Introduction
Angiogenetic factors play key roles in urological as
well as other types of cancer. In recent years, the expression of
angiogenic factors in solid human tumors has been widely reported
(1). Growth factors secreted by
tumor cells, such as fibroblast and transforming growth factors,
increase neovascularization in vivo and in vitro
(2). Studies have demonstrated
that peroxisome proliferator-activated receptor (PPAR)-γ ligands
inhibit the growth of cancer cells in vitro and in
vivo (3). PPAR-γ, a nuclear
hormone receptor, provides a strong link between lipid metabolism
and the regulation of gene transcription (4). PPAR-γ acts in the adipose tissue and
promotes lipogenesis under anabolic conditions. Recently, the
receptor has also been implicated in inflammation and
carcinogenesis. Significant evidence from many experimental systems
suggests that PPAR-γ plays an important role in carcinogenesis.
Angiotensin II is known as a key biological peptide
in the renin-angiotensin system, which regulates blood pressure and
renal hemodynamics, and angiotensin II receptor blockers (ARBs) are
widely used as anti-hypertensive drugs (5). It is well known that angiogenesis is
essential for tumor progression and metastasis (6,7).
Several studies have shown that angiotensin II induces
neovascularization and that ARBs inhibit vascular endothelial
growth factor (VEGF) production (8,9).
Benson et al discovered a structural resemblance between
telmisartan (a type of ARB) and a PPAR-γ ligand approved for the
treatment of type II diabetes, and reported that telmisartan has
PPAR-γ action (10).
Our previous research revealed the expression of
PPARs in human urological cancers and investigated the
administration of PPAR-γ ligands as an anti-cancer therapy
(11–15). With this background, the present
study aimed to evaluate the inhibitory effect of telmisartan on
human renal cell carcinoma (RCC), bladder cancer (BC), prostate
cancer (PC) and testicular cancer (TC) cell lines, and to determine
whether telmisartan induces apoptosis in such cells.
Materials and methods
Reagents and materials
RPMI-1640 was purchased from Nissui Pharmaceutical
Company (Tokyo, Japan). Fetal bovine serum (FBS) and
penicillin-streptomycin mixture were from Biowhittaker
(Walkersville, MD, USA). Trypsin/EDTA was from Gibco BRL
(Rockville, MD, USA). The angiotensin II receptor blockers
telmisartan, candesartan, valsartan and irbesartan were from
Toronto Research Chemicals, Inc. (Ontario, Canada). One of the
ARBs, losartan, was from Cayman Chemical (Ann Arbor, MI, USA).
Cell cultures
The human RCC cell line (Caki-1) was provided by Dr
Shinichi Ikemoto (Department of Urology, Osaka City University
School of Medicine, Osaka, Japan). The human BC cell line
(transitional cell carcinoma T24), PC cell lines (LNCaP, PC3 and
DU-145) and TC cell line (NEC-8) were obtained from the Health
Science Research Resources Bank (HSRRB, Osaka, Japan). Cells were
grown in a culture flask (Nunc, Roskilde, Denmark) in RPMI-1640
supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml
streptomycin in a humidified 5% CO2 atmosphere at 37°C.
The media were changed every 3 days, and the cells were separated
via trypsinization using trypsin/EDTA upon reaching
subconfluence.
Cell-proliferative studies
Approximately 1.0×104 cells placed onto
8×8-mm-diameter multichamber slides (Nunc, Copenhagen, Denmark)
were treated with telmisartan, and the other ARBs were dissolved in
ethanol. The final concentration of ethanol was <0.05%. Cell
viability was measured on day 1 by a microplate reader using a
modified
3-[4,5-dimethylthiazol-2-thiazolyl]-2,5-diphenyltetrazolium bromide
(MTT) assay (WST-1 assay; Dojindo, Kumamoto, Japan) and presented
as the percentage of cells under the control culture
conditions.
Flow cytometry
Annexin V and propidium iodide
staining
The effects of telmisartan and the other ARBs
(candesartan, valsartan, irbesartan and losartan) on the urological
cancer cells were determined by dual staining with Annexin V-FITC
and propidium iodide (PI) using the Annexin V-FITC Apoptosis
Detection kit I (Biosiences Pharmingen). Annexin V-FITC and PI were
added to the cellular suspension as per the manufacturer's
instructions, and a sample fluorescence of 1.0×104 cells
was analyzed by flow cytometry conducted with FACScan (Becton
Dickinson, Heidelberg, Germany).
Annexin V-FITC-positive and PI-negative cells were
identified as early apoptotic. Annexin V-FITC-positive and
PI-positive cells were identified as late apoptotic or
necrotic.
Identification of DNA
fragmentation
An assay was performed using the TdT-mediated dUTP
Nick End Labelling (TUNEL) method using the Apo-Direct™ kit (Becton
Dickinson). Following the experiments, the urological cancer cells
in suspension (1.0×106/ml) were fixed with 1% PBS,
washed in PBS and suspended in 70% (v/v) ice-cold ethanol, then
stored in ethanol at −20°C until use. Positive and negative
controls and the sample were stained with FITC-dUTP by incubation
in terminal deoxynucleotidyl transferase buffer as per the
manufacturer's instructions, and sample fluorescence of
1.0×104 cells was analyzed by flow cytometry (Becton
Dickinson). Results are expressed as the percentage (%) of
TUNEL-positive cells.
Detection of apoptosis
DNA chromatin morphology was assessed using Hoechst
staining. The urological cancer cells were incubated with 100 μM
telmisartan and the other ARBs for 24 h. Cells were washed with
RPMI-1640 and labeled with 8 mg/ml of Hoechst 33342 (Sigma-Aldrich
Japan K.K., Tokyo, Japan) for 10 min; PI (Sigma-Aldrich Japan K.K.)
was added (10 mg/ml final concentration), and the cells were
examined by fluorescence microscopy.
Results
Telmisartan-induced growth inhibition
in urological cancer cells determined by MTT
To investigate the effects of telmisartan and the
other ARBs on RCC, BC, PC and TC cell proliferation, we analyzed
cell viability in vitro using a modified MTT assay.
Telmisartan induced a reduction in cell viability
with a half-maximal concentration of growth inhibition in the
urological cancer cell lines (Table
I) in the range of 25–100 μM (Table I). Furthermore, in cells counted on
days 1, 2 and 3, marked inhibition of cell proliferation using 100
μM of telmisartan was clearly visible (Table II). Telmisartan arrested the growth
of all the urological cancer cells. However, the other ARBs had no
effect on cell proliferation in any of the urological cancer cell
lines (Table I).
 | Table I.Dose-dependent effects of angiotensin
II receptor blockers (ARBs) on the viability of human urological
cancer cell lines. |
Table I.
Dose-dependent effects of angiotensin
II receptor blockers (ARBs) on the viability of human urological
cancer cell lines.
| ARB dose
|
|---|
| 25 μM (%) | 50 μM (%) | 100 μM (%) |
|---|
| Telmisartan | | | | |
| RCC cell line | Caki-1 | 53.2% | 36.3% | 19.1% |
| BC cell line | T24 | 44.8 | 21.7 | 12.4 |
| PC cell lines | LNCaP | 32.0 | 18.7 | 12.7 |
| PC3 | 56.9 | 49.2 | 24.3 |
| DU-145 | 62.2 | 42.0 | 22.3 |
| TC cell line | NEC-8 | 43.3 | 32.6 | 32.6 |
| Candesartan | | | | |
| RCC cell line | Caki-1 | 92.7 | 90.6 | 102.3 |
| BC cell line | T24 | 99.5 | 91.9 | 75.1 |
| PC cell
lines | LNCaP | 128.0 | 129.0 | 141.5 |
| PC3 | 117.5 | 110.0 | 107.6 |
| DU-145 | 95.0 | 106.9 | 102.0 |
| TC cell line | NEC-8 | 118.0 | 122.1 | 120.1 |
| Valsartan | | | | |
| RCC cell
line | Caki-1 | 125.2 | 110.4 | 101.8 |
| BC cell line | T24 | 105.4 | 101.9 | 89.7 |
| PC cell
lines | LNCaP | 147.8 | 141.2 | 121.8 |
| PC3 | 109.5 | 98.1 | 123.1 |
| DU-145 | 97.5 | 86.7 | 68.8 |
| TC cell line | NEC-8 | 209.7 | 171.3 | 110.1 |
| Irbesartan | | | | |
| RCC cell
line | Caki-1 | 99.7 | 139.1 | 89.9 |
| BC cell line | T24 | 78.3 | 100.7 | 92.3 |
| PC cell
lines | LNCaP | 111.7 | 112.9 | 95.8 |
| PC3 | 118.9 | 106.8 | 111.3 |
| DU-145 | 88.4 | 109.1 | 82.2 |
| TC cell line | NEC-8 | 117.0 | 112.4 | 152.7 |
| Losartan | | | | |
| RCC cell
line | Caki-1 | 83.4 | 100.2 | 105.4 |
| BC cell line | T24 | 98.4 | 124.5 | 133.0 |
| PC cell
lines | LNCaP | 89.4 | 86.3 | 84.0 |
| PC3 | 102.6 | 106.4 | 112.5 |
| DU-145 | 129.6 | 114.0 | 110.2 |
| TC cell line | NEC-8 | 104.5 | 133.2 | 155.5 |
 | Table II.Effects of telmisartan on the cell
growth of human urological cancer cell lines in a time-dependent
manner. |
Table II.
Effects of telmisartan on the cell
growth of human urological cancer cell lines in a time-dependent
manner.
| | 0 h (%) | 24 h (%) | 48 h (%) | 72 h (%) |
|---|
| Control culture
(cell number) |
1.0×105 |
20.0×105 |
130.0×105 |
300.0×105 |
| Telmisartan | | | | | |
| RCC cell
line | Caki-1 | | 76.5 | 58.1 | 31.8 |
| BC cell line | T24 | | 34.3 | 12.7 | 18.3 |
| PC cell
lines | LNCaP | | 64.3 | 23.7 | 25.5 |
| PC3 | | 60.9 | 40.0 | 39.4 |
| DU-145 | | 60.9 | 11.1 | 29.2 |
| TC cell line | NEC-8 | | 29.2 | 21.5 | 18.3 |
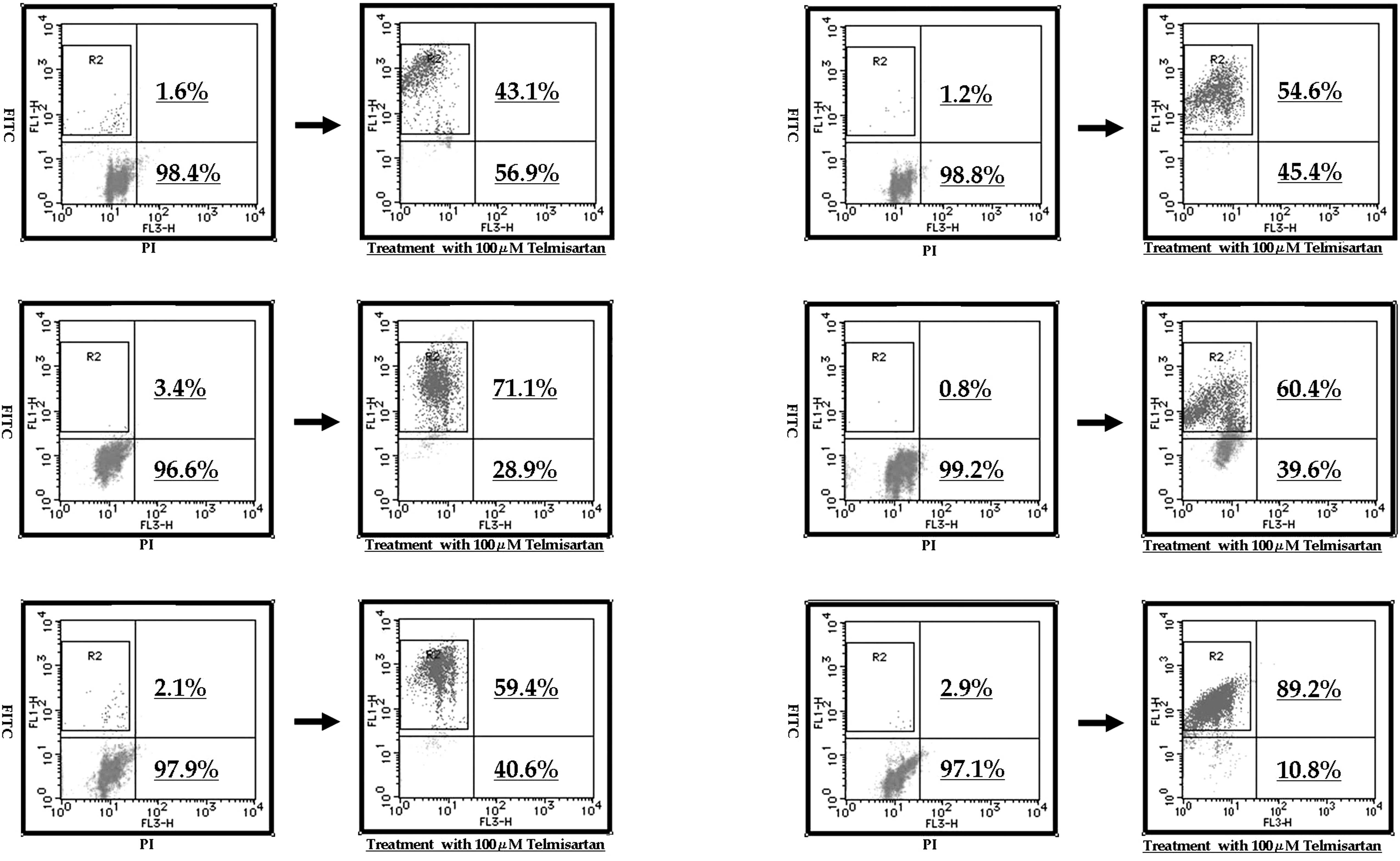
Telmisartan-induced apoptosis in
urological cancer cells determined by flow cytometry
To evaluate whether or not the cell death induced by
telmisartan and the other ARBs was achieved through apoptosis, flow
cytometry was used (Fig. 1).
Treatment with 100 μM telmisartan induced early apoptosis in almost
all the urological cancer cell lines.
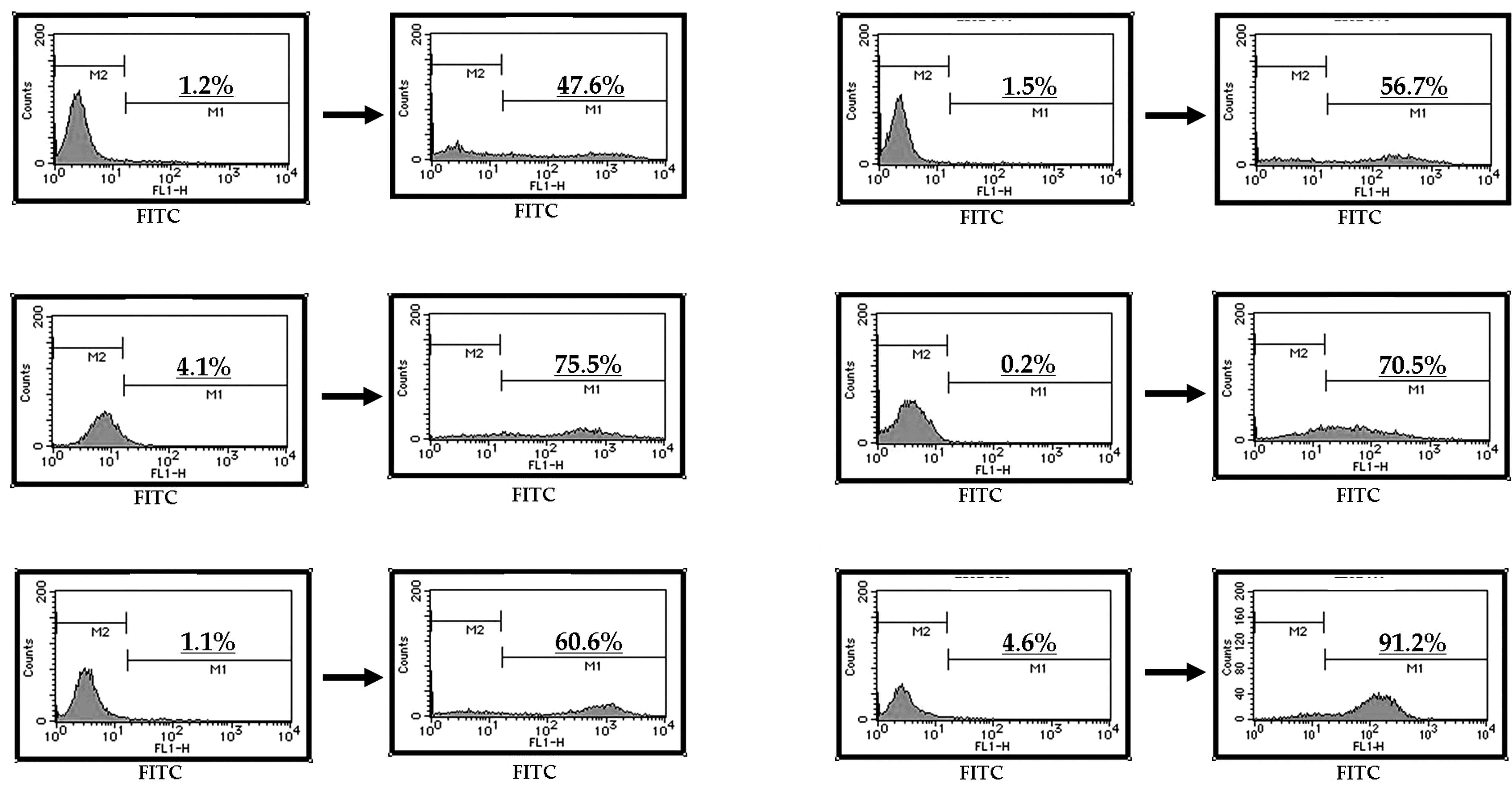
At a concentration of 100 μM, telmisartan induced
DNA fragmentation in all the urological cancer cell lines (Fig. 2A–F). In contrast, the other ARBs
did not induce DNA fragmentation in the urological cancer cells
(data not shown).
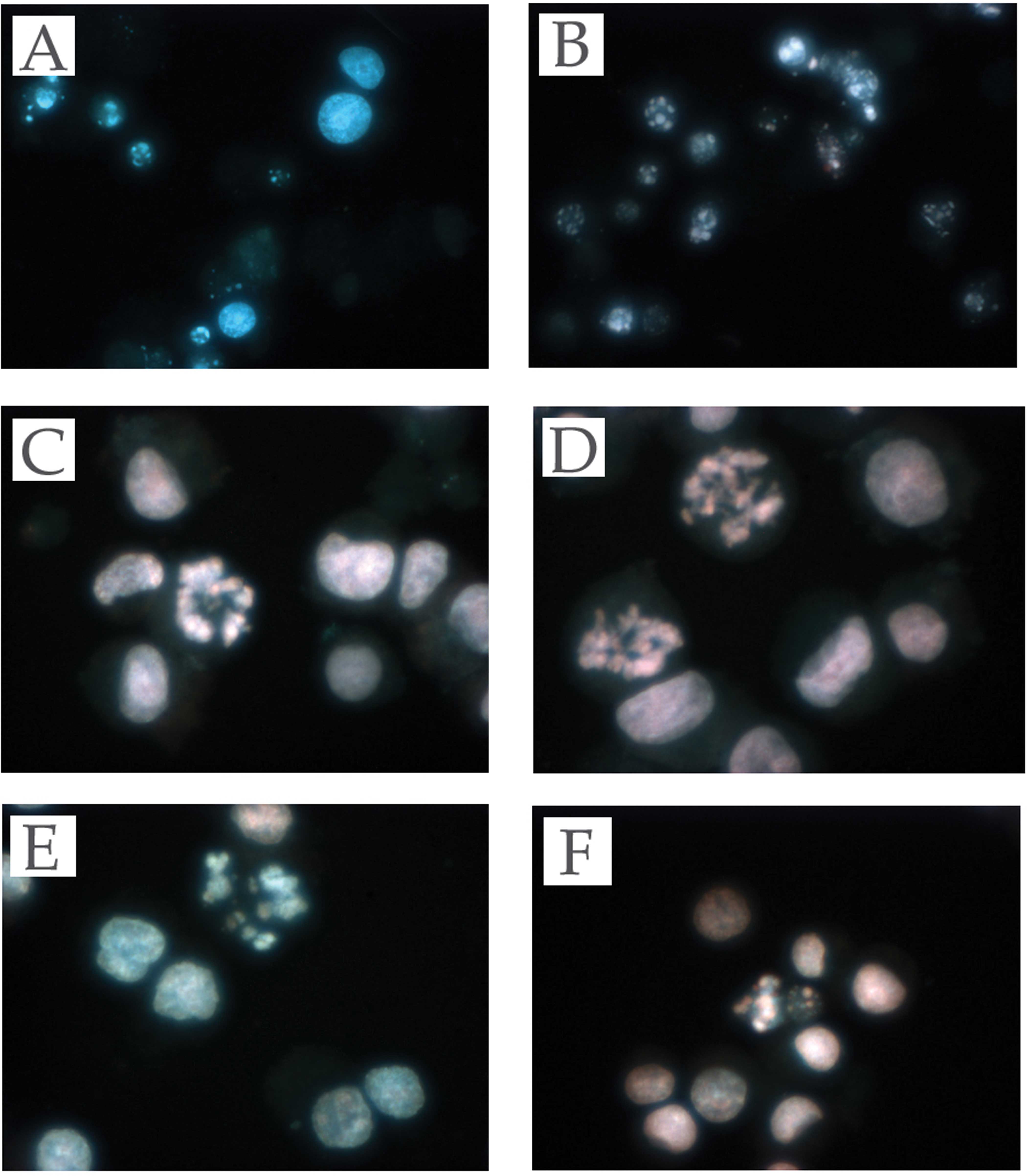
Effect of telmisartan on the induction
of apoptosis in urological cancer cells
To evaluate whether or not the cell death induced by
telmisartan was due to apoptosis, we evaluated the chromatin
morphology of the urological cancer cells using Hoechst 33342
staining. All the urological cancer cells treated with telmisartan
showed significant chromatin and cytoplasmic condensation, cellular
shrinkage and small membrane-bound (apoptotic) bodies, while the
urological cancer cells treated with the other ARBs did not show
any of the above characteristics. The former showed cellular
changes that were typically common characteristics of apoptosis
(Fig. 3A–F).
Discussion
ARBs have been synthesized and available for the
treatment of hypertension since the 1990s (16,17).
More recently, angiotensin II has been found to promote tumor
growth and angiogenesis, and ARBs have been considered a
significant anticancer and anti-angiogenesis therapeutic option
(18).
Some types of tumor cells, such as melanoma,
pancreatic cancer (19), RCC
(20,21), breast cancer (22), BC (23) and PC (24), have been reported to express the
angiotensin II receptor, and various studies have investigated the
anti-tumor effects caused by the anti-angiogenesis of ARBs. Some
researchers have demonstrated that candesartan (a type of ARB)
inhibited the production of VEGF, one of the most potent and
specific angiogenic factors, and decreased the growth of PC
(24,28). Kosaka et al (24) found that a specific ARB suppressed
VEGF production, resulting in reduced tumor angiogenesis and slower
progression of PC in a tumor xenograft model. Concerning other
tumor types, Kosugi et al (23) demonstrated that candesartan
prevented the pulmonary metastasis of RCC and BC by inhibiting
tumor angiogenesis through the suppression of VEGF in a xenograft
model. Uemura et al (27)
reported that, upon administering candesartan clinically to PC
patients with hypertension, the level of prostate-specific antigen
declined and the performance status of the patients improved.
However, they also reported that candesartan had no effects on
tumor growth in vitro, and did not detect apoptosis. Based
on their in vitro and in vivo experiments, they
suggested that the anti-tumor effect of ARB is not a result of
direct toxicity or apoptotic induction, but of its anti-angiogenic
effect.
The present study showed that candesartan and the
other ARBs (except telmisartan) did not induce a reduction in cell
viability and early apoptosis in urological cancer cells. Only
telmisartan induced a reduction in cell viability with a
half-maximal concentration of growth inhibition, and early
apoptosis and DNA fragmentation in urological cancer cells.
Benson et al discovered a structural
resemblance between telmisartan and pioglitazone, a PPAR-γ ligand
approved for the treatment of type II diabetes. They found that
telmisartan, not only blocks the angiotensin II receptor, but also
activates PPAR-γ. Telmisartan functioned as a moderately potent
selective PPAR-γ partial agonist, activating the receptor to 25–30%
of the maximum level achieved by the full agonists pioglitazone and
resiglitazone (10).
PPARs are members of the nuclear receptor
superfamily of ligand-activated transcriptional factors such as
steroids, thyroid hormones, vitamin D3 and retinoic acid. PPAR
binds to the peroxisome proliferator response element as a
heterodimer with the retinoic receptor in the regulation of PPAR
target genes. PPARs are considered important immunomodulatory
factors as well as fatty acid regulators. PPARs modulate these
activities in different immune cell types, such as
monocytes/macrophages, lymphocytes and endothelial cells (28).
PPAR-γ is expressed at a high level in adipose
tissue and is a critical regulator of adipocyte differentiation. It
is also expressed in the immune system, the spleen, monocyte
bone-marrow precursors and helper T-cell clones, and in
chondrocytes, synovial and bone tissues. Data have indicated that
PPAR-γ ligands lead to the inhibition of phorbol ester-induced
nitric oxide and macrophage-derived cytokines, such as tumor
necrosis factor-α, interleukin-1β and interleukin-6, chemokines and
adhesion molecules, in part by antagonizing the activities of
transcriptional factors (29). It
has been demonstrated that thiazolidinedione (a specific ligand for
PPAR-γ, a new class of anti-diabetic medication) regulates the
differentiation of cancer cells (30), and that nuclear-acting prostanoids
including 15-d-PGJ2 are potent activators of the PPAR-γ receptor
isoform (31,32). 15-d-PDJ2 induces apoptosis in
macrophages, endothelial and choriocarcinoma cells (33–35),
and thiazolidinedione induces fibroblast apoptosis (4).
We previously reported that PPAR-γ is strongly
expressed in urological cancer tissues. The extent and intensity of
PPAR-γ expression in urological cancer tissues were greater than in
normal urological tissues. PPAR-γ ligands strongly induced early
apoptosis in urological cancer cells as determined by flow
cytometry and Hoechst staining (11–15).
In this study, only telmisartan had a direct toxicity through
apoptosis. Thus, telmisartan may mediate potent anti-proliferative
effects against urological cancer cells through PPAR-γ. However, in
our study, that dose was not clinically achievable. Further studies
are needed to extend the use of telmisartan to clinical trials for
the treatment for human urological cancer.
References
|
1.
|
Weidner N, Folkman J, Pozza F, Bevilaqua
P, Allred EN and Moore DH: Tumor angiogenesis: a new significant
and independent prognostic indicator in early stage breast
carcinoma. J Natl Cancer Inst. 84:1875–1887. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Lafyatis R, Thompson NL, Remmers EF,
Flanders KC, Roche NS and Kim SJ: Transforming growth factor-beta
production by synovial tissues from rheumatoid patients and
streptococcal cell wall arthritic rats. Studies on secretion by
synovial fibroblast-like cells and immunohistologic localization. J
Immunol. 143:1142–1148. 1989.
|
|
3.
|
Kubota T, Koshizuka K, Williamson EA, et
al: Ligand for Peroxisome proliferator-activated receptor-γ
(troglitazone) has potent antitumor effect against human prostate
cancer both in vitro and in vivo. Cancer Res. 58:3344–3352.
1998.
|
|
4.
|
Spiegelman BM: PPAR-gamma: adipogenic
regulator and thiazolidinedione receptor. Diabetes. 47:507–514.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
See S and Stirling AL: Candesartan
cilexetil: an angiotensin II receptor blocker. Am J Health Syst
Pharm. 57:739–746. 2000.PubMed/NCBI
|
|
6.
|
Folkman J: Tumor angiogenesis: therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Le Noble FA, Hekking JW, van Straaten HW,
Slaaf DW and Struyker Boudier HA: Angiotensin II stimulates
angiogenesis in the chorio-allantoic membrane of the chick embryo.
Eur J Pharmacol. 195:305–306. 1991.PubMed/NCBI
|
|
9.
|
Le Noble FA, Schreurs NH, van Straaten HW,
et al: Evidence for a novel angiotensin II receptor involved in
angiogenesis in chick embryo chorioal-lantoic membrane. Am J
Physiol. 264:460–465. 1993.PubMed/NCBI
|
|
10.
|
Benson SC, Pershadsingh HA, Ho CI, et al:
Identification of telmisartan as a unique angiotensin II receptor
antagonist with selective PPARγ-modulating activity. Hypertension.
43:993–1002. 2004.
|
|
11.
|
Inoue K, Kawahito Y, Tsubouchi Y, et al:
Expression of peroxisome proliferator-activated receptor gamma in
renal cell carcinoma and growth inhibition by its agonists. Biochem
Biophys Res Commun. 287:727–732. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Yoshimura R, Matsuyama M, Segawa Y, et al:
Expression of peroxisome proliferator-activated receptors (PPARs)
in human urinary bladder carcinoma and growth inhibition by its
agonists. Int J Cancer. 104:597–602. 2003. View Article : Google Scholar
|
|
13.
|
Segawa Y, Yoshimura R, Hase T, et al:
Expression of peroxisome proliferator-activated receptor (PPAR) in
human prostate cancer. Prostate. 51:108–116. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Hase T, Yoshimura R, Mitsuhashi M, et al:
Expression of peroxisome proliferator-activated receptors in human
testicular cancer and growth inhibition by its agonists. Urology.
60:542–547. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yoshimura R, Matsuyama M, Hase T, et al:
The effect of peroxisome proliferator-activated receptor-γ ligand
on urological cancer cells. Int J Mol Med. 12:861–865. 2003.
|
|
16.
|
Burnier M: Angiotensin II type 1 receptor
blockers. Circulation. 103:904–912. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Dina R and Jafari M: Angiotensin
II-receptor antagonists. Am J Health-Syst Pharm. 57:1231–1241.
2000.PubMed/NCBI
|
|
18.
|
Abali H, Güllü IH, Engin H, Haznedaroğlu
IC, Erman M and Tekuzman G: Old antihypertensive as novel
antineoplastics: angiotensin-I-converting enzyme inhibitors and
angiotensin II type 1 receptor antagonists. Med Hypotheses.
59:344–348. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Fujimoto Y, Sasaki T, Tsuchida A and
Chayama K: Angiotensin II type 1 receptor expression in human
pancreatic cancer and growth inhibition by angiotensin II type 1
receptor antagonist. FEBS Lett. 495:197–200. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Miyajima A, Kosaka T, Asano T, et al:
Angiotensin II type 1 antagonist prevents pulmonary metastasis of
murine renal cancer by inhibiting tumor angiogenesis. Cancer Res.
62:4176–4179. 2002.PubMed/NCBI
|
|
21.
|
Goldfarb DA, Diz DI, Tubbs RR, Ferrario CM
and Novick AC: Angiotensin II receptor subtypes in the human renal
cortex and renal cell carcinoma. J Urol. 151:208–213.
1994.PubMed/NCBI
|
|
22.
|
Inwang ER, Puddefoot JR, Brown CL, et al:
Angiotensin II type 1 receptor expression in human breast tissues.
Br J Cancer. 75:1279–1283. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kosugi M, Miyajima A, Kikuchi E, Horiguchi
Y and Murai M: Angiotensin II type 1 receptor antagonist
candesartan as an angiogenic inhibitor in a xenograft model of
bladder cancer. Clin Cancer Res. 12:2888–2893. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Kosaka T, Miyajima A, Takayama E, et al:
Angiotensin II type I receptor antagonist as an angiogenic
inhibitor in prostate cancer. Prostate. 67:41–49. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Egami K, Murohara T, Shimada T, et al:
Role of host angiotensin II type 1 receptor in tumor angiogenesis
and growth. J Clin Invest. 112:67–75. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Koh WP, Yuan JM, van Den Berg D, Lee HP
and Yu MC: Polymorphisms in angiotensin II type 1 receptor and
angiotensin I converting enzyme genes and breast cancer risk among
Chinese women in Singapore. Carcinogenesis. 26:459–464. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Uemura H, Hasumi H, Kawahara T, et al:
Pilot study of angiotensin II receptor blocker in advanced
hormone-refractory prostate cancer. Int J Clin Oncol. 10:405–410.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Kawahito Y, Kondo M, Tsubouchi Y, et al:
15-deoxy-delta (12,14)-PGJ(2) induces synoviocyte apoptosis and
suppresses adjuvant-induced arthritis in rats. J Clin Invest.
106:189–197. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Tsubouchi Y, Sano H, Kawahito Y, et al:
Inhibition of human lung cancer cell growth by the peroxisome
proliferator-activated receptor-γ agonists through induction of
apoptosis. Biochem Biophys Res Commun. 270:400–405. 2000.
|
|
30.
|
Dreyer C, Krey G, Keller H, Givel F,
Helftenbein G and Wahli W: Control of the peroxisomal
beta-oxidation pathway by a novel family of nuclear hormone
receptors. Cell. 68:879–887. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Kliewer SA, Umesono K, Noonan DJ, Heyman
RA and Evans RM: Convergence of 9-cis retinoic acid and peroxisome
proliferator signalling pathways through heterodimer formation of
their receptors. Nature. 358:771–774. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Kliewer SA, Forman BM, Blumberg B, et al:
Differential expression and activation of a family of murine
peroxisome proliferator-activated receptors. Proc Natl Acad Sci
USA. 91:7355–7359. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Chinetti G, Griglio S, Antonucci M, et al:
Activation of proliferator-activated receptors alpha and gamma
induces apoptosis of human monocyte-derived macrophages. J Biol
Chem. 273:25573–25580. 1998. View Article : Google Scholar
|
|
34.
|
Altiok S, Xu M and Spiegelman BM:
PPARgamma induces cell cycle withdrawal: inhibition of E2F/DP
DNA-binding activity via down-regulation of PP2A. Genes Dev.
11:1987–1998. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Keelan JA, Sato TA, Marvin KW, Lander J,
Gilmour RS and Mitchell MD: 15-Deoxy-delta (12,14)-prostaglandin
J(2), a ligand for peroxisome proliferator-activated
receptor-gamma, induces apoptosis in JEG3 choriocarcinoma cells.
Biochem Biophys Res Commun. 262:579–585. 1999. View Article : Google Scholar : PubMed/NCBI
|