Introduction
It has been proposed that inflammation causes cancer
(1,2), and that approximately 18% of the
global cancer burden is attributable to infectious agents (3). Epstein-Barr virus (EBV) is a
ubiquitous DNA tumor virus, infecting almost all adults worldwide.
On the other hand, EBV has been thought to cause some restricted
tumors such as nasopharyngeal carcinoma, which occurs frequently in
Chinese populations, Burkitt’s lymphoma, which occurs in children
in East Africa, or approximately 10% of gastric carcinoma cases. It
is difficult to explain why the widely distributed EBV causes
endemic tumors in such restricted areas or races. We hypothesized
that EBV causes a wider variety of human tumors more frequently
than is thought at present. In studies using the mRNA in
situ hybridization method, it has been reported that EBV genes
are expressed in oral carcinoma (4), mesopharyngeal and hypopharyngeal
carcinoma (5), thyroid carcinoma
(6), renal cell carcinoma
(7), testicular tumors (8), uterine cervical carcinoma (9–11),
anaplastic large-cell lymphoma (12,13),
cutaneous T-cell lymphoma (14),
primary leptomeningeal lymphoma (15) and lymphoma originating in the lung
(16). Most of these tumors
expressed several EBV mRNAs and proteins. Other human tumors may
also be associated with EBV infection. This needs to be extensively
examined using several EBV mRNA probes, antibodies and primers. We
frequently used EBV-encoded non-polyadenylated RNA-1 (EBER1) in
situ hybridization in this study due to EBER expression in the
nucleus and macrophage CD68 expression in the cytoplasm, and so the
double staining was clearly visible. Other EBV mRNAs, for example
EBV nuclear antigen-2 (EBNA2), which is an oncogene of EBV, are
also important. In this study, we used four EBV probes:
BamHIW, EBNA2, EBV nuclear antigen leader protein (EBNA LP)
and EBER1. We detected EBNA2 mRNA and protein in almost all of the
tumors (4,6,7,10,14–16).
In the tumor cells mentioned above, the frequency of a correlation
with EBV was very high (more than 90%) in each disease. Through
these studies, the expression of EBV lytic infection protein BZLF1
by means of indirect immunofluorescence staining in the tumor cells
as well as infiltrating lymphocytes was frequently observed. It is
generally believed that EBV-related tumor cells express limited
genes which operate in tumorigenesis, but do not express lytic
proteins. However, Hoshikawa et al reported evidence of
lytic EBV infection in EBV-positive gastric carcinoma (17). Furthermore, Takasaka et al
observed EBV particles in established human gastric cancer cell
lines by employing electron microscopy (18). These reports suggest the presence
of productive EBV infection in human gastric cancer cells.
Therefore, not only EBV-carrying lymphocytes, but also tumor cells
may produce EBV. Lytic EBV infection of multiple tissues, may
provoke a strong inflammatory response, since cell lysis induced by
virus replication results in marked immune responses against viral
proteins.
Macrophages are derived from bone marrow
promonocytes, which develop into monocytes and infiltrate tissues.
There, they differentiate into a specific type of resident tissue
macrophage, such as microglial cells in the brain, Kupffer cells in
the liver and Langerhans’ cells in the skin. Their functions are to
protect the host from microbial infection, to regulate tissue
remodeling and to repair injury. Macrophages also comprise a major
component of the inflammatory infiltrate in tumors. Such cells are
termed tumor-associated macrophages (TAMs). TAMs can kill tumor
cells, but they also produce growth factors, angiogenic factors and
proteases which degrade the matrix. Through the action of these
macrophage-derived factors, tumor cell proliferation, angiogenesis,
tumor invasion and metastasis are accelerated (19).
EBV infection of a macrophage cell line was first
described by Revoltella et al (20). Furthermore, Savard et al
reported a lytic program of primary human macrophages induced by
EBV (21). We also showed the
expression and replication of EBV genes in cultured normal human
macrophages (22) and abnormal
histiocytes in Langerhans’ cell histiocytosis (LCH) (23,24).
Moreover, we revealed EBV expression in macrophages which had
infiltrated primary lung lymphoma (16). EBV infects macrophages as well as B
lymphocytes, T lymphocytes and epithelial cells. EBV-expressing
macrophages may play important roles in cancer-causing chronic EBV
infection and inflammation. To investigate the existence of
EBV-expressing macrophages in several human cancers, we studied
human cancer tissues that were already confirmed to express EBV
oncogenes and the lytic infection protein BZLF1.
Materials and methods
Patients
Five nasopharyngeal carcinoma, 5 oral cancer, 10
thyroid carcinoma, 2 renal cell carcinoma (RCC), 2 testicular
carcinoma, 11 uterine carcinoma, 4 cutaneous T-cell lymphoma, 2
anaplastic large-cell lymphoma cases and 1 case of chronic active
EBV infection were examined. EBV expression was previously detected
in the tumor cells. For a comparative study, 3 lichen planus, 2
Graves’ disease, 2 thyroid nodular hyperplasia, 1
glomerulosclerosis case and 3 cases of normal uterine cervix were
also examined. All samples used in this study have previously been
described (4–16). None of the patients had a history
or clinicopathological features indicative of an immunocompromised
state.
Probes
BamHIW probes were transcribed from 2.27-kb
EBV BamHIW fragments from which the ‘Alu-family’-like
sequence had been deleted. The BamHIW fragment of EBV is a
highly repetitive sequence that contains the mRNA leader sequence
for EBNAs. The fragment was cloned into the pBluescript II
SK+ vector. cDNA of the BamHIY1Y2 (EBNA LP)
region was also cloned into pBluescript II SK+. The size
of this cDNA was 153 bp. The sense and antisense probes were
labelled with digoxigenin-11-UTP by in vitro transcription
with T7 and T3 polymerases, respectively, using a commercial kit
(Boehringer Mannheim, Mannheim, Germany). EBNA2 cDNA
(14,802–48,583, including a spliced sequence) and EBER1 cDNA
(6,629–6,795) were synthesized through RT-PCR according to the
method reported by Tierney et al (25) and cloned into the pGEM-T Easy
Vector (Promega, Madison, WI, USA). The sizes of these cDNAs were
386 and 167 bp, respectively. The sense and antisense RNA probes
were labelled with digoxigenin-11-UTP by in vitro
transcription with T7 and SP6 polymerases, respectively, using a
commercial kit (Boehringer Mannheim). The labelled BamHIW
and EBNA2 riboprobes were then fragmented to ∼100 bases in length
by alkaline hydrolysis. The sense probe served as a negative
control.
Messenger RNA in situ hybridization
Paraffin sections were prepared from formalin-fixed
tissues from biopsied or surgically resected materials. Serial
sections were cut to 3–5 μm. After dewaxing and dehydration with
graded ethanol, slides were treated with 0.2 N HCl for 15 min at
room temperature (RT) and rinsed with phosphate-buffered saline
(PBS) for 5 min also at RT. They were then treated with 50 μg/ml
proteinase K in PBS for 15 min at 37°C and immersed in 2 mg/ml
glycine in PBS for 10 min at RT. Subsequently, the sections were
refixed with 4% paraformaldehyde in PBS for 15 min and washed twice
with PBS for 3 min at RT, then treated with 0.1 M triethanolamine
(pH 8.0) for 10 min at RT. After washing with PBS, the sections
were dehydrated with ethanol and hybridized for 40 h at 37°C for
EBNA2 and EBER1, 39°C for BamHIY1Y2 (EBNA LP) and 45°C for
BamHIW in 4X SSC, 50% formamide, 1X Denhardt’s solution, 5%
dextran sulfate, 0.5 mg/ml salmon sperm DNA, 0.5 mg/ml yeast tRNA
and 10 mM dithiothreitol. After hybridization, the sections were
washed twice with 2X SSC for 30 min and then twice with 0.5X SSC
for 20 min with gentle shaking at RT. They were subsequently
blocked with 1% skim milk (Difco) in 100 mM Tris and 0.15 M NaCl
(pH 7.5) for 30 min at RT. Next, they were reacted with 1:100 (for
EBNA2) and 1:200 diluted (for BamHIW, BamHIY1Y2 and
EBER1) alkaline phosphatase-labelled anti-DIG antibody (Boehringer
Mannheim) in blocking buffer for 2 h at RT. After washing, the
sections were incubated with nitroblue tetrazolium and X-phosphate
(Boehringer Mannheim) in buffer containing 0.1 M Tris, 0.1 M NaC1,
0.005 M MgCl2 and 1 mM levamisole (pH 9.6) for 16 h at
RT. The reaction was stopped with EDTA, and then the slides were
then dehydrated with graded ethanol and xylene and sealed with
malinol. These methods are a modified version of those we
previously reported (4–16).
Double staining with mRNA in situ
hybridization for EBV and immunostaining against macrophages
Tissue sections that had been prepared in the
previous studies, after in situ hybridization, were immersed
in 100% xylene, and the cover glasses were removed. After
rehydration with a series of descending concentrations of ethyl
alcohol and distilled water, the sections were exposed to
microwaves (500 W) for 5 min in 10 mM Tris and 1 mM EDTA (pH 9.0)
and digested with 0.05% trypsin in PBS at 37°C for 60 min. They
were subsequently immersed in 3% H2O2 in
methanol, washed with PBS and treated with x25 diluted anti-CD68
mouse monoclonal antibody (Dako, Glostrup, Denmark) for 60 min at
RT. Finally, the tissue sections were treated with a staining kit
(Histofine Simple Stain NAX-PO(M); Nichirei BioScience, Tokyo,
Japan) for 30 min.
Results
To clarify the presence of EBV expression in TAMs,
we carried out immunohistochemical staining using the anti-CD68
monoclonal antibody in sections already hybridized with EBV mRNA by
in situ hybridization. Tissue specimens of 5 nasopharyngeal
carcinoma, 5 oral cancer, 10 thyroid carcinoma (involving 4 cases
of papillary carcinoma, 1 of squamous cell carcinoma and 5 of
undifferentiated carcinoma), 2 renal cell carcinoma, 2 testicular
carcinoma, 3 uterine cervical intraepithelial neoplasia, 8 invasive
uterine carcinoma (involving 6 cases of cervical squamous cell
carcinoma and 2 of corpus adenocarcinoma), 4 cutaneous T-cell
lymphoma, 2 anaplastic large-cell lymphoma cases and 1 case of
chronic active EBV infection were prepared. For a comparative
study, oral mucosal tissues of 3 cases of lichen planus, thyroid
tissues of 2 cases (each of Graves’ disease and nodular
hyperplasia), kidney tissue of 1 case of glomerulosclerosis and
mucosal tissue of 3 cases of normal cervix were also prepared.
Hybridization signals appeared blue-purple in the nucleus with the
EBER1 probe and in the cytoplasm with the others (BamHIW,
EBNA2 and EBNA LP), whereas immunohistochemical staining with CD68
appeared dark brown in the cytoplasm. The results of double
staining with EBV mRNA in situ hybridization and CD68
immunostaining are summarized in Table
I and representative images are shown in Fig. 1. Double-stained TAMs were detected
in almost all tissues of the EBV-associated neoplasms examined.
Generally, the more macrophages were detected in the tissue, the
more they were double-stained. Although the number of TAMs was
variable between cases, the ratio of double-stained macrophages to
all macrophages was highest in the uterine cervical carcinoma, then
oral cancer, undifferentiated thyroid carcinoma and nasopharyngeal
carcinoma, when restricted to diseases for which we examined more
than 5 cases (Table I). In most
cases, macrophages also infiltrated into each comparative
non-cancerous tissue, whereas they were not double-stained
(Table I, Fig. 1c). In the nasopharyngeal carcinoma
of case 1, tissue around the cancer showed many EBV-expressing
epithelial cells (small arrowhead), a moderate number of
infiltrating macrophages (large arrowhead) and several
double-stained macrophages (double arrowhead). Since both signals
of BamHIW and CD68 staining were expressed in the cytoplasm,
the color of double-stained cells appeared black (Fig. 1a). In the oral cancer case 1, EBER1
signals were expressed in the nucleus and CD68 in the cytoplasm
(Fig. 1b). In the case of lichen
planus, a non-cancerous oral disease, there were many macrophages
stained with CD68; however, no double-stained macrophage was
observed (Fig. 1c). In the thyroid
carcinomas, undifferentiated carcinoma expressed more EBV RNA than
the papillary carcinoma cases (6);
however, the number of double-stained macrophages was not
significantly higher in the undifferentiated than in the papillary
carcinomas (Table I). The number
of TAMs that had infiltrated the uterine tissue was higher in the
uterine cervical than in the uterine corpus carcinoma cases, and
double staining was clearer in the former than in the latter
(Fig. 1f and g). EBV expression of
CIN was similar to invasive cervical carcinoma (9), and the number of double-stained
macrophages was not significantly different between CIN and
invasive carcinoma (Table I). The
normal cervix showed few macrophages in the tissue (Fig. 1h). TAMs dually expressing EBV and
CD68 were observed in the renal cell (Fig. 1i) and testicular (Fig. 1j) carcinomas. Moreover, in the
tissues of the cutaneous T-cell lymphoma and anaplastic large-cell
lymphoma, dually expressed TAMs were detected (Fig. 1k and l). Bone marrow macrophages
derived from chronic active EBV infection also showed double
staining for EBV and CD68 (Fig.
1m).
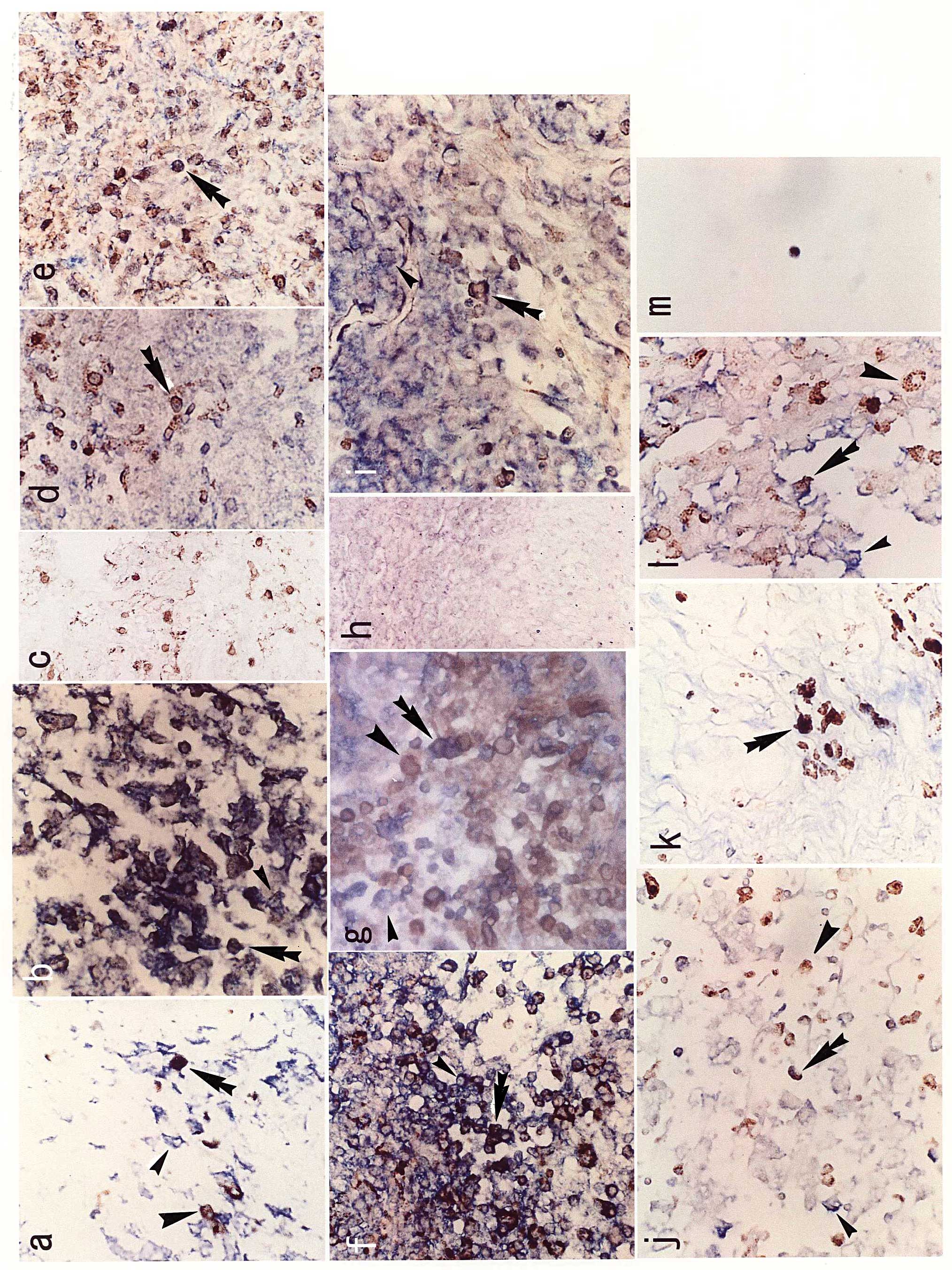 | Figure 1.Results of the double staining of EBV
mRNA on in situ hybridization and immunostaining with CD68.
EBV-expressing cells are indicated by the small arrowhead;
macrophages are indicated by the large arrowhead; double-stained
macrophages are indicated by the double arrowhead. (a)
Nasopharyngeal carcinoma, case 1, BamHIW antisense probe,
×40; (b) oral cancer, case 1, EBER1 antisense probe, ×40; (c)
lichen planus for non-cancerous control of oral cancer, case 1,
EBER1 antisense probe, ×20; (d) thyroid papillary carcinoma, case
3, EBER1 antisense probe, ×40; (e) thyroid undifferentiated
carcinoma, case 1, EBER1 antisense probe, ×40; (f) uterine cervical
carcinoma, case 1, EBNA2 antisense probe, ×40; (g) uterine corpus
carcinoma, case 1, EBNA2 antisense probe, ×40; (h) normal cervix,
case 1, EBER1 antisense probe, x20; (i) renal cell carcinoma, case
2, BamHIW antisense probe, x40; (j) testicular carcinoma,
case 2, BamHIW antisense probe, x40; (k) cutaneous T-cell
lymphoma, case 4, EBNA2 antisense probe, x40; (l) anaplastic
large-cell lymphoma, case 1, EBNA2 antisense probe, x40; (m)
chronic active EBV infection, case 1, EBNA LP antisense probe,
x40. |
 | Table I.Summary of results. |
Table I.
Summary of results.
| Disease | Histology | Case | Probe for ISH |
Double-stained/CD68-positivea | Rate | Average b |
|---|
| NPC | SCC | 1 | BamHIW | 9/35 | 0.26 | |
| SCC | 2 | BamHIW | 6/28 | 0.21 | |
| SCC | 3 | BamHIW | 7.5/31.5 | 0.24 | |
| SCC | 4 | BamHIW | 9/29 | 0.31 | |
| SCC | 5 | BamHIW | 3/28 | 0.11 | 0.226 |
| Oral cancer | SCC | 1 | EBER1 | 27/59.5 | 0.45 | |
| SCC | 2 | EBER1 | 12/30.6 | 0.39 | |
| SCC | 3 | EBER1 | 4.5/14 | 0.32 | |
| SCC | 3 | EBNA2 | 10/36 | 0.28 | |
| SCC | 4 | EBNA2 | 11/41 | 0.27 | |
| SCC | 5 | EBNA2 | 0/0 | 0 | 0.285 |
| Lichen planus | | 1 | EBER1 | 0/67 | 0 | |
| | 2 | EBNA LP | 0/24 | 0 | |
| | 3 | EBNA LP | 0/73 | 0 | 0 |
| Thyroid ca. | Pap. ca. | 1 | EBER1 | 5/20 | 0.25 | |
| Pap. ca. | 2 | EBER1 | 3/20 | 0.15 | |
| Pap. ca. | 3 | EBER1 | 7/34 | 0.21 | |
| Pap. ca. | 3 | BamHIW | 2/18 | 0.11 | |
| Pap. ca. | 4 | BamHIW | 1.5/14 | 0.12 | 0.168 |
| SCC | 1 | EBER1 | 6/53 | 0.11 | 0.11 |
| Undiff. ca. | 1 | EBER1 | 12.5/97.5 | 0.13 | |
| Undiff. ca. | 2 | EBER1 | 12.7/43 | 0.29 | |
| Undiff. ca. | 3 | EBER1 | 9.5/93 | 0.10 | |
| Undiff. ca. | 4 | EBER1 | 10/36 | 0.28 | |
| Undiff. ca. | 5 | EBER1 | 9/19.3 | 0.47 | 0.248 |
| Graves’
disease | | 1 | BamHIW | 0/12 | 0 | |
| | 2 | BamHIW | 0/13 | 0 | |
| Nodular
hyperplasia | | 1 | EBER1 | 0/45 | 0 | |
| | 2 | EBER1 | 0/51 | 0 | 0 |
| RCC | Clear cell | 1 | BamHIW | 1/9 | 0.11 | |
| Clear cell | 1 | EBNA LP | 15/65 | 0.23 | |
| Clear cell | 2 | BamHIW | 21/40 | 0.47 | |
| Clear cell | 2 | EBNA LP | 30/70 | 0.43 | 0.308 |
|
Glomerulosclerosis | | 1 | EBER1 | 0/4 | 0 | 0 |
| Testicular ca. | Seminoma | 1 | BamHIW | 2/12 | 0.17 | |
| Seminoma | 2 | BamHIW | 8/43 | 0.19 | 0.185 |
| Uterine CIN3 | SCC | 1 | EBER1 | 1/3.7 | 0.27 | |
| SCC | 1 | EBNA2 | 1/1.7 | 0.59 | |
| SCC | 2 | EBER1 | 5.5/10.75 | 0.51 | |
| SCC | 2 | EBNA2 | 3.7/15.7 | 0.24 | |
| SCC | 3 | EBER1 | 11/33.4 | 0.32 | |
| SCC | 3 | EBNA2 | 9.5/67.5 | 0.14 | 0.387 |
| Invasive uterine
cervical ca. | SCC | 1 | EBNA2 | 39/52 | 0.75 | |
| SCC | 2 | EBNA2 | 12/37 | 0.32 | |
| SCC | 3 | EBNA2 | 20.5/40.5 | 0.51 | |
| SCC | 4 | EBNA2 | 4/17 | 0.24 | |
| SCC | 5 | BamHIW | 26/46.5 | 0.56 | |
| SCC | 6 | EBER1 | 23.7/78.7 | 0.30 | |
| SCC | 6 | EBNA2 | 20.4/60.6 | 0.34 | 0.432 |
| Normal cervix | | 1 | EBER1 | 0/0 | 0 | |
| | 2 | EBER1 | 0/3 | 0 | |
| | 2 | EBNA2 | 0/7.5 | 0 | |
| | 3 | EBER1 | 0/13.5 | 0 | |
| | 3 | EBNA2 | 0/7 | 0 | 0 |
| Uterine cp.
ca. | Adenoca. | 1 | EBNA2 | 26.5/64 | 0.41 | |
| Adenoca. | 2 | EBER1 | 0/0 | 0 | 0.205 |
| CTCL | | 1 | EBER1 | 4.5/33 | 0.13 | |
| | 2 | EBER1 | 8.6/22 | 0.39 | |
| | 3 | EBNA2 | 10/37 | 0.27 | |
| | 4 | EBNA2 | 12.5/34 | 0.37 | 0.29 |
| ALCL | | 1 | BamHIW | 6/40 | 0.15 | |
| | 2 | BamHIW | 4/39 | 0.10 | 0.125 |
| Chr. ac. EBV
infect. | | 1 | EBNA LP | 2/2 | 1 | |
Discussion
In the present study, double-stained TAMs were
detected in almost all tissues of the EBV-associated neoplasms
examined. Tissues from normal controls or those from non-cancerous
disease cases sometimes contained many macrophages; however, they
were not TAMs and were never double-stained. On the other hand,
macrophages in a case with chronic active EBV infection (without
any neoplasms) were also double-stained (Fig. 1m). We previously reported EBV
expression in cultured macrophages from normal tissues of the
bronchus and testis, and in cultured epididymitis macrophages
(22). In the present study,
however, macrophages in the normal or non-cancerous tissues did not
show EBV expression. This may have been due to the selection of
EBV-carrying macrophages, which is very rare in normal or
non-cancerous tissues, but has a growth advantage in the process of
cell culture. We previously reported EBV-expressing macrophages in
the parotid tumor, non-Hodgkin’s lymphoma (22), LCH (23,24)
and primary lung lymphoma (16).
These results indicate that TAMs of EBV-related neoplasms as well
as macrophages infiltrating tissues with chronic active EBV
infection express EBV mRNA. In the case of chronic active EBV
infection, the selection of EBV-carrying macrophages, which is
similar to cell culture, may occur in the bone marrow.
Furthermore, double-stained TAMs were detected in
the thyroid papillary carcinoma and CIN at almost the same level as
in the thyroid undifferentiated and invasive cervical carcinomas,
respectively. This suggests the earlier association of TAMs in the
process of EBV oncogenesis.
As mentioned previously, most of these tumors were
already confirmed to express EBV oncogene EBNA2 and lytic infection
protein BZLF1; therefore, it can be said that TAMs in EBV-related
tumors involve EBV-carrying macrophages, and may also produce EBV.
We reported that childhood LCH expressed high levels of EBV lytic
infection protein, and that in one case the administration of
acyclovir resulted in complete remission (23). If inflammation caused by EBV
production in the tumor tissue is always as intense as we observed,
we can expect that the administration of an anti-herpesvirus drug
will be more effective and safer than the usual anti-cancer
chemotherapy. These lytic infections of TAMs may also be a target
of inflammation. We observed that the more macrophages were
detected in the tissue, the more they were double-stained by
insitu hybridization and CD68 immunostaining. This suggests
a strong correlation between EBV-carrying TAMs and inflammation.
Notably, a correlation between infiltrating macrophages and the
risk or poor prognosis of cervical intraepithelial neoplasia
(26), uterine endometrioid
adenocarcinoma (27) and RCC
(28) was reported, although it is
unknown whether EBV infection is associated with these
macrophages.
Chemical agent-associated chronic inflammation with
oxidative and nitrative DNA damage was reported by Kawanishi et
al. They described 8-nitroguanine as a potential biomarker for
evaluating the risk of inflammation-related carcinogenesis
(29). The correlation between
8-nitroguanine and EBV has been studied in cases of nasopharyngeal
carcinoma (30) and oral cancer
(31). Moreover, Ma et al
showed that the cells responsible for the reaction are macrophages
(30).
Recently, the suppression of HIV replication by
human herpesvirus 6 (32) or 7
(33) was reported. Furthermore,
it was reported that latently infected murine-γ herpesvirus 68,
which is genetically very similar to EBV, confers resistance
against Listeria monocytogenes and Yersinia pestis in
mice (34). Such virus-virus or
virus-microbe interactions may be important when considering
oncogenesis due to inflammation caused by viral infection. We
previously reported that EBV genes of BamHIW (9), EBNA2 (10) and EBNA LP (11) were expressed in uterine cervical
carcinoma tissue. The frequency of the correlation was higher with
EBV than with human papillomavirus (HPV) (9). Almost all cervical carcinoma samples
also carried the HPV16 gene (9),
and E6–E7 proteins of HPV16 were reported to induce uncontrollable
cell growth (35). Through this
study, TAMs in CIN as well as cervical and a part of corpus
carcinoma were shown to express EBV mRNA. Therefore, we hypothesize
that EBV infection may synergistically act with HPV to cause the
development or progression of cancer through the long-term
inflammation induced by infiltrating macrophages carrying EBV.
EBV-associated tumors other than uterine carcinoma were also
suspected to be caused by long-term inflammation with EBV alone or
EBV and another unidentified virus or microbe. Moreover, the role
of macrophages, not only in inflammation but also in the
interaction between viruses or viruses and microbes, should be
clarified. Through these studies of tumor and virus-related
inflammation involving macrophages, it may be possible to fully
elucidate the dynamic mechanism of EBV oncogenesis.
Abbreviations:
|
EBV
|
Epstein-Barr virus;
|
|
LCH
|
Langerhans’ cell histiocytosis;
|
|
TAM
|
tumor-associated macrophages;
|
|
NPC
|
nasopharyngeal carcinoma;
|
|
RCC
|
renal cell carcinoma;
|
|
CTCL
|
cutaneous T-cell lymphoma;
|
|
ALCL
|
anaplastic large-cell lymphoma;
|
|
EBER1
|
EBV-encoded non-polyadenylated
RNA-1;
|
|
EBNA2
|
EBV nuclear antigen-2;
|
|
EBNA LP
|
EBV nuclear antigen leader
protein;
|
|
BZLF1
|
BamHIZ coding leftward reading
frame-1;
|
|
CIN
|
cervical intraepithelial neoplasia
|
Acknowledgements
We thank Drs T. Sasagawa (Kanazawa
University), K. Kawahara (Osaka Prefectural Medical Center for
Respiratory and Allergic Diseases), T. Shinka, S. Tamura, H.
Nakamine (Wakayama Medical College), K. Horii (Osaka Dental
University), S. Yanoma (Yokohama City University), T. Kozuka and T.
Oka (Osaka National Hospital) for their generous gifts of
materials. This study was supported by a Grant-in-Aid for Cancer
Research from the Ministry of Health, Labour and Welfare of
Japan.
References
|
1.
|
Balkwill F and Mantovani A: Inflammation
and cancer: back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
IARC World Cancer Report: Chronic
Inflammations. IARC Press; Lyon: pp. 56–61. 2003
|
|
4.
|
Shimakage M, Horii K, Tempaku A, Kakudo K,
Shirasaka T and Sasagawa T: Association of Epstein-Barr virus with
oral cancers. Hum Pathol. 33:608–614. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Shimakage M, Sasagawa T, Yoshino K,
Yutsudo M, Kimura M, Yamamoto N and Yanoma S: Expression of
Epstein-Barr virus in mesopharyngeal and hypopharyngeal carcinomas.
Hum Pathol. 30:1071–1076. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Shimakage M, Kawahara K, Sasagawa T, Inoue
H, Yutsudo M, Yoshida A and Yanoma S: Expression of Epstein-Barr
virus in thyroid carcinoma correlates with tumor progression. Hum
Pathol. 34:1170–1177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Shimakage M, Kawahara K, Harada S,
Sasagawa T, Shinka T and Oka T: Expression of Epstein-Barr virus in
renal cell carcinoma. Oncol Rep. 18:41–46. 2007.PubMed/NCBI
|
|
8.
|
Shimakage M, Oka T, Shinka T, Kurata A,
Sasagawa T and Yutsudo M: Involvement of Epstein-Barr virus
expression in testicular tumors. J Urol. 156:253–257. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Sasagawa T, Shimakage M, Nakamura M,
Sakaike J, Ishikawa H and Inoue M: Epstein-Barr virus (EBV) genes
expression in cervical intraepithelial neoplasm and invasive
cervical cancer: a comparative study with human papillomavirus
(HPV) infection. Hum Pathol. 31:318–326. 2000. View Article : Google Scholar
|
|
10.
|
Shimakage M and Sasagawa T: Detection of
Epstein-Barr virus-determined nuclear antigen-2 mRNA by in situ
hybridization. J Virol Methods. 93:23–32. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Shimakage M, Harada S, Kawahara K, Oka T,
Yanoma S, Horii K and Sasagawa T: Detection of Epstein-Barr virus
nuclear antigen leader protein expression in various human cancers.
New Developments in Epstein-Barr Virus Research. Umar CS: Nova
Science Publishers; New York: pp. 261–276. 2006
|
|
12.
|
Shimakage M, Dezawa T, Tamura S, Tabata T,
Aoyagi N, Koike M, Inoue H, Yutsudo M, Hakura A and Ikegami N: A
Ki-1-positive cell line expressing Epstein-Barr virus antigen
established from a child with Ki-positive lymphoma. Intervirology.
36:215–224. 1993.PubMed/NCBI
|
|
13.
|
Shimakage M, Nakamine H, Tamura S,
Takenaka T, Yutsudo M and Hakura A: Detection of Epstein-Barr virus
transcripts in anaplastic large-cell lymphomas by mRNA in situ
hybridization. Hum Pathol. 28:1415–1419. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Shimakage M, Sasagawa T, Kawahara K,
Yutsudo M, Kusuoka H and Kozuka T: Expression of Epstein-Barr virus
in cutaneous T-cell lymphoma including mycosis fungoides.
Int J Cancer. 92:226–231. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Nakajima H, Shimakage M, Takeda Y,
Furutama D, Sugino M, Kimura F, Shibayama Y and Hanafusa T:
Epstein-Barr virus-associated primary leptomeningeal lymphoma. Eur
J Neurol. 13:e4–e6. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Shimakage M, Sakamoto H, Harada S,
Sasagawa T and Kodama K: Expression of the Epstein-Barr virus in
lymphoproliferative diseases of the lung. Oncol Rep. 17:1347–1352.
2007.PubMed/NCBI
|
|
17.
|
Hoshikawa Y, Satoh Y, Murakami M, Maeta M,
Kaibara N, Ito H, Kurata T and Sairenji T: Evidence of lytic
infection of Epstein-Barr virus (EBV) in EBV-positive gastric
carinoma. J Med Virol. 99:351–359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Takasaka N, Tajima M, Okinaga K, Satoh Y,
Hoshikawa Y, Katsumoto T, Kurata T and Sairenji T: Productive
infection of Epstein-Barr virus (EBV) in EBV-genome-positive
epithelial cell lines (GT38 and GT39) derived from gastric tissues.
Virology. 247:152–159. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Lewis CE and Pollard JW: Distinct role of
macrophages in different tumor microenvironments. Cancer Res.
66:605–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Revoltella RP, Vigneti E, Fruscalzo A,
Park M, Ragona G, Rocchi G and Calef E: Epstein-Barr virus DNA
sequences in precursor monocyte-macrophage cell line established
from the bone marrow of children with maturation defects of
haematopoiesis. J Gen Virol. 70:1203–1215. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Savard M, Belanger C, Tardif M, Gourde P,
Flamand L and Gosselin J: Infection of primary human monocytes by
Epstein-Barr virus. J Virol. 74:2612–2619. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Shimakage M, Kimura M, Yanoma S, Ibe M,
Yokota S, Tsujino G, Kozuka T, Dezawa T, Tamura S, Ohshima A,
Yutsudo M and Hakura A: Expression of latent and
replicative-infection genes of Epstein-Barr virus in macrophage.
Arch Virol. 144:157–166. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Shimakage M, Sasagawa T, Kimura M,
Shimakage T, Seto S, Kodama K and Sakamoto H: Expression of
Epstein-Barr virus in Langerhans’ cell histiocytosis. Hum Pathol.
35:862–868. 2004.
|
|
24.
|
Shimakage M: Langerhans cell histiocytosis
and its relationship with Epstein-Barr virus – reply. Hum Pathol.
37:1509–1511. 2006.
|
|
25.
|
Tierney RJ, Steven N, Young LS and
Rickinson AB: Epstein-Barr virus latency in blood mononuclear
cells, analysis of viral gene transcription during primary
infection and in the carrier state. J Virol. 68:7374–7385.
1994.PubMed/NCBI
|
|
26.
|
Hammes LS, Tekmal RR, Naud P, Edelweiss
MI, Kirma N, Valente PT, Syrjanen KJ and Cunha-Filho JS:
Macrophages, inflammation and risk of cervical intraepithelial
neoplasia (CIN) progression – clinicopathological correlation.
Gynecol Oncol. 105:157–165. 2007.PubMed/NCBI
|
|
27.
|
Soeda S, Nakamura N, Ozeki T, Nishiyama H,
Hojo H, Yamada H, Abe M and Sato A: Tumor-associated macrophages
correlate with vascular space invasion and myometrial invasion in
endometrial carcinoma. Gynecol Oncol. 109:122–128. 2008. View Article : Google Scholar
|
|
28.
|
Hamada I, Kato M, Yamasaki T, Iwabuchi K,
Watanabe T, Yamada T, Isoyama S, Ito H and Okada K: Clinical
effects of tumor-associated macrophages and dendritic cells on
renal cell carcinoma. Anticancer Res. 22:4281–4284. 2002.PubMed/NCBI
|
|
29.
|
Kawanishi S, Hiraku Y, Pinlaor S and Ma N:
Oxidative and nitrative DNA damage in animals and patients with
inflammatory diseases in relation to inflammation-related
carcinogenesis. Biol Chem. 387:365–372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Ma N, Kawanishi M, Hiraku Y, Murata M,
Huang GW, Huang Y, Luo DZ, Mo WG, Fukui Y and Kawanishi S: Reactive
nitrogen species-dependent DNA damage in EBV-associated
nasopharyngeal carcinoma: the relation to STAT3 activation and EGFR
expression. Int J Cancer. 122:2517–2525. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Chayarit P, Ma N, Hiraku Y, Pinlaor S,
Yangvanit P, Jintakanon D, Murata M, Oikawa S and Kawanishi S:
Nitrative and oxidative DNA damage in oral lichen planus in
relation to human oral carcinogenesis. Cancer Sci. 96:553–559.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Grivel J-C, Ito Y, Faga G, Santoro F,
Shaheen F, Malnati MS, Fitzgerald W, Lusso P and Margolis L:
Suppression of CCR5-but not CXCR4-tropic HIV-1 in lymphoid tissue
by human herpesvirus 6. Nat Med. 7:1232–1235. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Lisco A, Grivel JC, Biancotto A,
Vanopouille C, Origgi F, Malnati MS, Schols D, Lusso P and Margolis
LB: Viral interaction in human lymphoid tissue: human herpesvirus 7
suppresses the replication of CCR5-tropic human immunodeficiency
virus type 1 via CD4 modulation. J Virol. 81:708–717. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Barton ES, White DW, Cathelyn JS,
Brett-McClellan KA, Engle M, Diamond D, Miller VL and Virgin HW IV:
Herpesvirus latency confers symbiotic protection from bacterial
infection. Nature. 447:326–330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Sasagawa T: Human papillomavirus infection
and cervical cancer. Biomed Rev. 14:75–93. 2003. View Article : Google Scholar
|















