Introduction
Lung cancer is one of the leading causes of death by
cancer worldwide; approximately 80% of lung cancers can be
histologically classified as non-small cell lung cancers (NSCLCs).
Most patients present with locally advanced (37%) or metastatic
(38%) disease at the time of diagnosis (1). Despite advances in chemotherapy, the
average 5-year survival rate for patients with advanced NSCLC
remains extremely poor (2), thus
new agents are needed to establish an effective therapeutic
strategy against NSCLC. There is great interest in developing new
preventive and anti-tumor agents that are more effective and less
toxic. It has recently been suggested that deuterium-depleted water
(DDW) may play a potentially beneficial role in cancer prevention
(3).
In nature, the ratio between deuterium and hydrogen
(D/H) in ordinary water is approximately 1:6600 (4). It has been known for decades that the
mass difference between hydrogen and deuterium leads to differences
in the physical and chemical behavior of the two stable isotopes
(5,6). In biological systems, the effect of
replacing hydrogen with deuterium has also been well documented
(7,8). Early studies revealed that the life
span of mice with ascites tumors was prolonged by drinking 25–30%
deuterium water (deuterated water) (9), and the mortality caused by
60Co irradiation in mice was significantly decreased by
drinking 30% deuterated water (10). Gross and Spindel discovered that
high concentrations of deuterium in water induced stagnation
mitosis (11). Although the high
concentration of deuterium in water was able to inhibit cell
proliferation by mitosis arrest and to protect the cell from
radiation, it also reduced the life span of mice and even resulted
in death (12,13), which limits its clinical
application.
To date, research into the effects of deuterium in
organisms has focused primarily on deuterated water; little
research has been conducted on DDW. The possible role of naturally
occurring deuterium in biological systems was first investigated in
the early 1990s. DDW was shown to significantly decrease the growth
rate of L929 fibroblast cell lines in vitro, and
also inhibit tumor growth in xenotransplanted mice (14). Scientists have recently reported
the anti-tumor characteristics of DDW when the deuterium volume
fraction in normal water was reduced by 65% (15–17);
however, the mechanism underlying the anti-tumor effect of DDW is
still unknown. In this study, we investigated the in vivo
and in vitro effects of DDW on the growth of human lung
cancer and the possible mechanisms of these effects.
Materials and methods
Materials
DDW was provided by Shanghai Chitian DDWater
Bioengineering Co., Ltd. (Shanghai, China). A549 and H460 cells
were purchased from the Cell Research Institute of the Chinese
Medical Research Academy (Shanghai, China), and human embryonic
lung fibroblasts (HLF-1 cells) were purchased from the cell bank of
the Chinese Academy of Science (Shanghai, China).
Cell culture
The human lung carcinoma A549 and H460 cell lines
were maintained in RPMI-1640 medium (Gibco, USA) containing 10%
fetal bovine serum (FBS; Si Jiqing, HangZhou, China) at 37°C in 5%
CO2. For in vitro studies, the cells were seeded
in 25 ml cell culture bottles and grown in complete medium to 90%
confluence. Then, cells were washed with phosphate-buffered saline
(PBS) and incubated for 48 h at 37°C in 6 ml of serum-free medium
containing DDW.
HLF-1 cells were maintained in α-MEM medium (Genom,
HangZhou, China), containing 10% FBS at 37°C in 5%
CO2.
Analysis of cytotoxicity
The cytotoxicity of DDW was measured in A549 and
HLF-1 cells every 2 h for 24 h by the
3-(4,5-dimethyldiazol-2-yl)-2,5-diphenyltetrazolium-bromide (MTT;
Kai Ji Co. Ltd., Nan Jing, China) colorimetric assay. A preliminary
study was conducted to determine the optimal concentration of DDW
and the length of treatment. A549 and HLF-1 cells were cultured in
DDW (25, 50 or 105 ppm) and normal water in 96-well plates at
2×104 cells/100 μl well or 1×104 cells/100 μl
well, respectively. Cytotoxicity was determined 24, 48 and 72 h
after treatment. The MTT proliferation assay is based on the
ability of mitochondrial dehydrogenase in viable cells to convert
the MTT reagent into a soluble blue formazan dye. At the end of the
culture period, 50 μl of the MTT reagent was added, and cells were
incubated for 4 h at 37°C. After removal of the culture medium,
cells were lysed with dimethyl sulfoxide (DMSO) to determine the
amount of formazan product. The dishes were placed on a shaking
platform until the formazan crystals were dissolved. Absorption was
measured by a microplate reader (Multiskan MK3; Shanghai, China) at
550 nm, and the results were expressed as percent decrease in cell
viability compared to the controls (3). Each cell sample was measured three
times, and the mean was reported.
Transmission electron microscopy
(TEM)
A549 monolayer cells were treated with DDW at 50±5
ppm for 10 h, 72 h or 40 days and subsequently collected and fixed
with 25% glutaraldehyde in 0.2 M PBS (pH 7.4) at 4°C for 2 h. A549
cells were fixed with osmic acid and dehydrated in graded ethanol
solutions before embedding. After staining, samples were analyzed
using TEM (CM 120; Philips, The Netherlands).
Microscopy
After incubation with 50±5 ppm DDW for 40 days,
changes in morphology and structure of A549 cells were observed by
fluoroscope microscopy (Olympus, Japan). In addition, membrane
morphology was observed by SEM (Multimode Nanoscope IIIa; Digital
Instrument Co., USA). For SEM, A549 cells were collected and seeded
on a glass overnight and then fixed for 15 min with 0.25% glutaric
dialdehyde. Cells were dried and sprayed with gold after
dehydration.
Flow cytometric analysis
Cells were harvested by trypsinization after DDW
treatment (10 or 72 h), washed twice with PBS and then re-suspended
in ice-cold PBS and fixed with 70% ethanol. After the ethanol was
removed, the cells were washed once in PBS. The cells were then
centrifuged, and cell pellets were re-suspended in 1 ml propidium
iodide (PI)/Triton X-100 staining solution (0.1% Triton X-100 in
PBS, 0.2 mg/ml RNase A and 10 μg/ml PI) and incubated at least 30
min at room temperature. The stained cells were analyzed using a
FACScan flow cytometer in combination with BD Lysis II software (BD
Co., USA).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) staining
Cells were treated with 50 ppm DDW for 48 or 72 h,
seeded on polylysine-coated slides, fixed with 4% paraformaldehyde
in 0.1 M PBS for 1 h at 25°C, and then permeabilized with 1% Triton
X-100 in 0.01 M citrate buffer (pH 6.0). DNA fragmentation was
detected using a TUNEL detection kit (Kai Gene, Nan Jing, China),
which specifically labeled the 3′ hydroxyl terminus of DNA strand
breaks using fluorescein isothiocyanate (FITC)-conjugated dUTP. DNA
was also labeled with FITC DNA-binding dye for 5 min. FITC labels
were observed with a fluorescence microscope. As a positive
control, A549 cells were incubated in culture media without DDW for
48 h and treated with DNase I. The percentage of apoptotic cells
was calculated as the number of apoptotic cells divided by the
total number of cells.
Electrophoresis of DNA fragments
A549 cells were treated with 50 ppm DDW for 10, 24,
48 or 72 h. DNA and the DNA marker TrackIt 1 kb Plus Ladder (Kai
Gene) were separated on 1.5% agarose gels.
Animal experiment
Male BALB/c nude mice (weight 20±2 g) were purchased
from the ShangHai SLAC Laboratory Animal Co. Ltd. (Shanghai,
China). The 8-week-old mice used in this study were maintained in a
specific pathogen-free environment. Animal care and maintenance
were carried out in accordance with the Guide for the Care and Use
of Laboratory Animals by Long Hua Hospital, Shanghai, China.
For in vivo studies, the mice were randomly
divided into two groups of 8 animals each; the model group and
DDW-treated group. The model group mice and DDW-treated group mice
drank tap water and DDW, respectively. To construct the H460
xenograft model, cells were harvested after 14 days by
trypsinization, washed twice with PBS and re-suspended at
1×107 cells/ml. Approximately 2×106 H460
cells were injected subcutaneously into the right hind flank of all
mice. Animals were provided with DDW or tap water continually until
the end of the experiment.
Specimen preparation
Experimental pulmonary metastases were established
by inoculation of 2x106 H460 cells. Two months later,
the H460 xenograft model mice were sacrificed, and the tumors were
weighed. The tumor inhibition rate was calculated using the
following formula: tumor inhibition rate = (tumor weight of control
group - tumor weight of treatment group)/tumor weight of control
group × 100%.
Statistical analysis
Data are expressed as the mean ± SD. Differences
between groups were analyzed by analysis of variance (ANOVA) or the
Student’s t-test. Analyses were performed with SPSS software
version 13.0 (SPSS Inc., IL, USA). A P-value <0.05 was
considered statistically significant.
Results
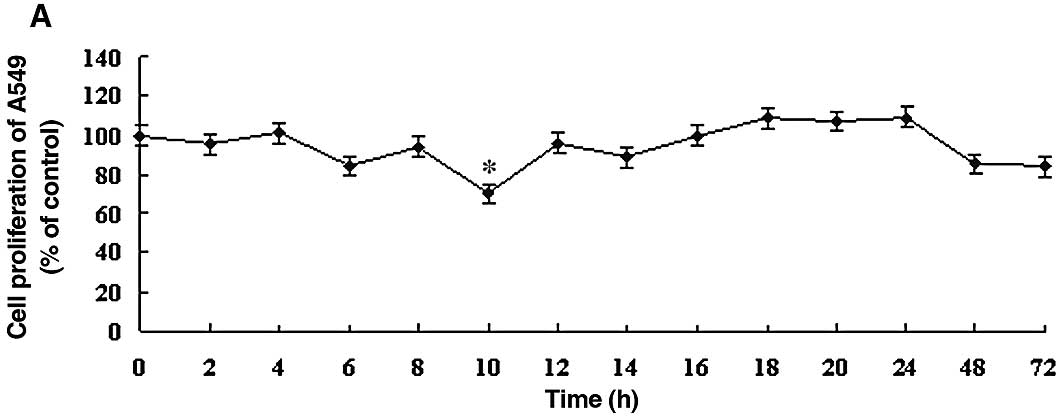
DDW inhibits the growth rates of A549 and
HLF-1 cells
To determine the optimal DDW treatment in the A549
human lung carcinoma cell line, the effect of DDW was assessed
using the MTT cell proliferation assay. The effects of DDW on the
growth and functional integrity of A549 cells are shown in Fig. 1. There were no significant changes
in the growth rate until 10 h of exposure to DDW. At 10 h, cell
viability of the treated cells (25, 50 or 105 ppm DDW) decreased
significantly to 70.39, 68.93 and 69.90%, respectively, compared to
the untreated controls (Fig. 1).
The cell growth rate subsequently returned to the level of the
controls at 48 h. After 72 h, cell viability at different DDW
concentrations decreased to 84.11, 75.23 and 86.44% of control
cells, respectively.
In contrast, DDW did not significantly alter the
growth of human embryonic lung fibroblast HLF-1 cells compared to
controls during the 72 h treatment (Fig. 2). Based on these results, we chose
to use 50 ppm DDW and A549 cells for subsequent experiments.
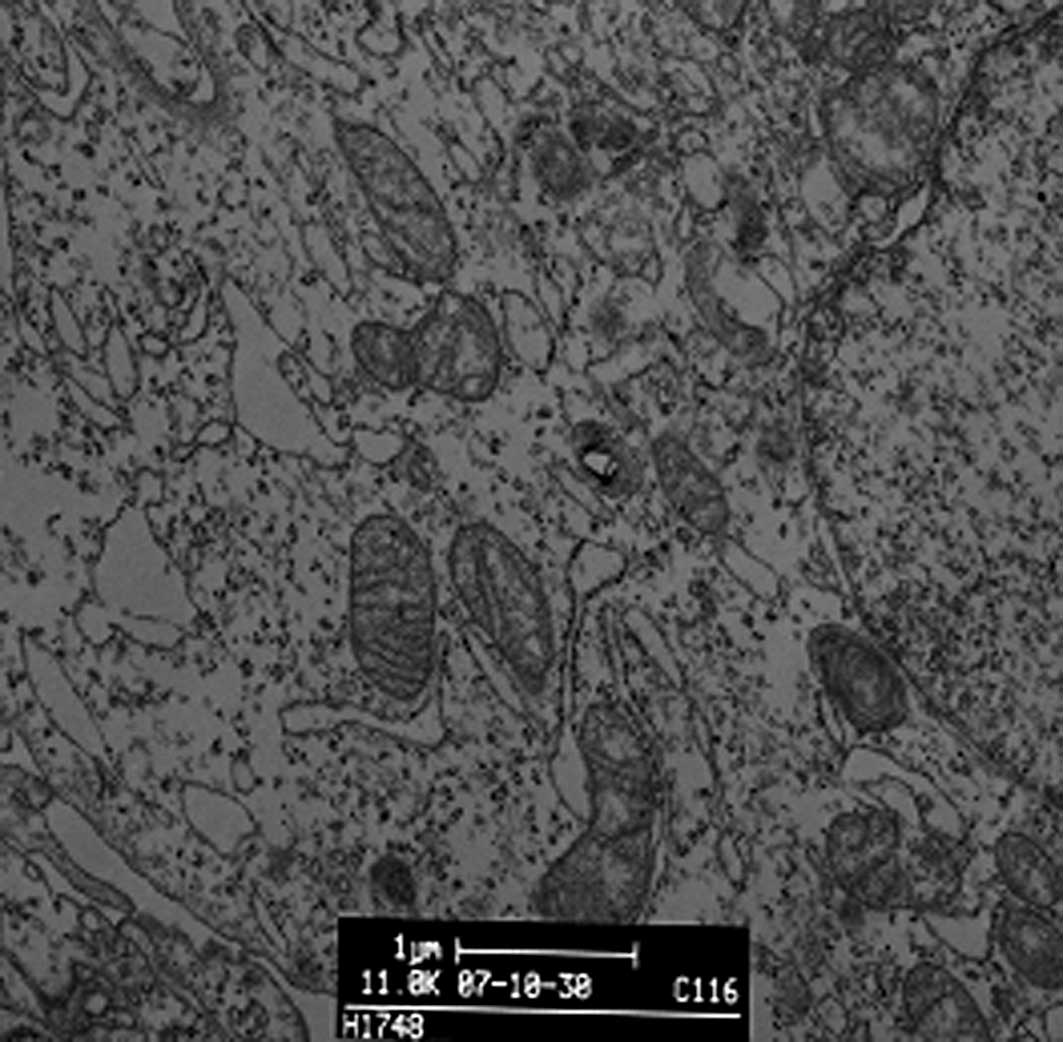
DDW treatment alters A549 cell morphology
and structure
We observed the morphology and structure of A549
cells by TEM. Untreated A549 cells showed a flattened profile of
cell morphology. There were no alterations to mitochondria, rough
or smooth endoplasmic reticulum, Golgi apparatus, lamellar bodies
or karyon (Fig. 3A). To observe
DDW-induced morphological changes, we incubated A549 cells with
50±5 ppm DDW for 10 or 72 h. After a 10-h treatment, a few myelin
bodies and physalides were observed in the cytoplasm (Fig. 3B), and more myelin bodies and
physalides were apparent after 72 h of treatment.
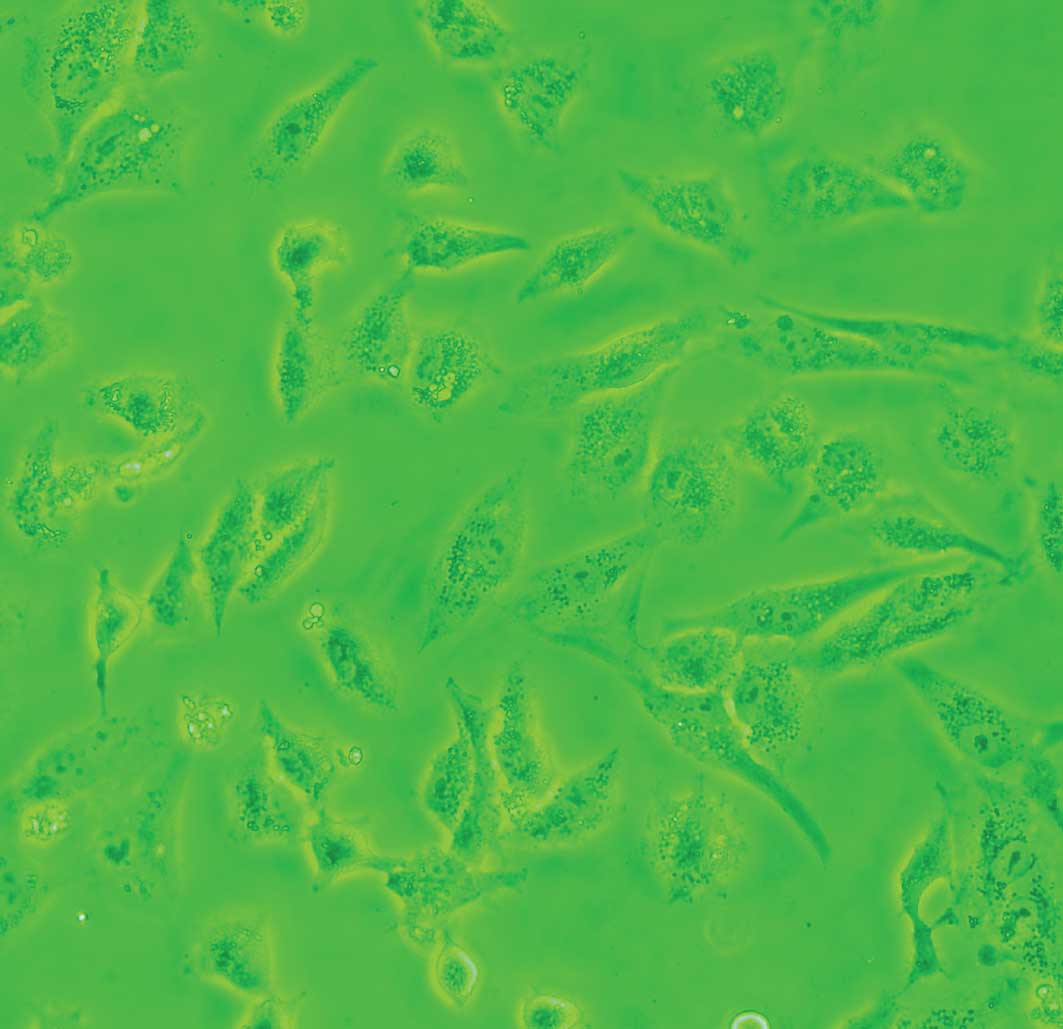
Cells exposed to DDW exhibited modified morphology,
which was more pronounced after 40 days of treatment. Untreated
control cells were shuttle-shaped or kidney-shaped (Fig. 4A), but they changed to an amorphous
polygon when treated with DDW (Fig.
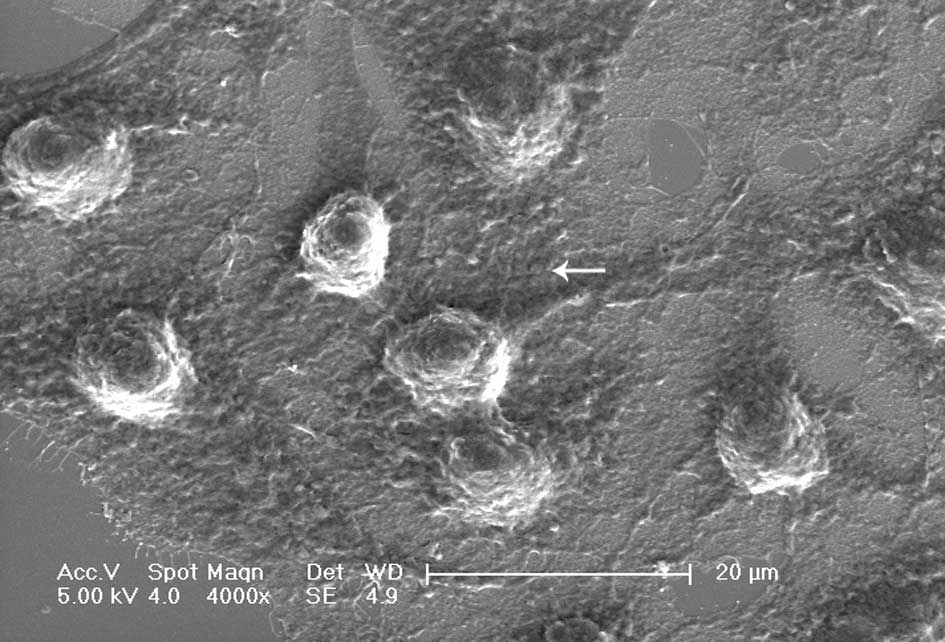
4B). Using SEM and TEM, we observed submicroscopic changes in
the morphology. Under SEM, untreated control cells showed a smooth
profile and more extracellular matrix than those exposed to DDW
(Fig. 5A and B). In contrast, A549
cells exposed to DDW had a rough profile and numerous microvilli on
the cell surface (Fig. 5C and D).
Under TEM, DDW-treated cells showed numerous myelin bodies in the
cytoplasm (Fig. 3D); however,
these changes were not observed in the control cells.
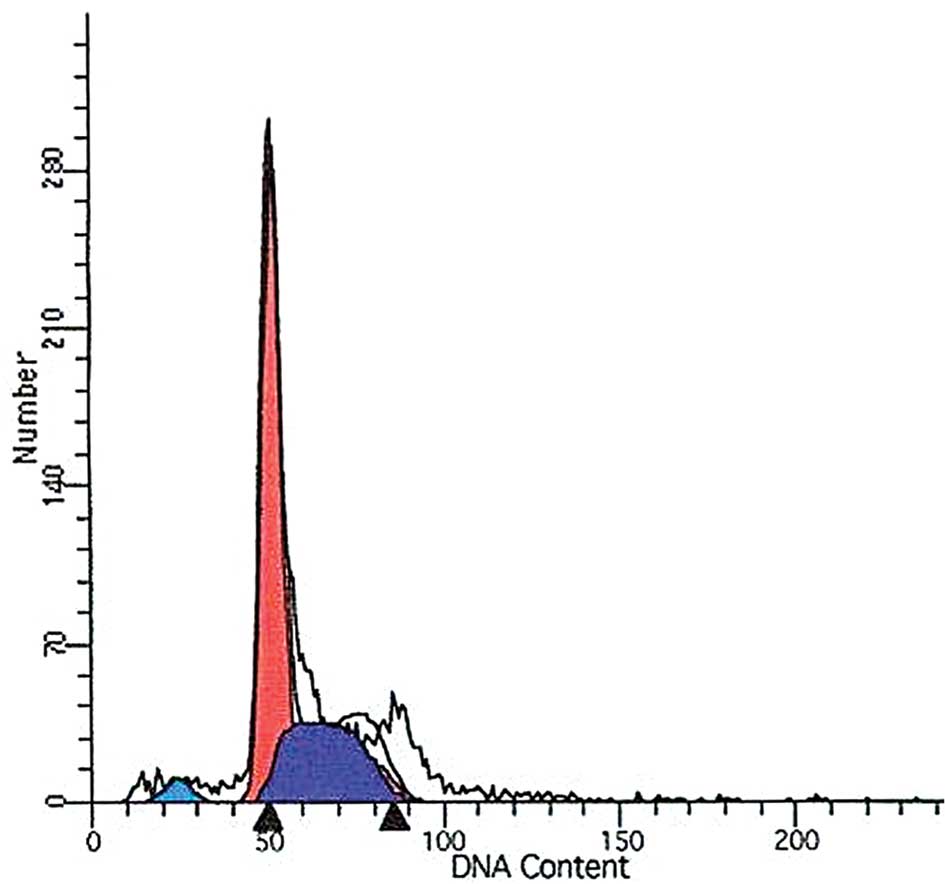
DDW treatment alters the cell cycle
To determine whether DDW alters the cell cycle in
A549 cells, we stained the cells with PI and used flow cytometry to
assess the sub-G1 population. Whereas no significant changes were
found in the proportion of cells in the sub-G1 phase between the
control cells and DDW-treated cells at 10 h (3.24 and 4.41%,
respectively), a significant increase in the proportion of cells in
the sub-G1 phase was observed in cells treated for 72 h (6.24 vs.
3.24%; Fig. 6). Meanwhile, cell
cycle alterations in DDW-treated cells were analyzed by flow
cytometry. The S phase increased whereas the G0 to G1 phase and G2
to M phase were reduced in DDW-treated cells compared to the
control cells (Table I; Fig. 6).
 | Table I.Cell cycle population in A549 cells
(mean ± SD). |
Table I.
Cell cycle population in A549 cells
(mean ± SD).
| Cycle | Control | 10 h | 72 h |
|---|
| Sub-G1 | 3.24±0.78 | 4.41±0.37a | 6.24±0.55a |
| G0–G1 | 60.11±2.15 | 58.63±2.40 | 55.23±1.47 |
| S phase | 32.65±0.78 | 38.47±0.29a | 44.03±0.35a |
| G2-M | 7.24±1.37 | 2.90±0.08a | 0.75±0.01a |
DDW-induced cell apoptosis
To ascertain whether DDW induces apoptosis in A549
cells, we treated cells with 50 ppm DDW and observed DDW-induced
apoptosis with the TUNEL assay (Fig.
7A). Apoptosis was evident in 31.39±2.54% of cells at 48 h and
25.38±3.90% at 72 h. The increased apoptosis was significant
compared with the untreated control group (10.87±1.11%; P<0.05,
Student’s t-test). DNA was extracted and analyzed by
electrophoresis. As shown in Fig.
7B, fragmented DNA was observed in cells treated with DDW for
48 or 72 h.
DDW influences tumor inhibition rates in
vivo
We investigated whether DDW inhibits the growth of
transplanted tumors in mice. After drinking DDW for 60 days, tumor
growth in nude mice was considerably reduced. We observed a
significant decrease by 30.80% on tumor inhibition rates in the DDW
group (Table II).
 | Table II.Tumor weight and inhibition rates of
nude mice. |
Table II.
Tumor weight and inhibition rates of
nude mice.
| Group | n | Tumor weight (g) | Inhibition rates
(%) |
|---|
| Control | 8 | 10.64±0.83 | - |
| DDW | 8 | 7.36±0.78a | 30.80 |
Discussion
In this study, we investigated the in vitro
effects of DDW on growth rate, morphology and structure of cells,
cell cycle distribution and apoptosis. We found that DDW
significantly suppressed the proliferation of A549 cells at 10 h.
This inhibitory effect disappeared from 12 to 24 h, but returned
during the prolonged 48- or 72-h treatment. In contrast, DDW
exerted no significant effects on HLF-1 cells, indicating a
cell-specific response to DDW treatment or a more rapid adaptation
for HLF-1 cells compared with A549 cells.
A previous study demonstrated that 30 ppm DDW
significantly decreased the growth rate of L929
fibroblast cells and also inhibited tumor growth in
xenotransplanted mice. Deuterium is crucial to the start of cell
proliferation as the lag period is 6–8 h longer in medium with low
D content (14). DDW also affects
seed germination; inhibition of germination was highest 5–6 days
after the beginning of germination, but the inhibition was not
observed after 10–12 days (18).
In addition, the biological effects of DDW on plant cells were
investigated in a previous study. In the first half hour of DDW
treatment, plants showed biochemical changes similar to those
induced by dark treatment; respiration increased, photosynthesis
stopped and intracellular pH became alkaline, whereas extracellular
pH became more acidic. Maximum effects were noted 30 min after
treatment, and then cells gradually returned to normal (19). The results in the present study are
remarkably similar to those of previous studies.
Cell division is sensitive to intracellular changes
in deuterium concentration, and a normal concentration of deuterium
is essential to initiate and to maintain normal cellular growth
(14). Our results appear to
support the hypothesis of Laskey et al, who hypothesized
that mechanisms exist in both animal and plant cells that detect
changes in deuterium concentration (19). It is necessary to reach the
threshold of intracellular D/H to initiate cell division. When
cells are cultured in a medium with low deuterium concentration,
proliferation is inhibited due to the increased time required to
reach the appropriate D/H ratio. In higher organisms, a regulatory
system has developed over millions of years, which is sensitive to
intracellular changes in D/H. The D/H ratio can increase more
rapidly in normal than in tumor cells (19). Tumor cells have a higher growth
rate than normal cells as a result of consuming a greater quantity
of deuterium (20). We observed
that in vitro proliferation of tumor cells was inhibited by
DDW, whereas proliferation of normal cells was not, suggesting that
DDW may influence the D/H ratio in tumor cells, which, in turn,
affects the growth rate.
In the present study, we found that DDW increases
the S phase cell population and inhibited the proliferation of A549
cells. A greater proportion of DDW-treated A549 cells were arrested
at S phase at 72 than at 10 h, as observed by flow cytometry. The
cell cycle regulatory system appeared to perceive the D/H ratio,
and at the threshold level it triggered the molecular mechanism
that finally caused the cell to enter into the S phase (19).
Cell apoptosis via intracellular mechanisms leads to
cell death. Many agents have been discovered to treat cancers by
inducing an abnormal cell cycle and apoptosis. Thus DDW may have
potential as a cancer therapy. Using TUNEL and DNA fragment
analyses we showed that DDW significantly increased the number of
apoptotic cells after 48 h, indicating that DDW may trigger a
molecular mechanism to induce cells to apoptosis. Gyongyi and
Somlyai found reduced expression of C-myc, Ha-ras and P53 in six
different organs (spleen, lung, thymus, kidney, liver and lymph
nodes) of nude mice in the DDW-treated group (21). They suggested that naturally
occurring deuterium may be involved in the regulation of genes that
play important roles in the cell cycle or tumor development
(21). Therefore, future studies
exploring the molecular mechanism of DDW-induced apoptosis are
vital to elucidate its tumor-inhibitory effects.
Our in vivo results revealed that DDW
significantly inhibited tumor growth. However, we do not know
whether the effect was caused by cell apoptosis. Further studies
are needed to elucidate the mechanism of DDW-induced tumor
inhibition in vivo.
In summary, we found that DDW exerts effects on the
cell cycle and changes in configuration and induces apoptosis in
vitro. We also found that DDW inhibits tumor growth in
xenotransplanted mice. Collectively, these findings suggest the
potential for DDW as an anti-tumor drug with clinical
application.
Acknowledgements
We acknowledge the financial support
from the Technology Centre of Luzhoulaojiao Co., Ltd. for the Top
Deuterium-Depleted Liquor Research Program.
References
|
1.
|
Carney DN: Lung cancer – time to move on
from chemotherapy. N Engl J Med. 346:126–128. 2002.
|
|
2.
|
Nishio K, Nakamura T, Koh Y, et al: Drug
resistance in lung cancer. Curr Opin Oncol. 11:109–115. 1999.
View Article : Google Scholar
|
|
3.
|
Swisher S and Roth JA: Clinical update of
Ad-p53 gene therapy for lung cancer. Surg Oncol Clin N Am.
11:521–535. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Criss RE: Principles of Stable Isotope
Distribution. Oxford University Press; New York: pp. 234–1999
|
|
5.
|
Collins CJ and Bowman NS: Isotope Effects
in Chemical Reactions. Van Nostrand Reinhold; New York: pp.
286–363. 1971
|
|
6.
|
Wiberg KB: The deuterium isotope effect.
Chem Rev. 55:713–743. 1955. View Article : Google Scholar
|
|
7.
|
Jancso G and van Hook WA: Condensed phase
isotope effects: especially vapor pressure isotope effects. Chem
Rev. 74:689–750. 1974. View Article : Google Scholar
|
|
8.
|
Rundel PW, Ehleringer JR and Nagy KA:
Stable Isotope in Ecological Research. Springer; New York: pp. 7–9.
1988
|
|
9.
|
Hughes AM, Tolbert BM, Lonberg-Holm K, et
al: The effect of deuterium oxide on survival of mice with ascites
tumor. Biochim Biophys Acta. 28:58–61. 1958. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Laissue JA, Bally E, Joel DD, et al:
Protection of mice from whole-body gamma radiation by deuteration
of drinking water. Radiat Res. 96:59–64. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Gross PR and Spindel W: Heavy water
inhibition of cell division: an approach to mechanism. Ann NY Acad
Sci. 90:500–522. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Katz JJ, Crespi HL, Czajka DM, et al:
Course of deuteriation and some physiological effects of deuterium
in mice. Am J Physiol. 203:907–913. 1962.PubMed/NCBI
|
|
13.
|
Katz JJ, Crespi HL and Hasterlik RJ: Some
observations on biological effects of deuterium with special
reference to effects on neoplastic processes. J Natl Cancer Inst.
18:641–659. 1957.PubMed/NCBI
|
|
14.
|
Somlyai G, Jancsó G, Jákli G, et al:
Naturally occurring deuterium is essential for the normal growth
rate of cells. FEBS Lett. 317:1–4. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Siniak IuE, Turusov VS, Grigorev AI, et
al: Consideration of the deuterium-free water supply to an
expedition to Mars. Aviakosam Ekolog Med. 37:60–63. 2003.PubMed/NCBI
|
|
16.
|
Turusov VS, Siniak IuE, Grigor’ev AI, et
al: Low-deuterium water effect on transplantable tumors. Vopr
Onkol. 51:99–102. 2005.PubMed/NCBI
|
|
17.
|
Tyrysov VS, Siniak IuE, Antoshina EE, et
al: The effect of preliminary administration of water with reduced
deuterium content on the growth of transplantable tumors in mice.
Vopr Onkol. 52:59–62. 2006.PubMed/NCBI
|
|
18.
|
Somlyai G: Defeating cancer! The
biological effect of deuterium depletion. Ramnicu Valcea, Romania
Conphys. 60–61. 2001.
|
|
19.
|
Laskay G, Somlyai G, Jancsó G, et al:
Reduced deuterium concentration of water stimulates
O2-uptake and electrogenic H+-efflux in the aquatic
macrophyte Elodea canadensis. Jpn J Deuterim Sci. 10:17–23.
2001.
|
|
20.
|
Berdea P, Cuna S, Cazacu M, et al:
Deuterium variation of human blood serum. Studia Universitatis
Babes-Bolyal, Physica. Special Issue. 256–258. 2001.
|
|
21.
|
Gyongyi Z and Somlyai G: Deuterium
depletion can decrease the expression of C-mys Ha-ras and p53 gene
in carcinogen-treated mice. In Vivo. 14:437–440. 2000.PubMed/NCBI
|