Contents
Introduction
Conventional treatments
Virus inactivation by arginine
Safety of arginine
Prospect of topical arginine applications
Advantage of virus inactivation by arginine
Introduction
The recent incident of influenza A virus pandemic
has reminded us of the high risk of sporadic influenza virus
infection during non-epidemic seasons. There is already indication
of a potential worldwide spread of influenza projected for this
coming autumn and winter. Although acquiring specific immunity
through vaccination is the most effective way to prevent virus
infection, daily routine preventive measures appear to be a simple,
yet effective approach against influenza A virus infection. Such
preventive measures are normally accomplished by the recommended
procedure of hand- and mouth-wash or wearing masks. These washes,
though effective, only remove viruses from the initial site of
contact. It is more effective when these washes are combined with a
reagent that can kill viruses. Disinfectants have been used to
inactivate viruses on contaminated surfaces or human fingers and
hands, but generally have severe cell and tissue toxicities and
hence cannot be practically used on mucosal membranes or injured
sites of the body (1–5). Less toxic acidic solvents have also
been used as disinfectants and, to a limited extent, as virus
inactivation agents for such sensitive surfaces (5,6).
When infected, there are several antiviral drugs
against influenza A virus, including neuraminidase inhibitors,
which are currently considered to be the most effective (7). There is, however, always the
potential risk of generating drug resistance when using
conventional antiviral drug treatments (8). Particularly in a pandemic infection,
the selective pressure in nature to generate a resistant virus is
much greater than the conventional epidemic mode of the infection.
Therefore, an additional treatment that utilizes different
mechanisms of antiviral strategy would be a valuable resource as an
alternative or addition to conventional drug treatments. Toward
these goals, i.e., preventive measures and therapeutic treatments,
we propose here that an aqueous arginine solution can be developed
as an effective virus inactivation agent. This is based on the
observations that an aqueous arginine solution inactivates
influenza A virus (9,10) and exhibits antiviral activities on
several enveloped viruses (11).
Due to the lack of safety concerns, an aqueous arginine solution
may be used, not only for hand and mouth wash as a disinfectant,
but also for the inactivation of viruses at the site of infection
in the form of a spray or mist. While acidic solvents provide, not
only physical removal but also inactivation of viruses (5,6),
arginine may have an edge over acidic solvents due to its safety,
or at least may provide an alternative option to acidic solvents or
antiviral drugs.
Conventional treatments
Preventive measures
Since no vaccine is available for new types of
influenza A viruses at an outbreak of a pandemic, the only
effective way to prevent the spread of infection is to reduce the
number of infecting viruses to the body. As described above,
recommended procedures have been hand and mouth wash to avoid the
contamination of hands as well as the removal of the contaminated
viruses in the nose and mouth. These procedures have been
considered highly effective and recommended in particular for the
recent swine flu incident. To enhance the effectiveness of washing
procedures, disinfectants and acidic solvents in different formats
have been developed (1–5).
Antiviral therapy
Antiviral drugs inhibit specific functions of viral
proteins required for the infection and virus multiplication.
Influenza virus infects cells in the upper respiratory tracts in
the initial phase of infection, causing rhinitis, pharyngitis,
laryngitis and simultaneously severe systemic symptoms, including
fever, chill, headache and muscle pains. There are two classes of
specific antiviral agents against influenza virus infections: M2
channel inhibitors (rimantadine and amantadine) and neuraminidase
inhibitors (zanamivir and oseltamivir) (7). M2 channel inhibitors block
dissociation (uncoating) of influenza A virus from the endosome at
the very early stage of infection and consequently inhibit virus
multiplication. Viral neuraminidase cleaves sialic acid of cell
surface glycoproteins at the late stage of infection and, as a
consequence, helps the release of progeny viruses from the infected
cells and allows the spread of the virus over the surface of the
respiratory epithelia. Thus, inhibition of neuramindase blocks the
spread of virus in the respiratory tracts, preventing further
development of the influenza symptoms. However, neuraminidase
inhibitors suffer the rapid appearance of drug-resistant mutants
(8). The problems of resistance
are inherent to drugs that bind to a single specific site of target
molecules. As discussed below, virus inactivation by arginine as
well as acidic solvents use a fundamentally different mechanism
through weak, multiple interactions with the viral components,
i.e., no single particular target site on the viral components.
Virus inactivation by arginine
We have shown that an aqueous arginine solution at a
low pH inactivates influenza A virus when incubated on ice for
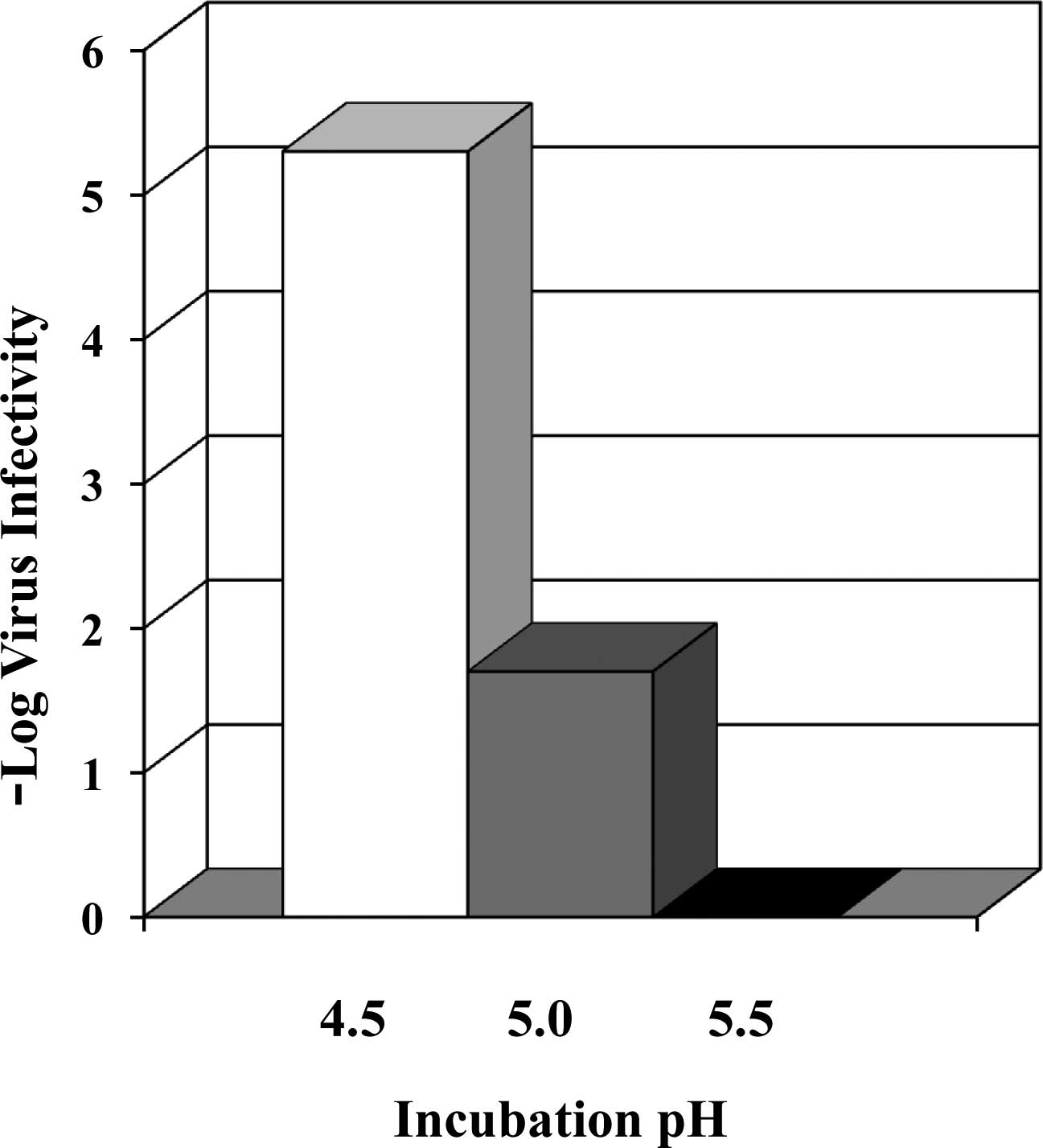
30–60 min (9). Fig. 1 shows virus inactivation by 0.7 M
arginine. The effects are extremely strong at pH 4.5, reaching a
greater than 5 log reduction of virus yield. As the pH is
increased, the effects rapidly decline, leading to no detectable
virus inactivation at pH 5.5. These virus inactivation effects are
significantly stronger than the acidic buffer alone. For example,
0.1 M citrate only leads to a 2 log reduction at pH 4.5,
approximately 1000-fold less effective than 0.7 M arginine. Such a
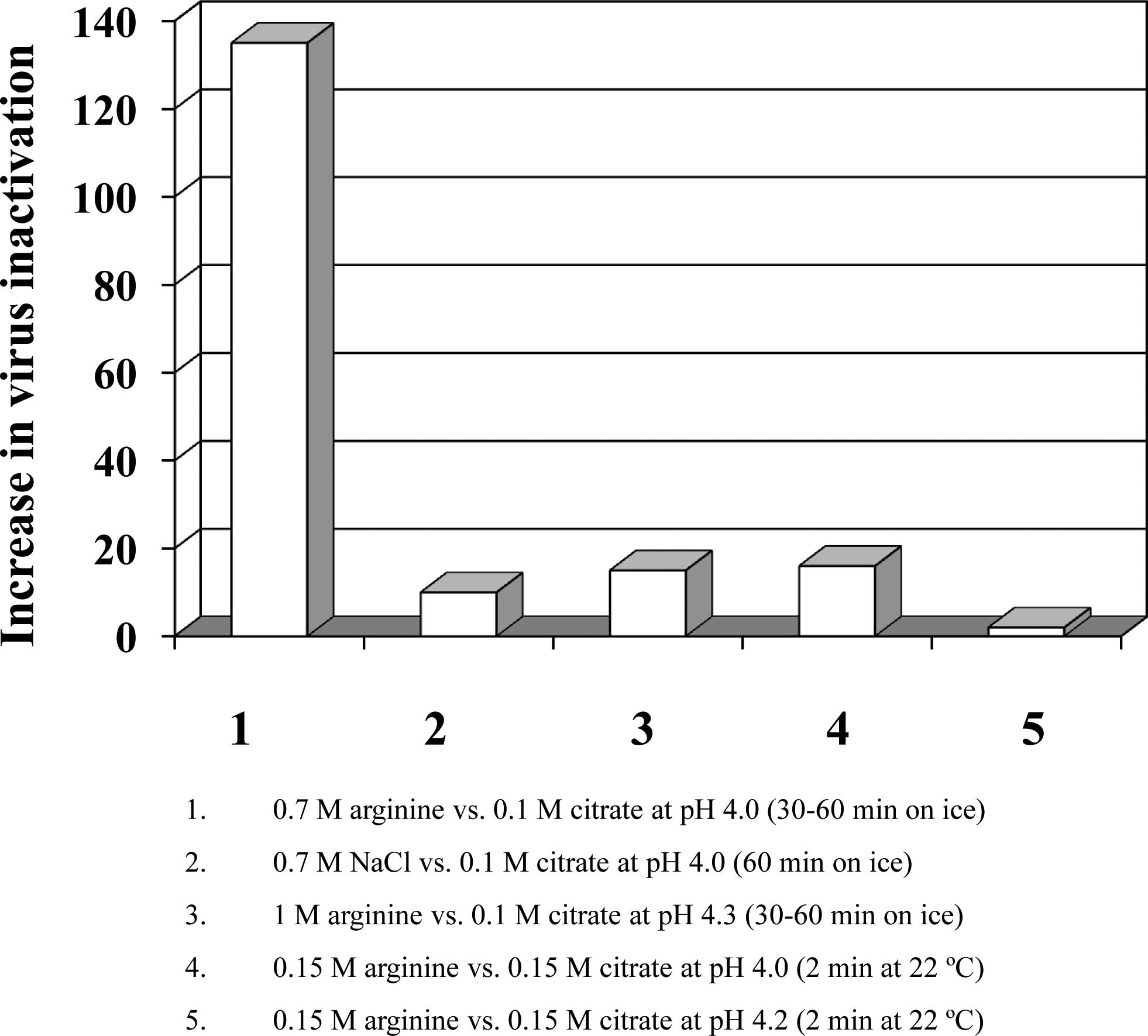
comparison is plotted in Fig. 2.
Column 1 shows that 0.7 M arginine is over 100-fold more
effective than 0.1 M citrate at pH 4.0. On the other hand, 0.7 M
NaCl is only 10-fold more effective than 0.1 M citrate (column 2),
indicating that higher ionic strength does contribute, but cannot
entirely explain, the strong virus inactivation effects of 0.7 M
arginine. Consistent with this, increasing the citrate
concentration to 0.7 M at pH 4.5 enhanced the effects by
approximately 100-fold over 0.1 M citrate (9), which is still 10-fold less than 0.7 M
arginine at the same pH. Nevertheless, it is evident that a higher
citrate concentration does enhance virus inactivation at pH 4.5.
The difference between arginine and citrate depends on pH, as at pH
4.3, 1 M arginine was only 15-fold more effective than 0.1 M
citrate (Fig. 2, column 3).
The above experiments were conducted on ice, and the
results hence may significantly differ from the effects at body or
room temperature, at which disinfecting procedures or in
vivo virus inactivation treatments take place (1–6).
When the influenza virus was treated at 37°C for 2 min with 0.7 M
arginine, pH 4.0, an approximately 4 log reduction was observed,
slightly less than the effect at pH 4.5, despite at a lower pH
(Tsujimoto et al, unpublished data). This indicates the
importance of incubation time. Under a identical condition, 0.1 M
citrate at pH 4.0 resulted in an approximately 2 log reduction,
thus still much weaker than 0.7 M arginine (4 log reduction) at the
same pH. At pH 5.0, 0.7 M arginine resulted in 0.06 virus
infectivity at 37°C for a 2-min incubation, which compares with
0.02 virus infectivity on ice for 60 min, again suggesting that a
longer exposure increases virus inactivation. Even at a
concentration of 0.15 M, arginine is stronger than citrate (Ejima
and Koyama, unpublished data). Column 4 in Fig. 2 shows an approximately 15-fold
stronger effect of arginine when the influenza virus was exposed to
the pH 4.0 solvent at 22°C for 2 min. However, the difference
between arginine and citrate is also dependent on pH at this
condition as well. At pH 4.2, 0.15 M arginine was only 2-fold more
effective than 0.15 M citrate (columns 4 and 5). We also observed
that a higher citrate concentration is highly effective against
influenza virus at elevated temperatures, a magnitude equal to or
exceeding the level achieved by 0.7 M arginine at an identical
acidic pH (Tsujimoto et al, unpublished data).
Virus inactivation by acidic arginine solutions has
been observed with other enveloped viruses when incubated on ice
for 60 min (9,10,12).
When the incubation time was reduced, the effects were
significantly reduced, meaning that virus inactivation is
time-dependent as described above. However, a higher incubation
temperature offsets the effects of a shorter incubation time.
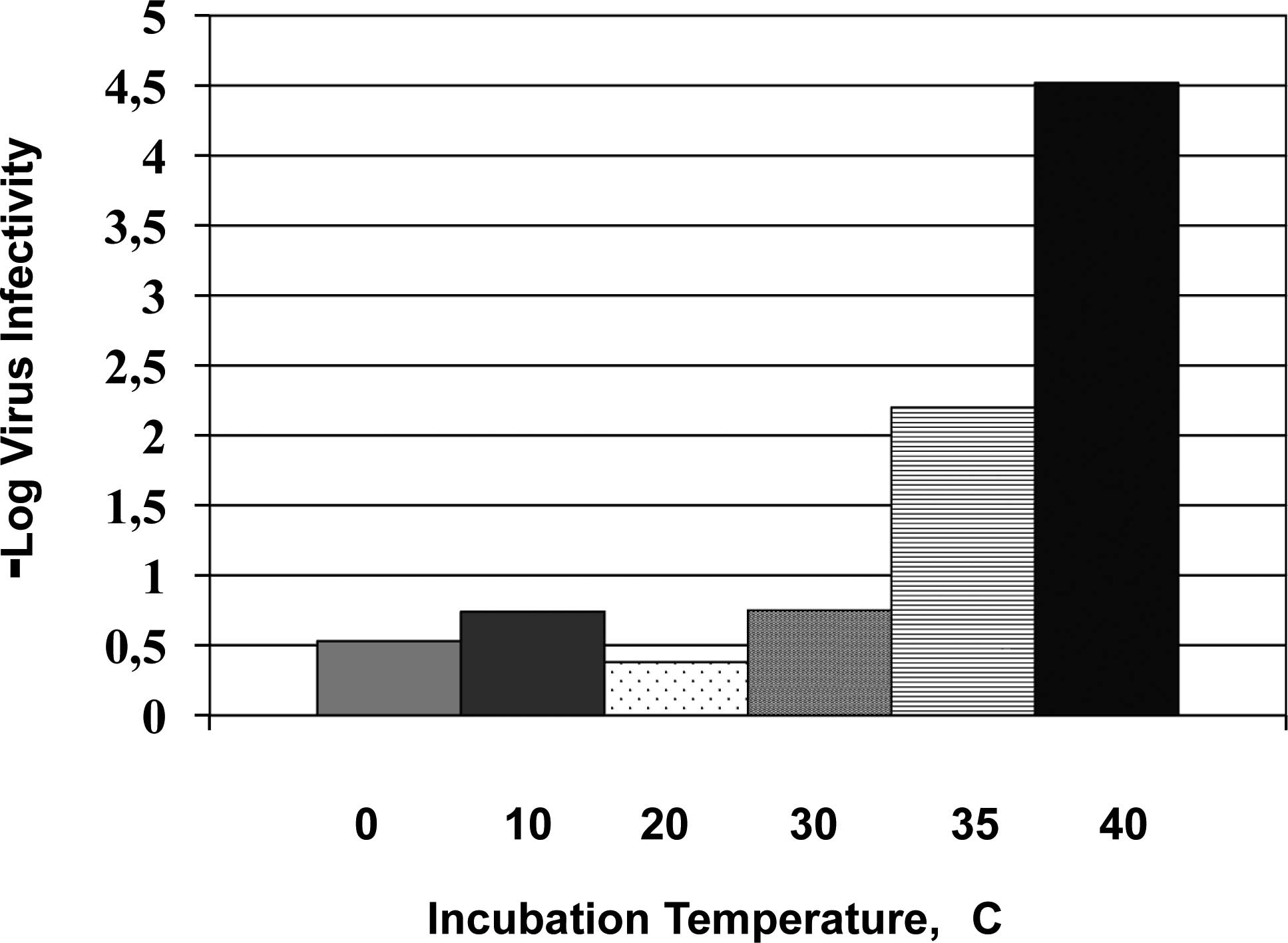
Fig. 3 shows the results of herpes
simplex virus type 2 (HSV-2) when incubated only for 5 min
(Tsujimoto et al, unpublished data). The virus inactivation
effects are marginal with 0.7 M arginine at pH 4.4 when incubated
on ice for 5 min. There is little change up to 30°C, above which
the virus inactivation sharply increases. Thus, even for a 5-min
incubation, a higher temperature close to the body temperature can
lead to extensive virus inactivation by 0.7 M arginine at pH
4.4.
A higher pH also reduces virus inactivation by
arginine. At a neutral pH as well, higher temperature offsets the
reduced virus-inactivating effects of arginine (12). Such temperature effects were tested
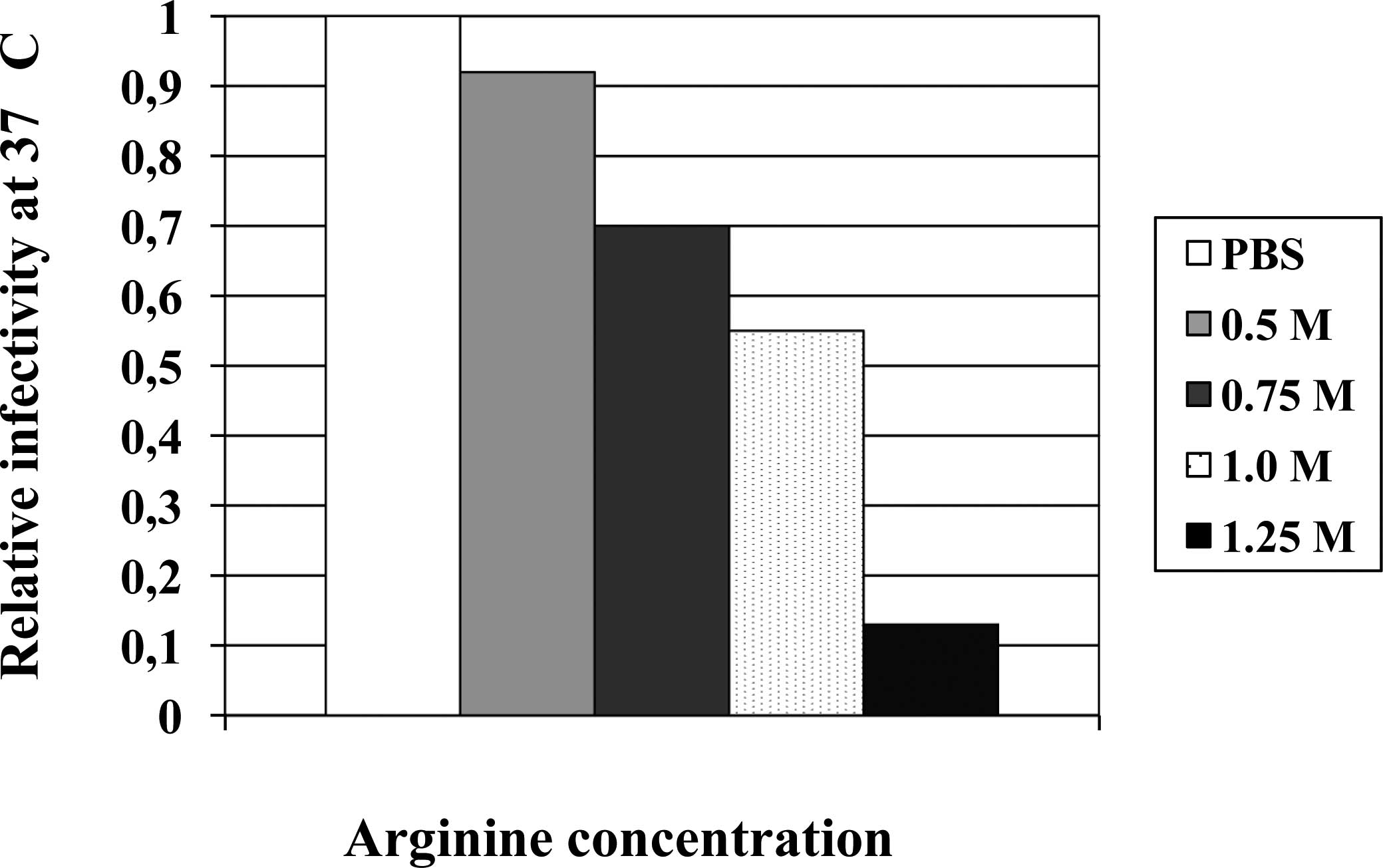
using herpes simplex virus type 1 (HSV-1). Fig. 4 summarizes the effects of arginine
on relative infectivity of HSV-1 at a neutral pH and a body
temperature of 37°C. Similar to the virus stability in PBS on ice,
a 37°C-exposure has no effect on virus infectivity, i.e., high
temperature alone is insufficient to inactivate HSV-1 in a
physiological solvent. A significant decrease in the infectivity
was observed in the presence of 0.5 M arginine; approximately 10%
of the virus was inactivated upon incubation at 37°C for 5 min.
Virus inactivation was enhanced with increasing concentrations of
arginine, reaching approximately 10% of the surviving virus with
1.25 M arginine. It is evident that even at a neutral pH, arginine
solution can inactivate HSV-1 at sufficiently high concentrations
and at 37°C. The effects are stronger at higher temperature and
with longer incubation.
A similar experiment showed that influenza A virus
cannot be inactivated by arginine under the conditions of 37°C, a
5–20 min incubation and 0.5–1.25 M arginine concentration at a
neutral pH (12). However, a
further increase in temperature resulted in significant virus
inactivation.
Safety of arginine
There are numerous applications of arginine,
including supplementary diets, sport drinks, pharmaceutical agents,
cosmetic ingredients and formulation reagents of protein and drug
substances. Thus, there is no doubt about the safety of arginine
administration. However, these applications are relatively used at
low doses. The applications of arginine as preventive measures and
topical virus inactivation require high concentrations. In other
words, effective virus inactivation requires arginine at high
concentrations. Our initial goal was to develop more effective
virus inactivation processes for pharmaceutical proteins. One of
the conventional procedures of virus inactivation has been a low pH
(13), which can damage proteins
(14). An idea behind the use of
arginine is that it may be able to raise the pH for virus
inactivation process, as arginine itself does not affect the
protein stability (15). In fact,
arginine at 0.3–1 M above pH 4.0 achieved virus inactivation that
could be achieved by a buffer at pH 3.5 without arginine (9). An acidic arginine solution at 0.3–1 M
above pH 4.0 is acceptable for virus inactivation of pharmaceutical
protein solutions, but may be too toxic for any in vivo
applications. It turns out that a low pH and high arginine
concentration appear to be tolerant for certain body surfaces such
as the mucosal layer of mouse genital organs and epithelial
keratinocytes in rabbit eyes (Ikeda et al, unpublished
data). The observed nontoxic nature of arginine is perhaps due to
its negligible effects on proteins; arginine does not denature
proteins (15). The preliminary
attempts to kill viruses by topical applications for herpetic
keratitis in rabbits showed that a high arginine concentration and
low pH are effective and tolerant for rabbit eyes (Ikeda et
al, unpublished data). This has opened a new window of
opportunity for the use of an arginine solution for the treatment
of influenza A virus infection.
Prospect of topical arginine
applications
Preventive measures
One of the most effective preventive measures for
influenza A virus infection is a hand- and mouth-wash routine. It
is simply removal, not inactivation, of the contaminated virus from
the primary infection site of the virus. It would be ideal if the
washing or rinsing step, not only physically removes, but also
kills the viruses. Along this line, an aqueous arginine solution
may be used in the form of a wet towel for hand wash or spray for
hand and mouth wash.
Therapeutic applications
The initial site of influenza A virus infection is
the epithelial cells of the upper respiratory tracts (6). The progeny viruses are released from
the infected cells to extracellular mucosal fluid on the epithelial
surface by the activity of neuraminidase as described above. It
might be possible to use an aqueous arginine solution in the form
of spray, as has been used for acidic solvent (6) or mist that can provide a burst of
concentrated arginine solution sufficient for inactivation of the
viruses in the mucosal fluid, preventing the spread of progeny
viruses to neighboring cells (i.e., similarly to the action of
anti-neuraminidase inhibitors), although both initial arginine
concentration and initial pH will change to the physiological
condition with time by dilution with body fluid. This dilution will
eventually abolish the virus inactivation effects of arginine.
However, arginine also has a weak, but significant, antiviral
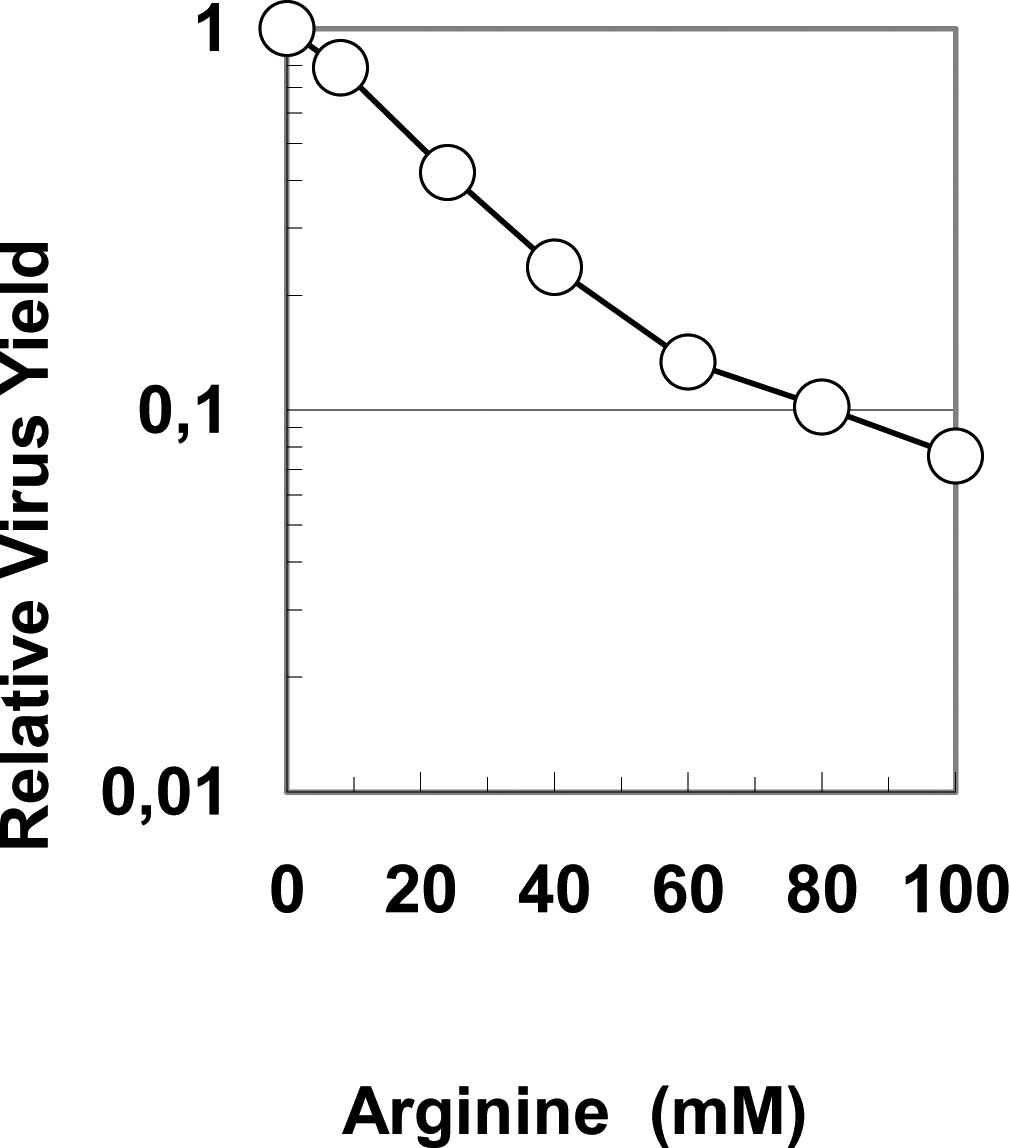
effect (11). As shown in Fig. 5, arginine below 100 mM does inhibit
virus growth of influenza A virus in cultured cells. This means
that even after an arginine solution is diluted below the level of
effective concentration and pH for virus inactivation, it can exert
antiviral actions.
Advantage of virus inactivation by
arginine
We demonstrated that an aqueous arginine solution
can inactivate viruses, including influenza A virus, when combined
with either low pH or elevated temperature or both. Although the
precise mechanism of the virus inactivation by arginine has not yet
been fully elucidated, one critical fact is that the virus
inactivation requires a high arginine concentration, e.g., higher
than 0.3 M and preferably 0.7–2 M. This requirement of high
concentration makes arginine qualitatively different from currently
available antiviral drugs that normally function at much lower
concentrations. Such a large difference in the effective
concentration inherently places a restriction on the utility of an
aqueous arginine solution, namely, only a topical application for
superficial infection. There is no way that arginine can be
systemically or orally administered to reach an effective
concentration in vivo.
However, such a difference in the effective
concentration between conventional antiviral drugs and an aqueous
arginine solution has a significant consequence in generating drug
resistance. The use of antiviral drugs can quickly result in
drug-resistant mutants, while the use of arginine does not. This is
due to the entirely different mechanisms of their functions. First,
antiviral drugs have a specific target, e.g., virus-coded enzymes
to which they bind and inhibit the activity of target molecules.
This leads to a mutation in the corresponding genes and loss of
inhibitory activities of the drugs. Conversely, arginine has no
specific viral or host cell components for its binding. Second,
drug-resistant mutations in general occur when the progeny viruses
are produced in the presence of suboptimal concentrations of
antiviral drugs, i.e., there is a consistent selection pressure to
escape from the inhibitory effects of antiviral drugs during the
course of virus multiplication in the presence of the antivirals at
subeffective concentrations. On the contrary, the mode of arginine
action is ‘all or none’. Once the virus is killed by arginine,
there is no chance to produce drug-resistant progeny virus. When
the virus infects the cells even in the presence of arginine at any
concentration, there is no selection pressure during virus
multiplication, and hence the progeny viruses are equally sensitive
to arginine. The virucidal mechanism of arginine eliminates the
possibility of generating a resistant mutant against arginine
treatments.
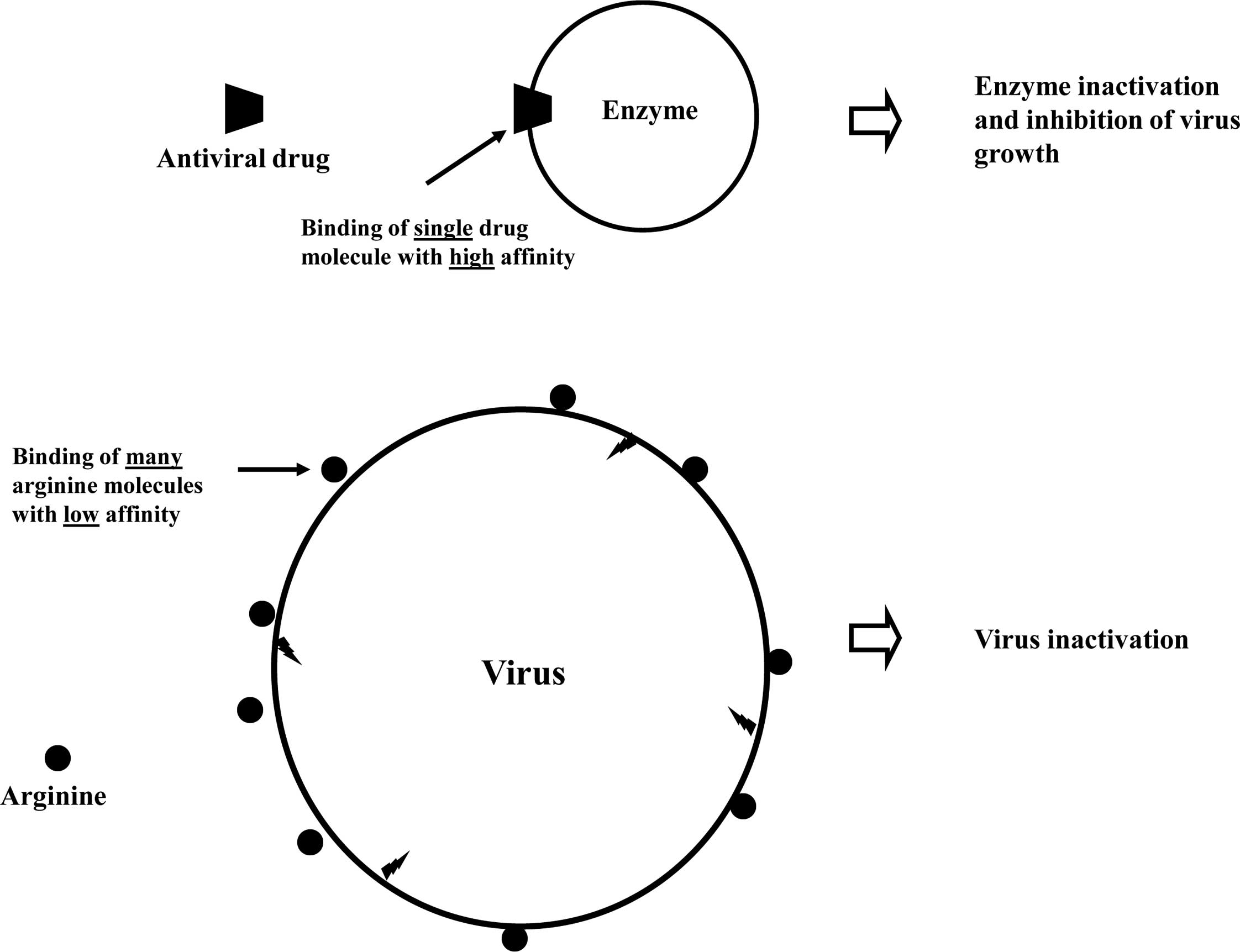
Requirement of a high concentration means that the
interactions of arginine with the virus are weak, i.e., arginine
interacts with the virus surface through weak interactions (16).
This is schematically depicted in Fig.
6. For comparison, the upper panel shows the binding of an
antiviral drug to the target enzyme that occurs with high affinity.
With such high affinity, the antiviral drugs can be systemically or
orally administered to reach the effective concentration. The lower
panel shows the plausible mechanism of virus inactivation by
arginine. Although the precise binding mechanism of arginine is
still unclear, arginine does appear to bind to proteins, aromatic
compounds, nucleic acids and lipids, but all with low affinity.
Significant effects of arginine are not normally observed unless
its concentration is higher than 0.1 M. Thus, the binding of
arginine to whatever is responsible for virus inactivation is weak.
Although no evidence exists, it is highly likely that multiple
arginine bindings occur on the virus surface, as there are many of
these arginine binding sites on the surface. Such multiple sites
would result in multiple damages to the virus, leading to virus
inactivation. Whether or not the damaged sites are the binding
sites of arginine remains to be ascertained, but one important
observation is the requirement of membrane for the mechanism of
arginine effects. The non-enveloped viruses studied so far showed
strong resistance to virus inactivation by arginine, implying that
either membranes are involved for arginine binding and the
resultant damage, or the damages occur on the membrane-protein
interface. These multiple bindings and damages on the virus
eliminate the possibility of generating resistant viruses against
arginine treatments. Another aspect of arginine binding is that
arginine binding to host proteins is most likely reversible, as
expected from such low affinity. Once the arginine concentration
decreases to 0.1 M, it dissociates from the proteins and becomes
ineffective on any perturbation that the high arginine
concentration may have caused. However, virus inactivation is not
reversible, as shown by in vitro virus inactivation
experiments. If the arginine effects were reversible, there should
be enough time for the viruses to regain infectivity between the
acid treatment and infection procedure under the experimental
conditions used (9,10).
Currently, there are acidic solvents used as
disinfectants or, to a limited extent, as virus inactivation agents
(6). We showed that a high
concentration of citrate is effective (9) and even more so than acidic arginine
solvents against influenza virus under certain conditions
(unpublished data). Clearly additional studies are required to
fully understand the disadvantages and advantages of each solvent
system, but the importance of such a solvent system cannot be
overemphasized. Acidic arginine solutions may provide an edge over
other solvent systems, or at least may be used as an alternative
option to other solvent treatments due to its safety. As the spread
of a new influenza virus pandemic is imminent, there is urgent need
for novel treatments, and the development of an arginine solution
as a disinfectant or as an in vivo virus inactivation agent
must be given proper attention.
References
|
1.
|
Wanaratana S, Tantilertcharoen R,
Sasipreeyajan J and Pakpinyo S: The inactivation of avian virus
subtype H5N1 isolated from chickens in Thailand by chemical and
physical treatments. Vet Microbiol. July 10–2009.(Epub ahead of
print).
|
|
2.
|
Patnayak DP, Prasad AM, Malik YS,
Ramakrishnan MA and Goyal SM: Efficacy of disinfectants and hand
sanitizers against respiratory viruses. Avian Dis. 52:199–202.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Grayson ML, Melvani S, Druce J, Barr IG,
Ballard SA, Johnson PD, Mastorakos T and Birch C: Efficacy of soap
and water and alcohol-based hand-rub preparations against live H1N1
influenza virus on the hands of human volunteers. Clin Infect Dis.
48:285–291. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Lombardi ME, Ladman BS, Alphin RL and
Benson ER: Inactivation of avian influenza virus using common
detergents and chemicals. Avian Dis. 52:118–123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Alphin RL, Johnson KJ, Ladman BS and
Benson ER: Inactivation of avian influenza virus using four common
chemicals and one detergent. Poult Sci. 88:1181–1185. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Rennie O, Bowtell P, Hull D, Charbonneau
D, Lambkin-Williams R and Oxford J: Low pH gel intranasal sprays
inactivate influenza viruses in vitro and protect ferrets against
influenza infection. Respir Res. 8:382007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Democratis J, Pareek M and Stephenson I:
Use of neuraminidase inhibitors to combat pandemic influenza. J
Antimicrob Chemother. 58:911–915. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
McKimm-Breschkin JL: Resistance of
influenza virus to neuraminidase inhibitors: a review. Antiviral
Res. 47:1–17. 2000. View Article : Google Scholar
|
|
9.
|
Yamasaki H, Tsujimoto K, Koyama AH, Ejima
D and Arakawa T: Arginine facilitates inactivation of enveloped
viruses. J Pharm Sci. 97:3067–3073. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Katsuyama Y, Yamasaki H, Tsujimoto K,
Koyama AH, Ejima D and Arakawa T: Butyroyl-arginine as a potent
virus inactivation agent. Int J Pharm. 361:92–98. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Naito T, Irie H, Tsujimoto K, Ikeda K,
Arakawa T and Koyama AH: Antiviral effect of arginine against
herpes simplex virus type 1. Int J Mol Med. 23:495–499. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Utsunomiya H, Ichinose M, Tsujimoto K,
Katsuyama Y, Yamasaki H, Koyama AH, Ejima D and Arakawa T:
Co-operative thermal virus inactivation of herpes simplex virus and
influenza virus by arginine and NaCl. Int J Pharm. 366:99–102.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Brorson K and Norling L: Current and
future approaches to ensure the viral safety of biopharmaceuticals.
Dev Biol. 118:17–29. 2004.PubMed/NCBI
|
|
14.
|
Vermeer AWP and Norde W: The thermal
stability of immunoglobulin: unfolding and aggregation of a
multi-domain protein. Biophys J. 78:394–404. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Arakawa T and Tsumoto K: The effects of
arginine on refolding of aggregated proteins: not facilitate
refolding, but suppress aggregation. Biochem Biophys Res Commun.
304:921–927. 2003. View Article : Google Scholar : PubMed/NCBI
|