Introduction
Recently, local therapies for hepatocellular
carcinoma (HCC), including percutaneous radiofrequency ablation
(RFA), have come to be widely employed. RFA has gained great
popularity as a treatment technique for small HCC because of its
feasibility, effectiveness, repeatability and safety (1–6). In
most HCC patients, treatment options are limited by liver
dysfunction due to underlying chronic inflammation and cirrhosis.
Although complete surgical resection of HCC offers the best chance
of long-term survival, cirrhosis may limit the extent of
parenchymal resection that can be tolerated, and also increases the
risk of postoperative liver failure and death (7). There is no significant difference in
overall or disease-free survival between surgical resection and RFA
when HCC patients are in Child-Pugh class B (8). However, some cases experiencing rapid
and aggressive HCC recurrence after RFA have recently been reported
(9–11). In a previous study, we reviewed 15
resected-HCC patients who developed local recurrence after RFA and
discussed the possibility of tumor dedifferentiation being induced
by RFA (12).
Increasing evidence indicates that
epithelial-mesenchymal transition (EMT) mediates tumor progression.
EMT is a key event in the tumor invasion process, whereby
epithelial cell layers lose polarity and cell-cell contacts and
undergo a dramatic remodeling of the cytoskeleton (13). The hallmark of EMT is loss of
E-cadherin (13) while N-cadherin
is expressed (the so-called cadherin switch) and vimentin
expression in epithelial cells, which is accompanied by loss of
tight cell-cell adhesion and acquisition of a fibroblastic
morphology (14).
This study aimed to examine the expression of
mesenchymal markers, E-cadherin, N-cadherin and vimentin, in
patients with local recurrence of HCC after RFA.
Materials and methods
Patients
Between January 2000 and December 2006, 174 HCC
patients underwent surgery at the Department of Gastroenterologic
Surgery of Kanazawa University Hospital (Kanazawa, Japan). Among
them, 15 patients, 12 men and 3 women with an average age of 62.5
years (range 44–79 years), developed local HCC recurrence after
RFA. Twenty-six cases who underwent HCC resection without local
therapy and were diagnosed with classical HCC by preoperative
radiological studies were selected as controls. All the patients
gave their informed consent for their samples to be used for
research purposes.
Pathological specimens
Formalin-fixed and paraffin-embedded specimens were
retrieved from the surgical pathology files of the Pathology
Department of Kanazawa University Hospital.
Immunohistochemical examination
For immunohistochemical staining, the Dako Envision
system, which uses dextran polymers conjugated with horseradish
peroxidase (Dako, Carpinteria, CA, USA) was employed to avoid any
endogenous biotin contamination. Tissues were fixed with 10%
formaldehyde in phosphate-buffered saline, embedded in paraffin and
cut into 5-μm tissue sections. The sections were
deparaffinized in xylene and rehydrated in a graded ethanol series.
Endogenous peroxidase was blocked by immersing the sections in 3%
H2O2 in 100% methanol for 20 min at room
temperature. Antigen retrieval was achieved by microwaving sections
at 95°C for 10 min in 0.001 M citrate buffer (pH 6.7). After
blocking the endogenous peroxidase, the sections were incubated
with Protein Block Serum-Free (Dako) at room temperature for 10 min
to block non-specific staining, and the sections were then
incubated for 2 h at room temperature with 1:50 diluted mouse
monoclonal antibodies against E-cadherin (Takara, Ohtsu, Japan),
N-cadherin (Takara) and vimentin (Santa Cruz Biotechnology, Santa
Cruz, CA, USA). Peroxidase activity was detected with enzyme
substrate 3-amino-9-ethyl carbazole. For negative controls, the
sections were incubated with Tris-buffered saline without the
primary antibodies. Samples in which at least 10% of tumor cells
were slightly counterstained with Mayer's hematoxylin were defined
as positive.
Statistical analysis
Categorical variables were compared using the
Chi-square test. For statistical analysis, P-values were calculated
using a two-tailed test, and P<0.05 was considered to indicate
statistical significance.
Results
Fifteen surgically resected specimens of locally
recurrent HCC after RFA were immunohistochemically examined for
E-cadherin, N-cadherin and vimentin expression and compared to
those of 26 HCC control specimens without local therapy (Table I).
 | Table I.Expression of EMT markers. |
Table I.
Expression of EMT markers.
| EMT markers | Local recurrence
after RFA (n=15) | Without local therapy
(n=26) | P-value |
|---|
| Loss of
E-cadherin | 9 (60.0%) | 5 (19.2%) | 0.02 |
| N-cadherin | 14 (93.3%) | 20 (76.9%) | NS |
| Vimentin | 3 (20.0%) | 0 (0.0%) | 0.08 |
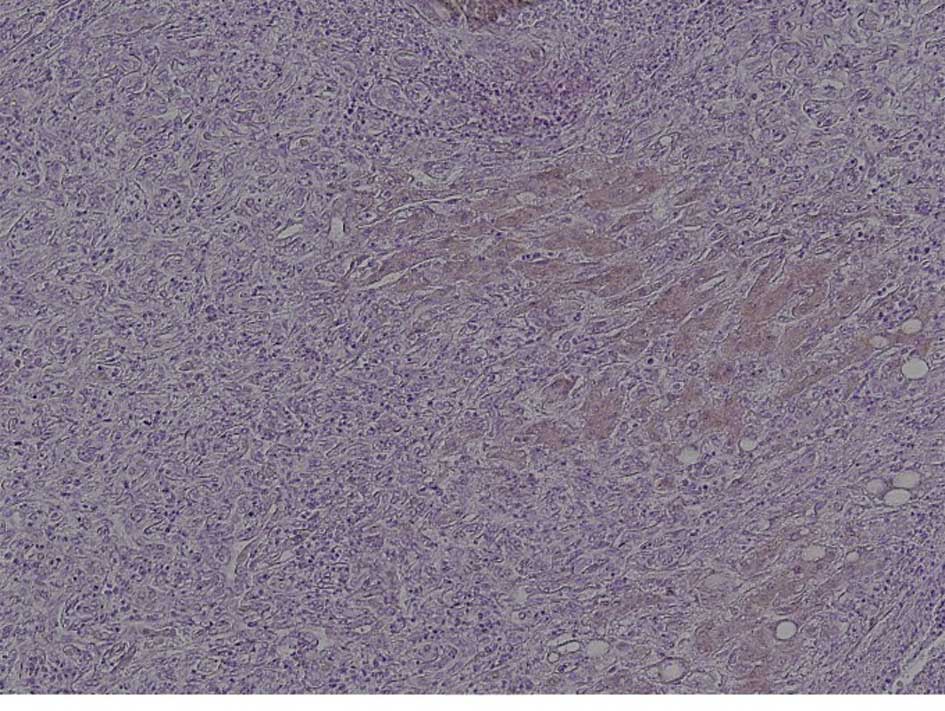
E-cadherin
In 9 of the 15 (60%) locally recurrent post-RFA
HCCs, loss of E-cadherin expression was noted. By contrast,
decreased E-cadherin expression was observed in only 5 of the 26
(19.2%) controls. The percentage of cases negative for E-cadherin
expression was significantly higher in the recurrent post-RFA HCC
than in the control cases (P=0.02) (Fig. 1).
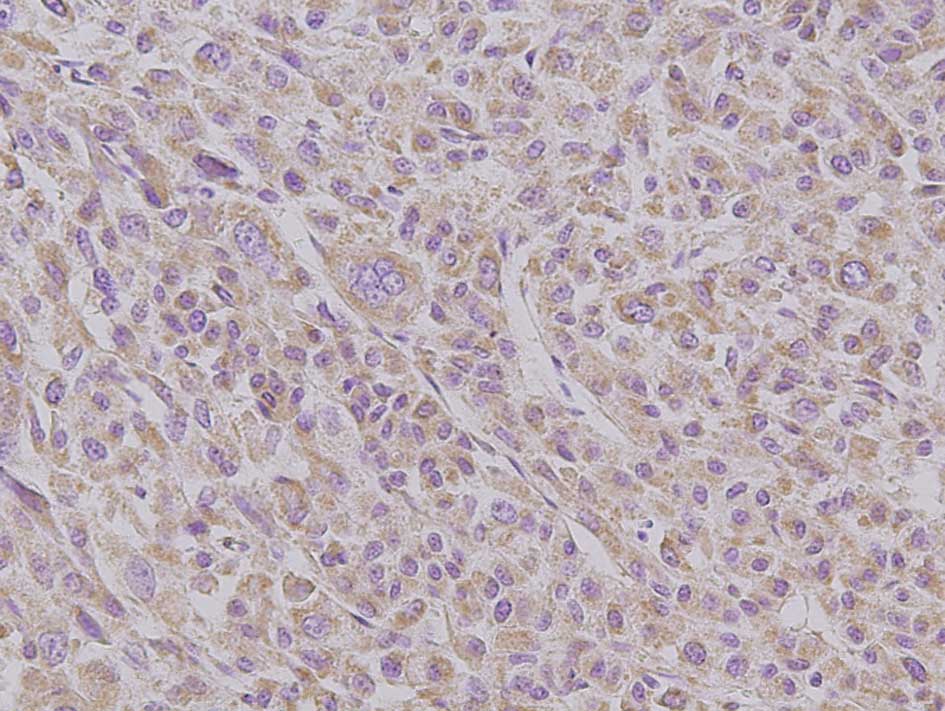
N-cadherin
Immunohistochemical analyses revealed membranous
N-cadherin expression in all non-tumoral liver tissues. N-cadherin
was expressed in hepatocytes and interlobular bile duct epithelia.
Immunoreactivity was increased in 14 of the 15 (93.3%) locally
recurrent post-RFA HCCs. However, 20 of the 26 (76.9%) control HCCs
also showed N-cadherin immunoreactivity. No difference was observed
in the intensity or the range of staining. In addition, there was
no association between E- and N-cadherin expression patterns, the
so-called cadherin switch (Fig.
2).
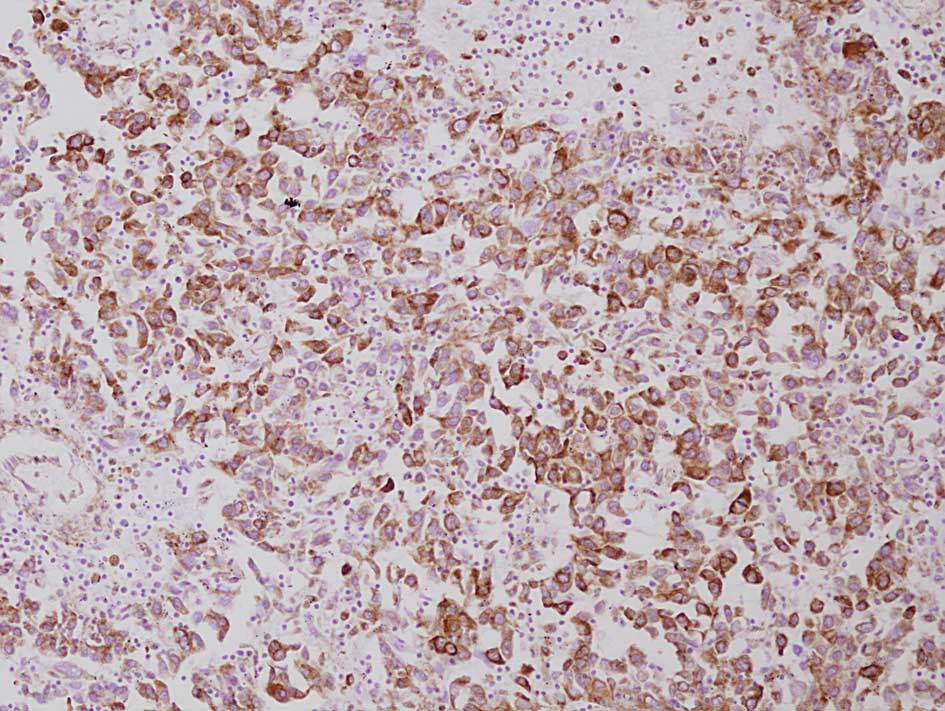
Vimentin
Vimentin was found to be diffusely positive in the
tumor cell cytoplasm in 3 of the 15 (20%) post-RFA recurrent HCCs.
In the control group, all 26 cases were negative for vimentin. The
percentage of the cases positive for vimentin was not significantly
higher in the locally recurrent post-RFA HCCs than in the controls
(P=0.08). In non-cancerous areas, vascular endothelial cells and
fibroblasts were positive for vimentin, while hepatocytes were
negative (Fig. 3).
Discussion
RFA has become a very popular technique for the
treatment of small HCC because of its feasibility, effectiveness,
repeatability and safety (1–6).
However, cases experiencing rapid and aggressive recurrence of HCC
after RFA have recently been reported (9–11).
Moreover, an autopsy case was reported in which well-differentiated
HCC developed into HCC with sarcomatous change after RFA (15). In a previous study, we reviewed 15
resected-HCC patients who developed local recurrence after RFA. In
patients with local HCC recurrence after RFA, the tumors showed
more invasive growth, more vascular invasion and less
differentiation than those in patients without RFA. We thus
concluded that tumor dedifferentiation can be induced by the heat
stress associated with RFA (12).
Recently, increasing evidence has shown that EMT, a
process first identified in embryogenesis (16), mediates tumor progression,
including local invasion, spread through the circulatory system and
metastasis. Hallmarks of EMT include the loss of E-cadherin
expression and the expression of mesenchymal markers, such as
N-cadherin and vimentin, in epithelial cells (13,14).
E-cadherin, a transmembranous glycoprotein that
mediates adherens junctions, is developmentally restricted to
polarized epithelial cells (17).
E-cadherin plays an essential role in maintaining the structural
integrity and polarization of the epithelia. Its loss consequently
allows destabilization of the structural integrity of the
epithelium and causes cells to dissociate from their neighbors and
lose polarity, as observed during gastrulation in the embryo and
also during wound repair and metastasis of carcinoma cells
(17). Decreased E-cadherin
immunoreactivity correlates with a lack of differentiation, tumor
aggressiveness and inhibited formation of nascent junctional
complexes among HCC cells (18).
On the other hand, E-cadherin has been recognized as an important
tumor biomarker and has been identified as a bona fide tumor
suppressor gene in diffuse gastric carcinomas (19).
N-cadherin, found primarily in neural tissues and
fibroblasts (20), is expressed in
the most invasive and dedifferentiated breast cancer cell lines and
in poorly differentiated areas of prostate cancers where E-cadherin
expression is negative or aberrant. Exogenous N-cadherin in tumor
cells enhances cellular motility, invasion and metastasis (21). Generally, cadherin subtype
switching from E- to N-cadherin in tumors, not only activates a
signaling program that promotes the invasive and survival
capabilities of tumor cells, but also fosters cooperation between
tumor cells and the surrounding microenvironment, a critical event
in metastatic progression (22).
However, an early study showed N-cadherin to be expressed in both
normal liver and HCC tissues (23). Moreover, a recent study identified
N-cadherin as frequently being overexpressed in HCC, and loss of
E-cadherin expression was not apparently a prerequisite for
N-cadherin expression (21). In
the present study, we found no correlation between the expression
patterns of E-cadherin and N-cadherin in either the post-RFA or
control cases with recurrence.
Vimentin, a cytoplasmic intermediate filament, is
characteristic of mesenchymal cells and is not usually expressed in
epithelial cells. The atypical expression of vimentin in epithelial
cancer cells may be associated with local invasiveness and the
potential for metastasis (24,25).
In the present study, although no statistically significant
difference was observed, vimentin expression was found only in
post-RFA recurrent HCCs (P=0.08).
Cancer cells suffer various forms of stress from
anticancer drugs, irradiation, hypo-oxygenation, hypo-nutrition and
heat treatment. Some recent studies suggest that these stresses
induce EMT of cancer cells (26,27),
and that this EMT is related to the invasive potential of cancer
cells (26). Moreover, heat stress
reportedly induces the tyrosine phosphorylation and activation of a
human carcinoma dedifferentiation modulator (28). Obara et al reported that
even a single heat treatment may induce transformation of an HCC
cell line and suggested that insufficient RFA treatment of HCC may
induce further malignant transformation in vivo (29). Accordingly, the thermal effect of
RFA may increase the risk of EMT and the transformation of residual
viable tumor cells.
In conclusion, to the best of our knowledge, the
present study is the first description of EMT induction by heat
stress associated with RFA. Based on our findings, we suggest that
EMT is closely related to aggressive recurrence and
dedifferentiation of HCC after RFA. The impact of the complications
reported herein may temper the optimistic outcomes reported in some
initial series (30), suggesting
that caution is needed when determining the indications for and
applications of RFA, particularly in patients who are also
appropriate surgical candidates. The risk of aggressive recurrence
after RFA must be considered when assessing the treatment options
for HCC. Further experimental research is needed before final
conclusions can be drawn.
References
|
1.
|
Rossi S, Garbagnati F, Lencinoni R, et al:
Percutaneous radio-frequency thermal ablation of non-resectable
hepatocellular carcinoma after occlusion of tumor blood supply.
Radiology. 217:119–126. 2000. View Article : Google Scholar
|
|
2.
|
Livraghi T, Goldberg SN, Lazzaroni S, et
al: Small hepatocellular carcinoma: treatment with radio-frequency
ablation versus ethanol injection. Radiology. 210:655–661. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Allgaier HP, Deibert P, Zuber I, et al:
Percutaneous radio-frequency interstitial thermal ablation of small
hepatocellular carcinoma. Lancet. 353:1676–1677. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Nicoli N, Casaril A, Marchiori L, et al:
Intraoperative and percutaneous radiofrequency thermal ablation in
the treatment of hepatocellular carcinoma. Chir Ital. 52:29–40.
2000.PubMed/NCBI
|
|
5.
|
Nicoli N, Casaril A, Marchiori L, et al:
Treatment of recurrent hepatocellular carcinoma by radio-frequency
thermal ablation. J Hepatobil Pancreat Surg. 8:417–421. 2001.
View Article : Google Scholar
|
|
6.
|
Solbaiati L, Livraghi T, Goldberg SN, et
al: Percutaneous radio-frequency ablation of hepatic metastasis
from colorectal cancer: long-term result in 117 patients.
Radiology. 221:159–166. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Steven AC, Francesco I, Lee ME, Nicolas V
and Paolo V: Radiofrequency ablation of hepatocellular cancer in
110 patients with cirrhosis. Ann Surg. 232:381–391. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Vivarelli M, Guglielmi A, Ruzzenente A, et
al: Surgical resection versus percutaneous radiofrequency ablation
in the treatment of hepatocellular carcinoma on cirrhotic liver.
Ann Surg. 204:102–107. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Nicola N, Andrea C, Moh'd AH, et al: A
case of rapid intrahepatic dissemination of hepatocellular
cartinoma after radiofrequency thermal ablation. Am J Surg.
188:165–167. 2004. View Article : Google Scholar
|
|
10.
|
Nazario P, Guido AMT, Maurizio R, et al:
Aggressive recurrence after radiofrequency ablation of liver
neoplasms. Hepatogastroenterology. 50:2179–2184. 2003.PubMed/NCBI
|
|
11.
|
Takada Y, Kurata M and Ohkohchi N: Rapid
and aggressive recurrence accompanied by portal tumor thrombus
after radio-frequency ablation for hepatocellular carcinoma. Int
Clin Oncol. 8:332–335. 2003. View Article : Google Scholar
|
|
12.
|
Tajima H, Ohta T, Okamoto K, et al:
Radiofrequency ablation induces dedifferentiation of hepatocellular
carcinoma. Oncol Lett. 1:91–94. 2010.PubMed/NCBI
|
|
13.
|
Yang J, Mani SA, Donaher JL, et al: Twist,
a master regulator of morphogenesis, plays an essential role in
tumor metastasis. Cell. 117:927–939. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Hay ED and Zuk A: Transformations between
epithelium and mesenchyme: normal, pathological and experimentally
induced. Am J Kidney Dis. 26:678–690. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Koda M, Maeda Y, Matsunaga Y, et al:
Hepatocellular carcinoma with sarcomatous change arising after
radiofrequency ablation for well-differentiated hepatocellular
carcinoma. Hepatol Res. 27:163–167. 2003. View Article : Google Scholar
|
|
16.
|
Soo K, O'Rourke MP, Khoo PL, et al: Twist
function is required for the morphogenesis of the cephalic neural
tube and the differentiation of the cranial neural crest cells in
the mouse embryo. Dev Biol. 247:251–270. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Gumbiner BM: Cell adhesion: the molecular
basis of tissue architecture and morphogenesis. Cell. 84:345–357.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Du GS, Lu JX, Ma CQ, et al: Expression of
P-aPKC-I, E-cadherin and β-catenin related to invasion and
metastasis in hepatocellular carcinoma. Ann Surg Oncol.
16:1578–1586. 2009.
|
|
19.
|
Perl AK, Wilgenbus P, Dahl U, et al: A
causal role for E-cadherin in the transition from adenoma to
carcinoma. Nature. 392:190–193. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Hatta K and Takeichi M: Expression of
N-cadherin adhesion molecules associated with early morphogenetic
events in chick development. Nature. 320:447–449. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Seo DD, Lee HC, Kim HJ, et al: Neural
cadherin overexpression is a predictive marker for early
postoperative recurrence in hepatocellular carcinoma patients. J
Gastroenterol Hepatol. 23:1112–1118. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Hazan RB, Qiao R, Keren R, Badano I and
Suyama K: Cadherin switch in tumor progression (Review). Ann NY
Acad Sci. 1014:155–163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Ihara A, Koizumi H, Hashizume R and
Uchikoshi T: Expression of epithelial cadherin and alpha- and
beta-catenins in nontumoral livers and hepatocellular carcinomas.
Hepatology. 23:1441–1447. 1996.PubMed/NCBI
|
|
24.
|
Kakizoe S, Kojiro M and Nakashima T:
Hepatocellular carcinoma with sarcomatous change. Cancer.
59:310–316. 1987. View Article : Google Scholar
|
|
25.
|
Park MY, Kim K, Park HS, et al: Expression
of the serum response factor in hepatocellular carcinoma:
Implications for epithelial-mesenchymal transition. Int J Oncol.
31:1309–1315. 2007.PubMed/NCBI
|
|
26.
|
Takamoto H, Shibata K, Kajiyama H, et al:
Irradiation-induced epithelial-mesencymal transition (EMT) related
to invasive potential in endometrial carcinoma cells. Gynecol
Oncol. 107:500–504. 2007. View Article : Google Scholar
|
|
27.
|
Yang AD, Fan F, Camp ER, et al: Chronic
oxaliplatin resistance induces epithelial-mesenchymal transition in
colorectal cancer cell lines. Clin Cancer Res. 12:4147–4153. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Yang SD, Lee SC and Chang HC: Heat stress
induces tyrosine phosphorylation/activation of kinase FA/GSK-3
alpha (a human carcinoma dedifferentiation modulator) in A431
cells. J Cell Biochem. 66:16–26. 1997. View Article : Google Scholar
|
|
29.
|
Obara K, Matsumoto N, Okamoto M, et al:
Insuffcient radio-frequency ablation therapy may induce further
malignant transformation of hepatocellular carcinoma. Hepatol Int.
2:116–123. 2008. View Article : Google Scholar
|
|
30.
|
Llovet JM, Vilana R, Brú C, et al:
Increased risk of tumor seeding after percutaneous radiofrequency
ablation for single hepatocellular carcinoma. Hepatology.
33:1124–1129. 2001. View Article : Google Scholar : PubMed/NCBI
|