Contents
Introduction
The calpain family
Calpain in pathophysiology
Calpain in colon cancer
Biological inhibitors of calpain
Conclusion
Introduction
Calpain is an intracellular
Ca2+-dependent cysteine protease that is ubiquitously
distributed throughout the body. Calpain plays a significant role
in various cellular functions such as signal transductions and cell
morphogenesis (1,2). Various reports have suggested that
humans have 15 genes that encode a calpain-like protease domain.
These generate diverse types of calpain homologues with
combinations of several functional domains, such as
Ca2+-binding and the Zn-finger domains (1,2). The
purpose of this review is to summarize the cross-talk between
m-calpain and its inhibitors, calpastatin and high molecular
weight calmodulin-binding protein (HMWCaMBP), in human colorectal
cancer.
The calpain family
Calpains are Ca2+-activated cysteine
proteases that act as major mediators for Ca2+ signals
in many biological systems (1,2).
There are two types of calpains, I or μ- and II or
m-calpain, which require a micromolar and millimolar
concentration of Ca2+ for activation, respectively
(1,2). Both calpains are heterodimers
consisting of a common small subunit (28 kDa) with a regulatory
function and a distinct large catalytic subunit (80 kDa). Different
mechanisms responsible for m-calpain regulation have been
reported, and an important role has been ascribed to the specific
inhibitor calpastatin (3–5). In addition, an endogenous calpain
inhibitor, HMWCaMBP, was identified and characterized in our
laboratory (6). Based on sequence
homology, amino acid analysis, antibody reactivity and calpain
inhibition, we demonstrated that HMWCaMBP is homologous to
calpastatin, an endogenous inhibitor of calpains (7).
Calpain has catalytic and regulatory subunits that
can be divided into 4 and 2 domains, respectively (1–5). The
N-terminus of domain I of the large subunit is autolyzed upon
activation by Ca2+ in order to have a lower
Ca2+ requirement, and the autolysis results in the
dissociation of the subunits. Therefore, autolysis is involved in
the regulation of calpain activity and specificity (1). Three-dimensional structural studies
revealed that the protease domain in the absence of Ca2+
is divided into two sub-domains, domains IIa and IIb, which are
folded into one domain upon Ca2+ binding (1–4).
This domain is most conserved among calpain family members,
suggesting it has indispensable functions. The protease domain of
μ- and m-calpains without other domains showed
Ca2+-dependent protease activity. This is supported by
3-D-structural studies of the protease domain in the presence of
Ca2+, which showed Ca2+ binding to domains
IIa and IIb. Thus, the Ca2+-dependency of calpains is
controlled as a whole molecule, since all domains (IIa, IIb, III,
IV and VI) bind at least one Ca2+ with different
affinities (1–4).
Calpain in pathophysiology
Recently, the importance of calpain in the
metastatic process has received a great deal of attention. Calpain
may be involved in cell adhesion, spreading, migration, myoblast
fusion, cell cycle control and mitosis (8–10).
Calpain-mediated proteolysis represents a major pathway of
post-translational modification that influences various aspects of
cell physiology, including apoptosis, cell migration and cell
proliferation (1). Calpains cause
limited proteolysis of substrates, resulting in the alteration of
substrate activity. PEST sequences are believed to be the
intramolecular signals for rapid proteolytic degradation by
m-calpain. Lakshmikuttyamma et al (11,12)
reported that the increase in calcineurin (CaN) activity and strong
immunostaining in ischemic/perfused rat hearts may be due to the
m-calpain-mediated proteolysis of CaN. In addition, the
interaction of CaN with m- and μ-calpains was strong in
epileptic chickens compared to normal birds (13,14).
It has also been reported that N-myristoyltransferase (NMT)
interacts with m-calpain in epileptic chickens (14,15).
Among two forms of NMTs (NMT1 and NMT2), a higher interaction of
m-calpain with NMT2 was observed (14,15).
Calpain in colon cancer
Calpains result in the proteolysis of a broad
spectrum of cellular proteins (2),
including multiple signaling enzymes, protein kinase C,
pp60c-Src and tyrosine phosphatase 1B (16–18).
Most of the substrate proteins of calpains have been implicated in
the pathogenesis of human tumors, suggesting an important
regulatory role of calpains in malignant diseases. The role of
calpains in carcinogenesis and tumor progression has yet to be
explored. In human renal cell carcinomas, significantly higher
levels of μ-calpain expression were found in tumors that had
metastasized to peripheral lymph nodes compared to tumors that
apparently had not metastasized (19). It has been reported that the
epigenetic activation of calpain II plays an important role in the
invasion of human prostate cancer and can be targeted to reduce
tumor progression (20).
Gastric-specific calpain-9 is down-regulated in carcinomas, and its
relation to differentiation status or tumorigenesis remains unclear
(21,22). The activity and expression of
μ-calpain were significantly increased in chronic lymphocytic
leukemia cells compared to non-malignant cells, whereas the
activity and expression of m-calpain and calpastatin were
unchanged (23).
The proto-oncogenes c-fos and c-jun,
several cytoskeletal proteins, the tumor suppressor protein p53 and
signaling molecules protein kinase C and focal adhesion kinase
(FAK; a non-receptor kinase) are substrates for calpain (24–26).
Calpain-mediated cleavage of FAK and focal adhesion disassembly
accompany v-Src-induced morphological transformation (26). v-Src-induced oncogenic
transformation is characterized by alterations in cell morphology,
adhesion, motility, survival and proliferation (27,28).
In response to v-Src activation, Carragher et al (29) demonstrated an increase in the total
protein levels of calpain II and decreased levels of calpastatin in
chicken embryo fibroblasts. Furthermore, the data suggested a
feed-back loop mechanism of calpain activation initiated in
response to the activation of the v-Src oncogene (29). Activation of Src, which has
intrinsic tyrosine kinase activity, has been demonstrated in human
solid tumors such as colorectal and breast carcinomas (30,31).
We observed that the increased activity and expression of
m-calpain corresponded to a decrease in the expression of
calpastatin and HMWCaMBP in adenocarcinoma (32). Selvakumar et al (33) reported that the protein-protein
interaction of NMTs revealed that m-calpain interacts with
NMT1, while caspase-3 interacts with NMT2 in human colorectal
adenocarcinomas. Previously, our laboratory reported that
m-calpain proteolyses NMT1 and abolishes the enzyme activity
(34).
Cell death by apoptosis is a fundamental process
controlling the normal development and homeostasis of multicellular
organisms. Decreased apoptotic susceptibility contributes to the
pathogenesis of several diseases including cancer (1–5).
Calpains are involved in controlling the level and duration of
transduction signals leading to either proliferation or apoptosis
in multiple cell systems (1,3).
However, their role in the development and course of apoptosis is
controversial. A central regulator of apoptotic susceptibility is
the tumor suppressor protein p53, whose level is regulated by
several stress conditions, cell adhesion and the expression of
several oncogenes (35,36). It has also been reported that p53
is proteolytically cleaved in vitro by calpains (25,37).
The elevated expression of m-calpain in colorectal cancer
may act on p53, and is followed by a decrease in the event of
apoptosis. Likewise, the calpain-mediated cleavage of Bax promotes
the pro-apoptotic effect of Bax (38), and the calpain cleavage of
pro-caspase-7 and procaspase-3 leads to the activation of these
proteases (39,40). Chen et al (41) reported a reduction in the protein
levels of caspase-3, -7 and -9 in human colon cancer specimens. The
apoptosis promoting caspase system is activated after calpain
inhibition with calpain inhibitor II in neoplastic lymphoid cells
(42). Cross-talk between calpain
and caspases appears to be important for the regulation of
apoptosis in colon tumors.
Various reports, including those from our
laboratory, have suggested that m-calpain is involved in the
progression of metastasis (26,32).
In order to analyze the role of m-calpain in human
colorectal adenocarcinoma, calpain activity and protein expression
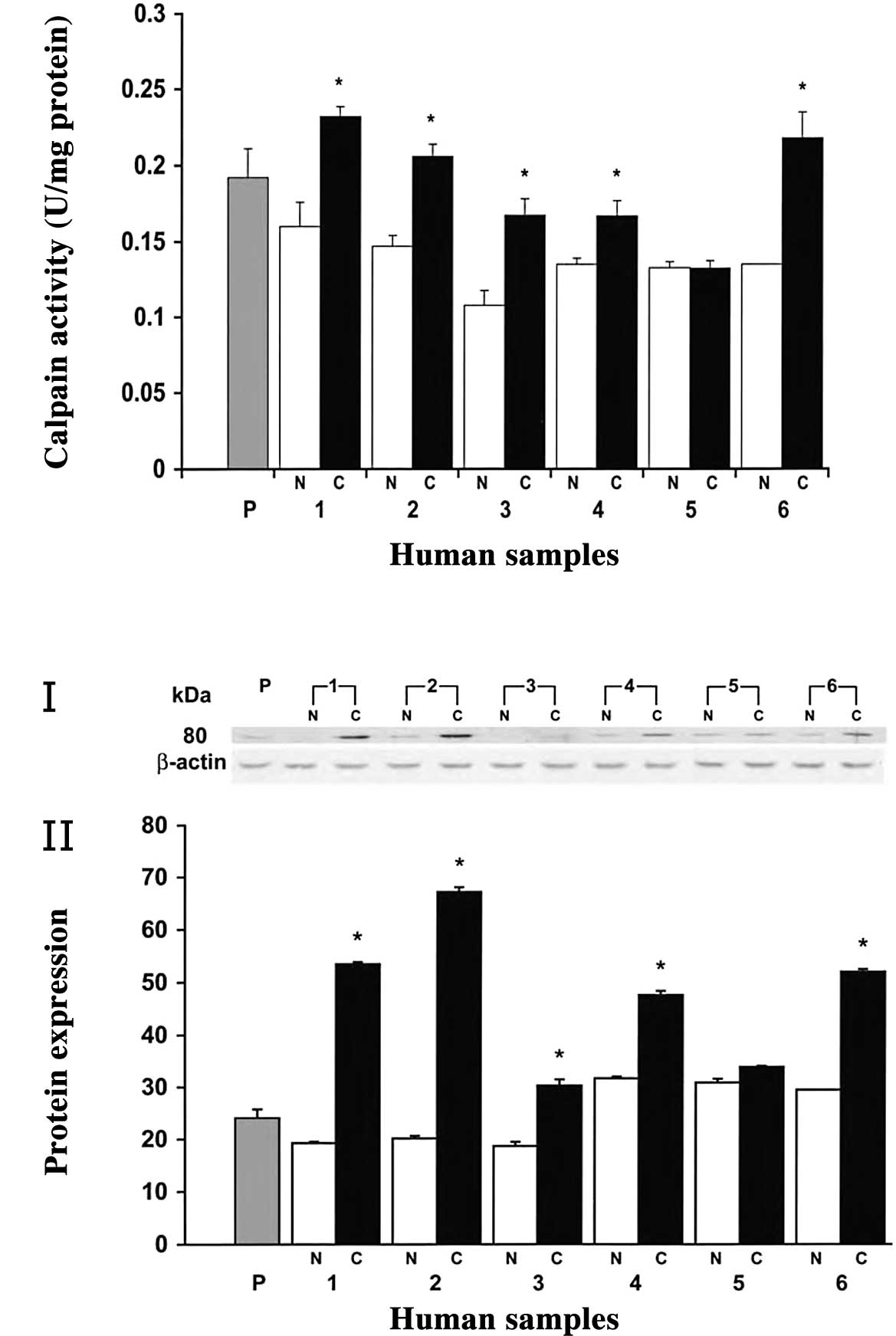
was examined in human tissue samples. In most of the cases, the
calpain activity was significantly higher in human colorectal
adenocarcinoma than in normal mucosa (83% of cases, P<0.05)
(Fig. 1). Notably,
m-calpain activity was higher in polyps than normal tissues,
though not as high as in cancerous tissues (Fig. 1A). Furthermore, a higher expression
of m-calpain was found in cancerous tissues, whereas it was
poorly expressed in normal mucosa as determined by Western blot
analysis (Fig. 1B, panel I).
Quantitative analysis of the 80-kDa band revealed a 2- to 3-fold
higher expression (P<0.05) of m-calpain in colorectal
tumors compared to their respective normal mucosa (Fig. 1B, panel II). However, no change in
expression was observed at the 28 kDa small subunit of
m-calpain (unpublished data). In polyps, the expression of
m-calpain was higher than in normal tissues, while no
significant change was observed in the remaining normal tissues
(Fig. 1B, panel II).
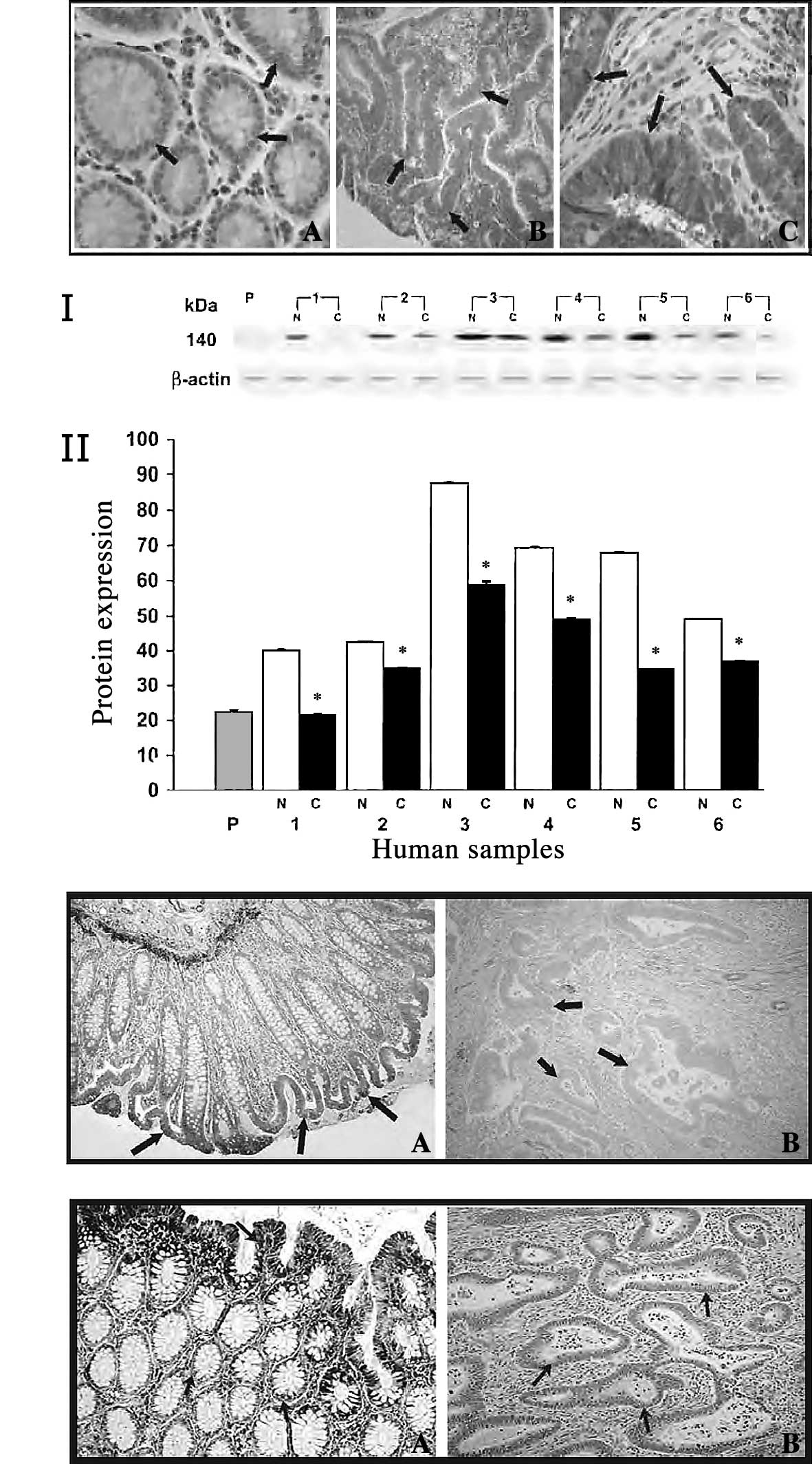
Immunohistochemical analysis showed strong staining
for m-calpain in colorectal adenocarcinoma (Fig. 2A-c) by the avidin-biotin complex
method graded as described previously (43,44).
Mild reactivity (<10% of protein expression) was observed in
mucosal sections taken distant from the cancerous tissues (Fig. 2A-a). In polyps, moderate staining
was observed, and the degree of immunoreactivity was less than in
tumor tissue (Fig. 2A-b). From
these studies and various reports, it is clear that
m-calpain is a major player during the process of various
diseases, including cancer, cardiovascular and neurological
diseases.
Biological inhibitors of calpain
Calpain activity is tightly regulated by its
ubiquitously expressed endogenous inhibitor, calpastatin (3–5). We
also monitored the expression of the calpain inhibitor,
calpastatin, to determine whether these endogenous inhibitors
regulate calpain activity in colorectal adenocarcinoma (32). Western blot analysis of calpastatin
revealed strong expression in normal mucosa, whereas weak
expression was observed in colorectal tumors (Fig. 2B, panel I). Quantitative analysis
of calpastatin revealed an approximately 2-fold increased
expression in normal tissues compared to cancerous tissues
(Fig. 2B, panel II). The weak
expression of calpastatin in colon tumors was further confirmed by
immunohistochemical analysis (Fig.
2C-b). Moderate-to-strong staining was observed for normal
mucosa, while a weak cytoplasmic positivity was observed for
calpastatin in invasive carcinoma, with decreased intensity in the
invasive component (Fig.
2C-a).
We also investigated the role of another calpain
endogenous inhibitor, HMWCaMBP, which was identified and
characterized at our laboratory in human colorectal adenocarcinomas
(32). Similar to calpastatin,
Western blot analysis was carried out using anti-HMWCaMBP and
showed a weakly expressed immunoreactive band with an apparent
molecular mass of 140 kDa in colon tumors. Significantly higher
staining with anti-HMWCaMBP was observed in normal mucosa
(unpublished data). Furthermore, immunohistochemical studies
revealed mild reactivity of HMWCaMBP in colorectal adenocarcinoma
(Fig. 2D-b). However, the mucosal
sections taken distant from the tumor showed strong staining
(Fig. 2D-a). We observed that the
increased activity and expression of m-calpain was
correlated with the decreased expression of calpastatin and
HMWCaMBP in human colorectal adenocarcinoma.
Conclusion
Apart from the known regulatory functions of
calpains, various reports, including ours, suggest that increased
m-calpain expression may directly contribute to the
development of cell progression in colorectal adenocarcinoma. The
determination of the mechanisms causing the increase in calpain
expression and its action on cell signaling may yield data critical
for addressing many unanswered questions about cell proliferation
in colon tumors. Increased activity and moderate staining of
m-calpain in polyps demonstrate the usage of this enzyme as
a marker for the early detection of colorectal adenocarcinoma using
immunological approaches. The overexpression of calpastatin or
HMWCaMBP, which are specific for calpain inhibition, may be used as
a specific molecular target for the treatment of colon cancer.
Acknowledgements
This study was supported by the
Canadian Institutes of Health Research, Canada. The authors are
thankful to Dr J.R. Dimmock, College of Pharmacy and Nutrition,
University of Saskatchewan, for the critical reading of the
manuscript and suggestions, and to Mr. Mark F. Boyd and Mr. Todd
Reichert for the technical and photographic work.
References
|
1.
|
Sorimachi H, Ishiura S and Suzuki K:
Structure and physiological function of calpains. Biochem J.
328:721–732. 1997.
|
|
2.
|
Goll DE, Thompson VF, Li H, Wei W and Cong
J: The calpain system. Physiol Rev. 83:731–801. 2002. View Article : Google Scholar
|
|
3.
|
Ono Y, Sorimachi H and Suzuki K: Structure
and physiology of calpain, an enigmatic protease. Biochem Biophys
Res Commun. 245:289–294. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Maki M, Ma H, Takano E, Adachi Y, Lee WJ,
Hatanaka M and Murachi T: Calpastatins: biochemical and molecular
biological studies. Biomed Biochim Acta. 50:509–516.
1991.PubMed/NCBI
|
|
5.
|
Carragher NO: Calpain inhibition: a
therapeutic strategy targeting multiple disease states. Curr Pharm
Des. 12:615–638. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Sharma RK: Purification and
characterization of novel calmodulin-binding protein from cardiac
muscle. J Biol Chem. 265:1152–1157. 1990.PubMed/NCBI
|
|
7.
|
Kakkar R, Raju RV, Mellgren RL, Radhi J
and Sharma RK: Cardiac high molecular weight calmodulin binding
protein contains calpastatin activity. Biochemistry.
36:11550–11555. 1997. View Article : Google Scholar
|
|
8.
|
Carragher NO and Frame MC: Calpain: a role
in cell transformation and migration. Int J Biochem Cell Biol.
34:1539–1543. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Huttenlocher A, Palecek SP, Lu Q, Zhang W,
Mellgren RL, Lauffenburger DA, Ginsberg MH and Horwitz AF:
Regulation of cell migration by the calcium-dependent protease
calpain. J Biol Chem. 272:32719–32722. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Patel YM and Lane MD: Mitotic clonal
expansion during pre-adipocyte differentiation: calpain-mediated
turnover of p27. J Biol Chem. 275:17653–17660. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Lakshmikuttyamma A, Selvakumar P, Kakkar
R, Kanthan R, Wang R and Sharma RK: Activation of calcineurin
expression in ischemia-reperfused rat heart and in human ischemic
myocardium. J Cell Biochem. 90:987–997. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Lakshmikuttyamma A, Selvakumar P, Sharma
AR, Anderson DH and Sharma RK: In vitro proteolytic degradation of
bovine brain calcineurin by m-calpain. Neurochem Res. 29:1913–1921.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Lakshmikuttyamma A, Selvakumar P,
Charavaryamath C, Singh B, Tuchek J and Sharma RK: Expression of
calcineurin and its interacting proteins in epileptic fowl. J
Neurochem. 96:366–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Lakshmikuttyamma A, Selvakumar P, Tuchek J
and Sharma RK: Myristoyltransferase and calcineurin: novel
molecular therapeutic target for epilepsy. Prog Neurobiol.
84:77–84. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Selvakumar P, Lakshmikuttyamma A,
Charavaryamath C, Singh B, Tuchek J and Sharma RK: Expression of
myristoyltransferase and its interacting proteins in epilepsy.
Biochem Biophys Res Commun. 335:1132–1139. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kishimoto A, Kajikawa N, Shiota M and
Nishizuka Y: Preoteolytic activation of calcium-activated,
phospholipid-dependent protein kinase by calcium-dependent neutral
protease. J Biol Chem. 258:1156–1164. 1983.
|
|
17.
|
Oda A, Drucker BJ, Ariyoshi H, Smith M and
Salzman E: pp60src is an andogenous substrate for
calpain in human blood platelets. J Biol Chem. 268:12603–12608.
1993.
|
|
18.
|
Frangioni JV, Oda A, Smith M, Salzman EW
and Neel BG: Calpain-catalyzed cleavage and subcellular relocation
of protein phosphotyrosine phosphatase 1B PTB-1B in human
platelets. EMBO J. 12:4843–4856. 1993.
|
|
19.
|
Braun C, Engel M, Seifert M, Theisinger B,
Seitz G, Zang KD and Welter C: Expression of calpain I messenger
RNA in human renal cell carcinoma: correlation with lymph node
metastasis and histological type. Int J Cancer. 84:6–9. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Mamoune A, Luo JH, Lauffenburger DA and
Wells A: Calpain-2 is a target for limiting prostate cancer
invasion. Cancer Res. 63:4632–4640. 2003.PubMed/NCBI
|
|
21.
|
Yoshikawa Y, Mukai H, Hino F, Asada K and
Kato I: Isolation of two novel genes, down regulated in gastric
cancer. Jpn J Cancer Res. 91:459–463. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Liu K, Li L and Cohen SN: Antisense
RNA-mediated deficiency of the calpain protease, nCL-4, in
NH3T3cells is associated with neoplastic transformation and
tumorigenesis. J Biol Chem. 275:31093–31098. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Witkowski JM, Zmuda-Trzebiatowska E,
Swiercz JM, Cichorek M, Ciepluch H, Lewandowski K, Bryl E and
Hellmann A: Modulation of the activity of calcium-activated neutral
proteases calpains in chronic lymphocytic leukemia B-CLL cells.
Blood. 100:1802–1809. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Carillo S, Pariat M, Steff AM, Roux P,
Etienne-Julan M, Lorca T and Piechaczyk M: Differential sensitivity
of FOS and JUN family members to calpains. Oncogene. 9:1679–1689.
1994.PubMed/NCBI
|
|
25.
|
Kubbutat MH and Vousden KH: Proteolytic
cleavage of human p53 by calpain: a potential regulator of protein
stability. Mol Cell Biol. 17:460–468. 1997.PubMed/NCBI
|
|
26.
|
Carragher NO, Fincham VJ, Riley D and
Frame MC: Cleavage of focal adhesion kinase by different proteases
during SRC-regulated transformation and apoptosis. Distinct roles
for calpain and caspases. J Biol Chem. 276:4270–4275. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Johnson D, Frame MC and Wyke JA:
Expression of the v-Src oncoprotein in fibroblasts disrupts normal
regulation of the CDK inhibitor p27 and inhibits quiescence.
Oncogene. 16:2017–2028. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Kellie S: Cellular transformation,
tyrosine kinase oncogenes and the cellular adhesion plaque.
Bioessays. 8:25–30. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Carragher NO, Westhoff MA, Riley D, Potter
DA, Dutt P, Elce JS, Greer PA and Frame MC: v-Src-induced
modulation of the calpain-calpastatin proteolytic system regulates
transformation. Mol Cell Biol. 22:257–269. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Bolen JB, Veillette A, Schwartz AM, DeSeau
V and Rosen N: Activation of pp60c-src protein kinase
activity in human colon carcinoma. Proc Natl Acad Sci USA.
84:2251–2255. 1987.PubMed/NCBI
|
|
31.
|
Ottenhoff-Kalff AE, Rijksen G, van Beurden
EA, Hennipman A, Michels AA and Staal GE: Characterization of
protein tyrosine kinases from human breast cancer: involvement of
the c-src oncogene product. Cancer Res. 52:4773–4778.
1992.PubMed/NCBI
|
|
32.
|
Lakshmikuttyamma A, Selvakumar P, Kanthan
R, Kanthan SC and Sharma RK: Overexpression of m-calpain in human
colorectal adenocarcinomas. Cancer Epidemiol Biomarkers Prev.
13:1604–1609. 2004.PubMed/NCBI
|
|
33.
|
Selvakumar P, Smith-Windsor E, Bonham K
and Sharma RK: N-myristoyltransferase 2 expression in human colon
cancer: cross-talk between the calpain and caspase system. FEBS
Lett. 580:2021–2026. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Raju RV, Kakkar R, Datla RS, Radhi J and
Sharma RK: Myristoyl-coA: protein N-myristoyltransferase from
bovine cardiac muscle: molecular cloning, kinetic analysis and in
vitro proteolytic cleavage by m-calpain. Exp Cell Res. 241:23–35.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Levine AJ: p53, the cellular gatekeeper
for growth and division. Cell. 88:323–331. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Giaccia AJ and Kastan MB: The complexity
of p53 modulation: emerging patterns from divergent signals. Genes
Dev. 12:2973–2983. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Gonen H, Shkedy D, Barnoy S, Kosower NS
and Ciechanover A: On the involvement of calpains in the
degradation of the tumor suppressor protein p53. FEBS Lett.
406:17–22. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Toyota H, Yanase N, Yoshimoto T, Moriyama
M, Sudo T and Mizuguchi J: Calpain-induced Bax-cleavage product is
a more potent inducer of apoptotic cell death than wild-type Bax.
Cancer Lett. 189:221–230. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Blomgren K, Zhu C, Wang X, Karlsson JO,
Leverin AL, Bahr BA, Mallard C and Hagberg H: Synergistic
activation of caspase-3 by m-calpain after neonatal
hypoxia-ischemia: a mechanism of ‘pathological apoptosis’? J Biol
Chem. 276:10191–10198. 2001.PubMed/NCBI
|
|
40.
|
Ruiz-Vela A, Gonzalez de Buitrago G and
Martinez AC: Implication of calpain in caspase activation during B
cell clonal deletion. EMBO J. 18:4988–4998. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Chen T, Yang I, Irby R, Shain KH, Wang HG,
Quackenbush J, Coppola D, Cheng JQ and Yeatman TJ: Regulation of
caspase expression and apoptosis by adenomatous polyposis coli.
Cancer Res. 63:4368–4374. 2003.PubMed/NCBI
|
|
42.
|
Zhu DM and Uckun FM: Calpain inhibitor II
induces caspase-dependent apoptosis in human acute lymphoblastic
leukemia and non-Hodgkin's lymphoma cells as well as some solid
tumor cells. Clin Cancer Res. 6:2456–2463. 2000.PubMed/NCBI
|
|
43.
|
Raju RV, Moyana TN and Sharma RK:
N-myristoyltransferase overexpression in human colorectal
adenocarcinomas. Exp Cell Res. 235:145–154. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Moyana TN and Xiang J: Expression of
tumor-associated polymorphic epithelial mucin and carcinoembryonic
antigen in gastrointestinal carcinoid tumors. Cancer. 75:2836–2843.
1995. View Article : Google Scholar
|