Introduction
Colorectal cancer still represents a major medical
challenge. Besides improving current available therapies, new
treatment methods must be evaluated in order to provide improved
outcomes for patients with advanced stages of the disease. A
variety of plant-derived drugs have recently been explored for
anticancer efficacy (1). It has
been demonstrated that thymoquinone (TQ), which is the major
compound of black seed (Nigella sativa) oil, traditionally
used in Mediterranean and Arab medicine, possesses significant
anticancer effects in various cancer models (2).
We previously showed that TQ induces apoptosis
through p53-dependent pathways in human colon cancer cells and
animal models (3,4). Although LD50 values for TQ
in mice and rats indicate that high doses are tolerable in
vivo (5), the high
concentrations required impair these positive effects. In addition,
due to its chemical structure
(2-isopropyl-5-methylbenzo-1,4-quinone), TQ is unstable under
physiologic conditions and in aqueous solutions, and shows only a
low capacity to penetrate through biologic membranes. Recently, we
demonstrated that the chemical modification of TQ by attachment of
saturated and unsaturated fatty acid side chains enhanced the
biological efficacy of TQ by increasing ROS production and inducing
apoptosis in HL-60 leukaemia and 518A2 melanoma cells (6). Besides having a cytotoxic effect, TQ
has been demonstrated to interfere with the cell cycle by
inhibiting the activity of polo-like kinase 1 (PLK1), which is a
key regulator of mitosis progression and is itself regulated by p53
(7).
Based on these findings, we developed further TQ
derivatives which, in the present study, were investigated for
their cell cycle regulating activity in HCT116 colon cancer cells
and the human hepatoma cell line HepG2. Dependent on p53 status,
these new molecules induced a cytostatic effect at low
concentrations by the up-regulation of p21cip1/waf1 and
the suppression of cyclin E.
Materials and methods
Design and synthesis of thymoquinone
derivatives
The thymoquinone hydrazones (TQ-H) were prepared
from TQ and α-linolenic acid or hexadecanoic acid, respectively,
according to a previously applied general procedure (6).
Cell growth and treatment
Human HCT116 colon cancer cells (wild-type and
derivatives lacking p53) and human HepG2 hepatocellular carcinoma
cells were cultivated in RPMI-1640 medium supplemented with 10%
fetal bovine serum (FBS), 1% penicillin and 0.5% streptomycin in an
atmosphere of 5% CO2 at 37°C. Cell cultures were grown
on Nunc EasyFlasks (Thermo Fisher Scientific, Roskilde, Denmark).
Cell culture media and supplements were obtained from Biochrom,
Berlin, Germany. Cell lines were obtained from the German
Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig,
Germany); HCT116p53−/− cells were a gift from B.
Vogelstein (Johns Hopkins University, Baltimore, MD, USA).
For 24–72 h of treatment, 105 or
5×104 cells were seeded in 6-well plates and allowed to
adhere overnight. TQ derivatives were added at different
concentrations (0.01–10 μM) for the indicated time
points.
Cell number and cell cycle analysis
Treated cells were washed with phosphate-buffered
saline (PBS; Biochrom) and lysed by incubation with trypsin/EDTA
(Biochrom). Cell number was determined by counting the viable cells
after trypan blue staining in a Neubauer chamber as described
previously (8). Apoptosis and DNA
content were determined by flow cytometry on a FACSCalibur
fluorescence activated cell sorter (BD Bioscience, Heidelberg,
Germany) after staining with hypotonic propidium iodide solution
(0.1% sodium citrate, 0.1% Triton X-100 and 50 μg/ml
propidium iodide; all from Sigma, Deisenhofen, Germany) as
described previously (8). For each
sample, 10,000 events were collected, and the percentage of cells
with a subdiploid DNA content in different phases of the cell cycle
was determined using CellQuest software (BD Bioscience). Results
displayed in the graphs and diagrams are the mean and standard
deviation of three independent experiments.
Protein extraction and Western blot
analysis
Total protein was extracted after treatment under
the indicated conditions using Jie's protein extraction buffer as
described previously (9). Proteins
were quantified using the Pierce BCA Protein Assay kit (Thermo
Scientific, Rockford, IL, USA) according to the manufacturer's
protocol. Samples were subjected to gel electrophoresis on NuPAGE
Bis-Tris Gels (Invitrogen, Carlsbad, CA, USA) for 50 min at 200 V
and 125 mA. Proteins were then transferred to a nitrocellulose
transfer membrane (Whatman, Dassel, Germany) at 90 V for 30 min.
Membranes were blocked at 4°C overnight using PBS with 0.1%
Tween-20 and 5% low fat milk powder, and then probed with primary
antibodies (Table I) for 90 min at
room temperature (RT). Membranes were washed three times with
blocking buffer and incubated with appropriate secondary antibodies
for 1 h at RT (Table I). Reactive
bands were visualized using enhanced chemiluminescence (ECL) and
exposure to X-ray films. Densitometry analysis was performed using
GelScan 5 software (BioSciTec, Frankfurt, Germany). All membranes
were stripped with glycine-buffer (pH 2.0) and reprobed with an
anti-β-actin antibody to show equal loading of the lanes.
 | Table I.Western blot antibodies. |
Table I.
Western blot antibodies.
| Antigen | Manufacturer;
Dilution | Second antibody;
Dilution |
|---|
|
p21cip1/waf1 | BD Bioscience,
mouse monoclonal; 1:500 | Mouse, 1:1000 |
| p53 | BD Bioscience,
mouse monoclonal; 1:500 | Mouse; 1:1000 |
| Cyclin A | Abcam, mouse
monoclonal; 1:500 | Mouse; 1:1000 |
| Cyclin D | Abcam, rabbit
monoclonal; 1:200 | Rabbit; 1:1000 |
| Cyclin E | Abcam, rabbit
polyclonal; 1:200 | Rabbit; 1:1000 |
| β-actin | Sigma, mouse
monoclonal; 1:2000 | Mouse; 1:1000 |
RNA extraction, cDNA synthesis and
quantitative real-time PCR
Total RNA was extracted using peqGOLD RNA Pure
(Peqlab, Erlangen, Germany) according to the manufacturer's
instructions. cDNA synthesis was performed as described previously
using oligo(dT)15 primer and random hexamer primer (both from
Promega, Mannheim, Germany) with 100 U SuperScript II reverse
transcriptase (Invitrogen). Relative transcript levels were
quantified by real-time RT-PCR using 2 μl of template cDNA
on a thermal cycler system (CFX96 Real Time System, C1000 Thermal
Cycler; BioRad). Quantitect Primers for human CCNA2, CCND2, CCNE2,
TP53, CDKN1A as well as GAPDH were obtained from Qiagen (Hilden,
Germany). PCR was performed with the Absolute SYBR Green
Fluorescein Mix (Thermo Scientific, ABgene House, Epsom, Surrey,
UK) according to the manufacturer's instructions. Data were
analyzed with Bio-Rad CFX Manager software. Results were normalized
to GAPDH levels. Samples were analyzed in duplicate.
Statistical analysis
Significance was calculated using the t-test for
paired samples. P<0.05 was regarded as significant.
Results
Thymoquinone hydrazone derivatives
inhibit proliferation of human colon cancer cells in vitro
Human HCT116 colon cancer cells and the derivative
lacking p53 (HCT116p53−/−), as well as the human HepG2
liver cancer cell line, were exposed to different concentrations of
thymoquinone hydrazone derivatives (TQ-H) for 24–96 h at
concentrations of 0.1 to 10 μM, thus not exceeding cytotoxic
doses (IC50, 40 μM), as reported previously
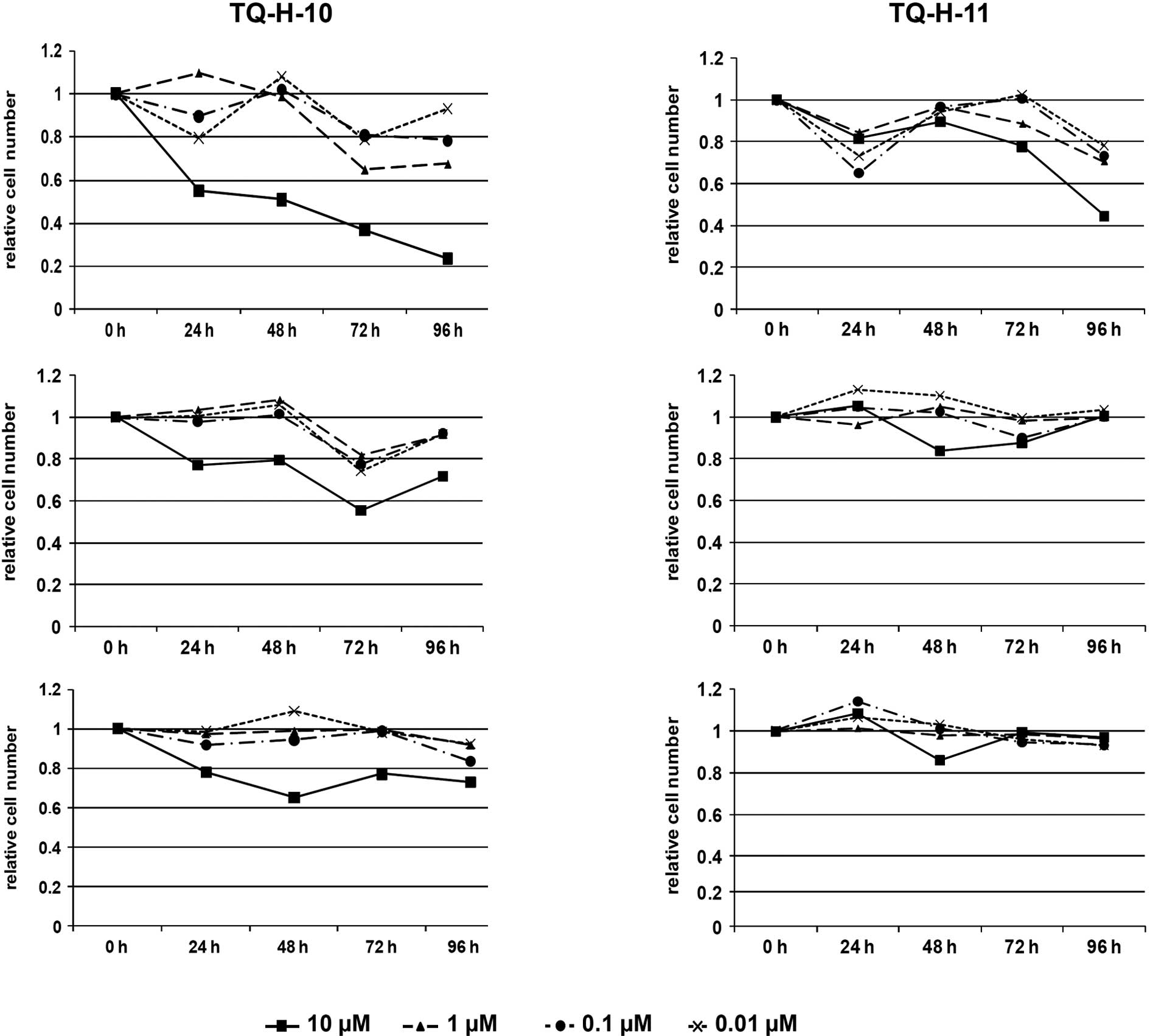
(10). As shown in Fig. 2, the viable cell count revealed
significant effects of the two investigated TQ-H derivatives.
HCT116 cells proved to be most sensitive to
treatment with either thymoquinone-4-α-linolenoylhydrazone
(TQ-H-10) or thymoquinone-4-palmitoylhydrazone (TQ-H-11) (Fig. 2A and D). At 10 μM, both
compounds significantly decreased the number of viable cells to 24%
of the untreated controls for TQ-H-10 and 45% for TQ-H-11 after 96
h. Lower concentrations also reduced cell proliferation in this
cell line to 65 and 80% at 1 and 0.1 μM, respectively, for
TQ-H-10, and to ∼75% for TQ-H-11. In contrast, reduction in cell
proliferation was less pronounced for HCT116p53−/− cells
(Fig. 2B and E). In these cells,
only 10 μM of TQ-H-10 led to a decrease in cell number of
∼58% after 72 h and 72% after 96 h; lower concentrations of TQ-H-10
did not affect cell proliferation. Surprisingly, TQ-H-11 was
ineffective even at 10 μM. A similar pattern was observed
for the human HepG2 liver cancer cell line (Fig. 2C and F). Only 10 μM of
TQ-H-10 led to a significant decrease in cell number at 48–96 h,
ranging from 65 to 75% of the untreated controls. Lower
concentrations of TQ-H-10 as well as all tested concentrations of
TQ-H-11 were ineffective.
TQ-H derivatives induce changes in the
distribution of cell cycle phases
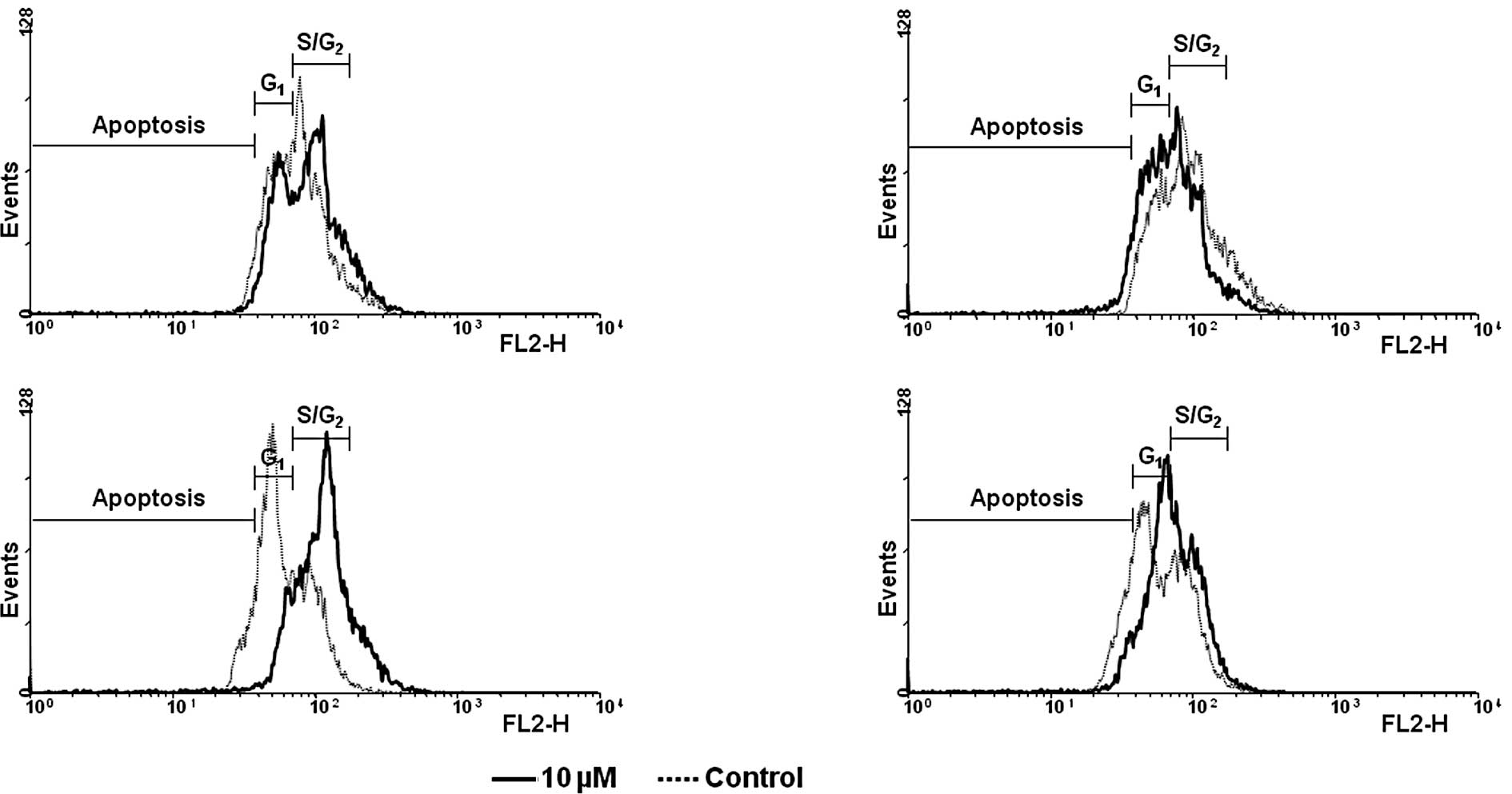
Cell cycle distribution and apoptosis were
determined by flow cytometry after propidium iodide staining. In
parallel to cell counting experiments, HCT116 cells proved to be
most sensitive to treatment with 10 μM TQ-H-10 or TQ-H-11
(Fig. 3A and D). Both compounds
induced a significant shift in the cell cycle distribution towards
the S/G2 phase after 72 and 96 h. In detail, the amount
of cells in the S/G2 phase increased from 67.8 to 89.1%
and from 62.4 to 88.6% after 72 or 96 h of treatment with TQ-H-10.
TQ-H-11 increased this parameter from 72.3 to 88.1% and from 49.2
to 84.5%, respectively. Representative flow cytometry scans are
shown in Fig. 4. Similar to the
results described above, neither TQ-H-10 nor TQ-H-11 influenced the
distribution of the cell cycle at 10 μM in either the
HCT116p53−/− (Fig. 3B and
C) or HepG2 cells (Fig. 3E and
F).
Molecular analysis of cell cycle
regulating factors after TQ-H treatment
To investigate which factors are involved in
TQ-H-mediated cell cycle arrest and to determine the influence of
p53 status on the observed results, we performed quantitative
real-time RT-PCR and Western blotting on all tested cell lines
after 48 and 72 h of incubation with 10 μM TQ-H-10 and
TQ-H-11.
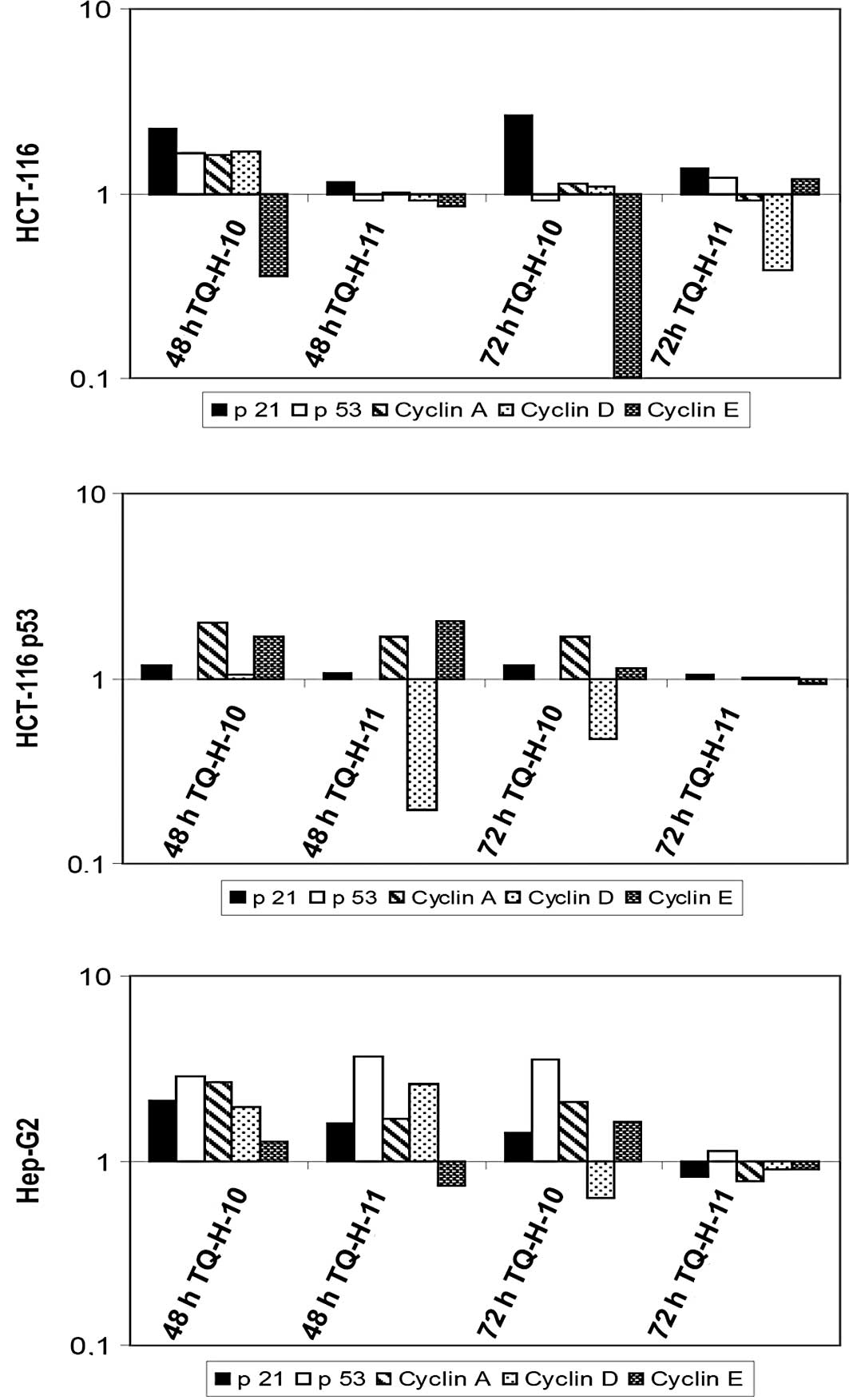
Compared to untreated controls, TQ-H-10 induced a
significant increase in the mRNA levels of p21cip1/waf1
and a pronounced down-regulation of cyclin E in HCT116 cells
(Fig. 5A). TQ-H-11 led only to a
down-regulation of cyclin D after 72 h, while all other parameters
remained unchanged. In line with the view that
p21cip1/waf1 is a transcriptional target of p53
(11), no significant increase in
p21cip1/waf1 was observed in HCT116p53−/−
cells (Fig. 5B). However, both
compounds led to a suppression of cyclin D mRNA levels after 48 h
(TQ-H-11) or 72 h (TQ-H-10). In HepG2 cells, which showed the
greatest resistance to TQ-H treatments, no significant
down-regulation of cell cycle-associated genes was observed
(Fig. 5C). In this cell line, the
increased expression of p53 and cyclins A, D and E was observed,
which supports the findings regarding cell death and cell
proliferation.
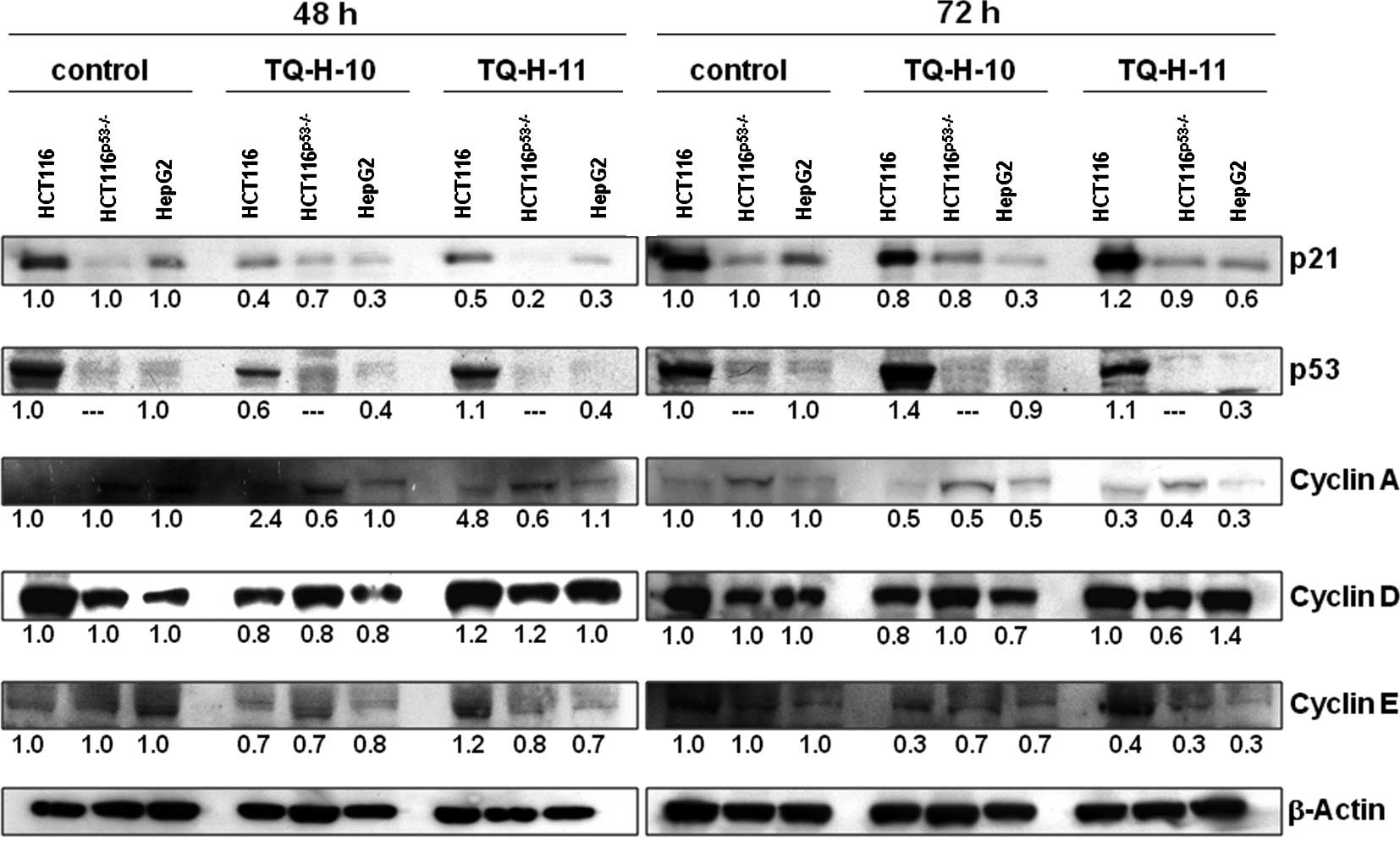
To confirm these results, quantitative Western
blotting was performed (Fig. 6).
In line with the previously described findings, the most resistant
HepG2 cells showed a pronounced down-regulation of
p21cip1/waf1 and p53, while cyclin levels were mostly
unaffected at 48 h. In the sensitive HCT116 cell line, we observed
no increase in p21cip1/waf1 protein, but found a
pronounced down-regulation of cyclins A and E, particularly after
72 h of incubation with both TQ derivatives. At the protein level,
HCT116p53−/− cells also showed a down-regulation of
cyclins A and E after a 72-h treatment with 10 μM TQ-H-11,
while other parameters remained largely unaffected. Again,
expression of p21cip1/waf1 was unchanged in these
p53-deficient cells after treatment with either of the TQ
compounds.
Discussion
Black seed (Nigella sativa) and its oil have
traditionally been used in Arab and Mediterranean medicine for a
variety of diseases, and have also become popular as food
supplements in Western countries. Thymoquinone (TQ) was identified
as the major active constituent of black seed essential oil
(1), and has been demonstrated to
exert anticancer effects in various models of human cancer
(2,3,10,12–14).
Several molecular pathways of TQ activity in cancer cells have
recently been described, for example, activation of caspases or
p53-dependent mechanisms of cell growth control (4,15–17).
Although these pre-clinical and experimental data provide
encouraging evidence for a clinical application of TQ, its use in
humans is limited due to its low chemical stability and poor
solubility in aqueous solutions. We previously showed that the
modification of TQ structure by attaching fatty acids enhances the
pro-apoptotic and antiproliferative properties of the molecule
(6). In the present study, we
investigated the molecular effects associated with unsaturated
(TQ-H-10) and saturated (TQ-H-11) fatty acid modifications of TQ in
the human HCT116 colorectal cancer cell line and in a derivative
lacking p53 (HCT116p53−/−), as well as in the human
HepG2 hepatocellular carcinoma cell line (p53 wild-type).
Our previous work showed that TQ effectively
inhibits the proliferation of HCT116 cells at concentrations of 40
μM or higher (4,10). Modification of TQ with an
unsaturated fatty acid enhanced this effect and resulted in
significant growth inhibition, even at 10 μM, in
p53-competent HCT116 cells (Fig.
2). This was associated with an increase in the S/G2
cell population (Figs. 3 and
4). Notably, this effect was not
observed in either HCT116p53−/− or HepG2 cells,
indicating a differential intracellular metabolism in liver and
colon cell types as well as a dependency on p53 to inhibit cell
cycle progression. Although previous studies have demonstrated a
good apoptotic response to TQ in p53-deficient cells as well
(15,17), these results were obtained at
higher concentrations of native TQ, which may also induce a
non-specific cytotoxic reaction, for example, due to formation of
oxidative stress which we demonstrated previously (6).
A molecular pathway analysis by quantitative RT-PCR
and Western blotting revealed stable expression of the cell cycle
inhibitor p21cip1/waf1 in responsive HCT116 cells, but
not in the other investigated cell lines (Figs. 5 and 6). Although an increase in p53 mRNA was
observed in HepG2 cells, this up-regulation was not observed at the
protein level, indicating a post-transcriptional processing of p53
mRNA (18). The observed growth
inhibition and redistribution of cells to the S/G2 phase
was confirmed using PCR based on the observed strong
down-regulation of mRNA for cyclins D and E. Notably, TQ has
previously been shown to induce G0/G1 arrest
in various cancer cell lines (12,16),
suggesting a different interaction caused by the unsaturated fatty
acid residue in TQ-H-10. Although this observation is in line with
the known cell cycle inhibition properties of
p21cip1/waf1 (19,20),
the down-regulation, particularly of cyclin E, which is crucial for
progression from the G1 to S phase (20,21),
is in contrast to our results. However, recent reports suggest the
possibility for cells to enter the S phase even when lacking
CDK2/cyclin E complex activity (19,20,22),
and knockout mice for either cyclin E or CDK2 also showed normal
development (23). It is currently
assumed that other cyclins can rescue the lack of CDK2/cyclin E in
this setting, and we observed a slight increase in cyclin A
expression, which might be sufficient to promote cell cycle
progression. As cyclin E overexpression is commonly observed in
human malignancies and has also been proposed as a prognostic
marker (21,22,24–28),
our findings indicate a beneficial effect, especially on cyclin
E-positive tumors, by treatment with TQ-H derivatives. This effect
is enhanced by covalent linkage to unsaturated fatty acid
structures, resulting in extensive antiproliferative effects as
described above.
Acknowledgements
The excellent technical assistant of
Astrid Taut and Isabel Zeitträger is gratefully acknowledged.
References
|
1.
|
Amin A, Gali-Muhtasib H, Ocker M and
Schneider-Stock R: Overview of major classes of plant-derived
anticancer drugs. Int J Biomed Sci. 5:1–11. 2009.PubMed/NCBI
|
|
2.
|
Worthen DR, Ghosheh OA and Crooks PA: The
in vitro anti-tumor activity of some crude and purified components
of blackseed, Nigella sativa L. Anticancer Res.
18:1527–1532. 1998.PubMed/NCBI
|
|
3.
|
Gali-Muhtasib H, Diab-Assaf M, Boltze C,
et al: Thymoquinone extracted from black seed triggers apoptotic
cell death in human colorectal cancer cells via a p53-dependent
mechanism. Int J Oncol. 25:857–866. 2004.PubMed/NCBI
|
|
4.
|
Gali-Muhtasib H, Kuester D, Mawrin C, et
al: Thymoquinone triggers inactivation of the stress response
pathway sensor CHEK1 and contributes to apoptosis in colorectal
cancer cells. Cancer Res. 68:5609–5618. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Al-Ali A, Alkhawajah AA, Randhawa MA and
Shaikh NA: Oral and intraperitoneal LD50 of thymoquinone, an active
principle of Nigella sativa, in mice and rats. J Ayub Med
Coll Abbottabad. 20:25–27. 2008.PubMed/NCBI
|
|
6.
|
Breyer S, Effenberger K and Schobert R:
Effects of thymoquinone-fatty acid conjugates on cancer cells.
ChemMedChem. 4:761–768. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Martin BT and Strebhardt K: Polo-like
kinase 1: target and regulator of transcriptional control. Cell
Cycle. 5:2881–2885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Okamoto K, Neureiter D, Alinger B, et al:
The dual EGF/VEGF receptor tyrosine kinase inhibitor AEE788
inhibits growth of human hepatocellular carcinoma xenografts in
nude mice. Int J Oncol. 33:733–742. 2008.PubMed/NCBI
|
|
9.
|
Jabari S, Meissnitzer M, Quint K, et al:
Cellular plasticity of trans- and dedifferentiation markers in
human hepatoma cells in vitro and in vivo. Int J
Oncol. 35:69–80. 2009.PubMed/NCBI
|
|
10.
|
Gali-Muhtasib H, Ocker M, Kuester D, et
al: Thymoquinone reduces mouse colon tumor cell invasion and
inhibits tumor growth in murine colon cancer models. J Cell Mol
Med. 12:330–342. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Ocker M and Schneider-Stock R: Histone
deacetylase inhibitors: signalling towards p21cip1/waf1. Int J
Biochem Cell Biol. 39:1367–1374. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Shoieb AM, Elgayyar M, Dudrick PS, Bell JL
and Tithof PK: In vitro inhibition of growth and induction
of apoptosis in cancer cell lines by thymoquinone. Int J Oncol.
22:107–113. 2003.
|
|
13.
|
Tan M, Norwood A, May M, Tucci M and
Benghuzzi H: Effects of (-)epigallocatechin gallate and
thymoquinone on proliferation of a PANC-1 cell line in culture.
Biomed Sci Instrum. 42:363–371. 2006.PubMed/NCBI
|
|
14.
|
Edris AE: Anti-cancer properties of
Nigella spp. essential oils and their major constituents,
thymoquinone and beta-elemene. Curr Clin Pharmacol. 4:43–46.
2009.
|
|
15.
|
El-Mahdy MA, Zhu Q, Wang QE, Wani G and
Wani AA: Thymoquinone induces apoptosis through activation of
caspase-8 and mitochondrial events in p53-null myeloblastic
leukemia HL-60 cells. Int J Cancer. 117:409–417. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Gali-Muhtasib HU, Abou Kheir WG, Kheir LA,
Darwiche N and Crooks PA: Molecular pathway for
thymoquinone-induced cell-cycle arrest and apoptosis in neoplastic
keratinocytes. Anticancer Drugs. 15:389–399. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Roepke M, Diestel A, Bajbouj K, et al:
Lack of p53 augments thymoquinone-induced apoptosis and caspase
activation in human osteosarcoma cells. Cancer Biol Ther.
6:160–169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Kruse JP and Gu W: Modes of p53
regulation. Cell. 137:609–622. 2009. View Article : Google Scholar
|
|
19.
|
Sanchez I and Dynlacht BD: New insights
into cyclins, CDKs and cell cycle control. Semin Cell Dev Biol.
16:311–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Sherr CJ and Roberts JM: CDK inhibitors:
positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Ekholm-Reed S, Mendez J, Tedesco D,
Zetterberg A, Stillman B and Reed SI: Deregulation of cyclin E in
human cells interferes with prereplication complex assembly. J Cell
Biol. 165:789–800. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Hwang HC and Clurman BE: Cyclin E in
normal and neoplastic cell cycles. Oncogene. 24:2776–2786. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Mendez J: Cell proliferation without
cyclin E-CDK2. Cell. 114:398–399. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Keck JM, Summers MK, Tedesco D, et al:
Cyclin E over-expression impairs progression through mitosis by
inhibiting APC(Cdh1). J Cell Biol. 178:371–385. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Schraml P, Bucher C, Bissig H, et al:
Cyclin E overexpression and amplification in human tumours. J
Pathol. 200:375–382. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Muller-Tidow C, Metzger R, Kugler K, et
al: Cyclin E is the only cyclin-dependent kinase 2-associated
cyclin that predicts metastasis and survival in early stage
non-small cell lung cancer. Cancer Res. 61:647–653. 2001.PubMed/NCBI
|
|
27.
|
Erlanson M and Landberg G: Prognostic
implications of p27 and cyclin E protein contents in malignant
lymphomas. Leuk Lymphoma. 40:461–470. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Wingate H, Puskas A, Duong M, et al: Low
molecular weight cyclin E is specific in breast cancer and is
associated with mechanisms of tumor progression. Cell Cycle.
8:1062–1068. 2009. View Article : Google Scholar : PubMed/NCBI
|