lymphadenectomy may be considered as an appropriate surgical
treatment.
References
|
1.
|
Cortese AF, Zahn D and Cornell GN:
Perforation in gastric malignancy. J Surg Oncol. 4:190–206. 1972.
View Article : Google Scholar
|
|
2.
|
Adachi Y, Mori M, Maehara Y, et al:
Surgical results of perforated gastric carcinoma: an analysis of
155 Japanese patients. Am J Gastroenterol. 92:516–518.
1997.PubMed/NCBI
|
|
3.
|
Japanese Gastric Cancer: A Japanese
Classification of Gastric Carcinoma. 2nd English edition. Gastric
Cancer. 1:10–24. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Adachi Y, Aramaki M, Shiraishi N, et al:
Long-term survival after perforation of advanced gastric cancer:
case report and review of the literature. Gastric Cancer. 8:180–83.
1998.PubMed/NCBI
|
|
5.
|
Kasakura Y, Ajani JA, Fujii M, et al:
Management of perforated gastric carcinoma: a report of 16 cases
and review of world literature. Am Surg. 68:434–440.
2002.PubMed/NCBI
|
|
6.
|
Gertsch P, Chow LW, Yuen ST, et al:
Long-term survival after gastrectomy for advanced bleeding or
perforated gastric carcinoma. Eur J Surg. 162:723–727.
1996.PubMed/NCBI
|
|
7.
|
Gertsch P, Yip SK, Chow LW, et al: Free
perforation of gastric carcinoma. Results of surgical treatment.
Arch Surg. 130:177–181. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Lehnert T, Buhl K, Dueck M, et al:
Two-stage radical gastrectomy for perforated gastric cancer. Eur J
Surg Oncol. 26:780–784. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Roviello F, Rossi S, Marrelli D, et al:
Perforated gastric carcinoma: a report of 10 cases and review of
the literature. World J Surg Oncol. 4:192006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Jwo SC, Chien RN, Chao TC, et al:
Clinicopathological features, surgical management and disease
outcome of perforated gastric cancer. J Surg Oncol. 91:219–225.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Hayashi N, Hasuike Y, Fujiwara S, et al: A
case of perforated gastric cancer treated with an emergency
laparoscopic occlusion followed by radical resection. J Jpn Soc
Endosc Surg. 12:61–65. 2007.
|
|
11.
|
Kawase H, Ebihara Y, Kitashiro S, et al: A
case of gastric perforation of early gastric cancer treated by
two-step radical surgery after laparoscopic omentopexy. J Jpn Surg
Assoc. 66:2426–2430. 2005. View Article : Google Scholar
|
|
12.
|
Fukuda N, Wada J, Takahashi S, et al:
Perforated gastric carcinoma treated with laparoscopic omental
patch repair followed by open radical surgery – report of a case. J
Jpn Surg Assoc. 66:2431–2435. 2005.
|