Introduction
Despite improvements in diagnostic methods and
antimicrobial agents, the incidence of infective endocarditis (IE)
remains high in specific populations of individuals, such as
hemodialysis patients and drug abusers (1,2). The
prognosis of IE has been shown to be strongly influenced by the
complication of congestive heart failure, neurologic events,
systemic embolism and prolonged fever (3). Glomerulonephritis, secondary to
endocarditis, is a rare diagnosis usually associated with blood
culture-positive bacteria, particularly staphylococci and
streptococci (4). In some cases,
IE induces rapidly progressive glomerulonephritis and results in
end-stage renal failure, which is associated with poor patient
prognosis (5). Apart from
antibiotic therapy, effective strategies include surgery, steroid
therapy, immunosuppressive therapy and dialysis (6,7).
However, the appropriate therapy for IE associated with renal
injury has not been adequately defined. Here, we report a case of
IE associated with acute renal failure (ARF) for which the clinical
symptoms were successfully improved by treatment with antibiotics,
surgery and continuous ambulatory peritoneal dialysis (CAPD)
therapy. Most importantly, we performed repeat biopsy to elucidate
the prognosis and turnover of this condition.
Case presentation
A 25-year-old male with a history of intravenous
drug abuse was admitted to our hospital due to high fever,
bilateral thoracalgia, lower limb edema and deterioration of renal
function [creatinine (CR), 528 μmol/l; blood urea nitrogen (BUN),
12.2 mmol/l]. There was no history of skin rashes, joint swelling,
digestive symptoms, respiratory symptoms or hypertension.
One month before admission, the patient was admitted
elsewhere with high fever, shivers, general malaise, bilateral
thoracalgia and a squeezing feeling. Laboratory tests revealed
798.7 μmol/l of serum CR, 23.4 mmol/l of BUN and
15.08×109/l of white blood cells with 73.9% neutrophilic
granulocytes. The blood specimen for culture demonstrated
Staphylococcus aureus. His chest CT showed hematogenous
dissemination of pulmonary abscesses, and his echocardiogram
demonstrated vegetation on the tricuspid valve associated with
severe regurgitation. The sizes of the left atrium, right atrium
and right ventricle were mildly increased. The patient was treated
with antibiotics, hemodialysis and anticoagulation therapy.
After being transferred to our hospital for further
treatment, his condition was reevaluated. His temperature was 37°C,
pulse and respiratory rates were 88 and 20/min, respectively, and
blood pressure was 105/65 mmHg. From his physical examination, the
patient was diagnosed with an expanded heart boundary, weak heart
beat and systolic murmur of Levine II/IV. A systolic murmur was
noted at the auscultation area of the tricuspid and the second
auscultation area of the aortic. The lungs were clear, and the
abdomen was soft with no distension, tenderness or palpable masses.
The liver was palpable in the left subcostal region and was not
tender. The rest of the physical examination was normal. Our
overall laboratory findings were anemia (Hb, 73 g/l; HCT, 0.234);
white blood cells, 10.61×109/l (neutrophilic
granulocytes 63.1%); BUN, 12.2 mmol/l and CR, 528 μmol/l. The
patient was positive for the hepatitis C antibody (HCV-Ab). Other
parameters, such as serum complement levels, HIV, hepatitis B
surface antigen, antinuclear antibody, antiglomerular basement
membrane antibody and anti-neutrophil cytoplasmic antibody were all
negative or normal. Echocardiogram again showed vegetation on the
tricuspid valve with severe regurgitation and expansion of the
right atrium and ventricle boundary, accompanying mild pulmonary
hypertension and mild regurgitation of the bicuspid and pulmonary
valves.
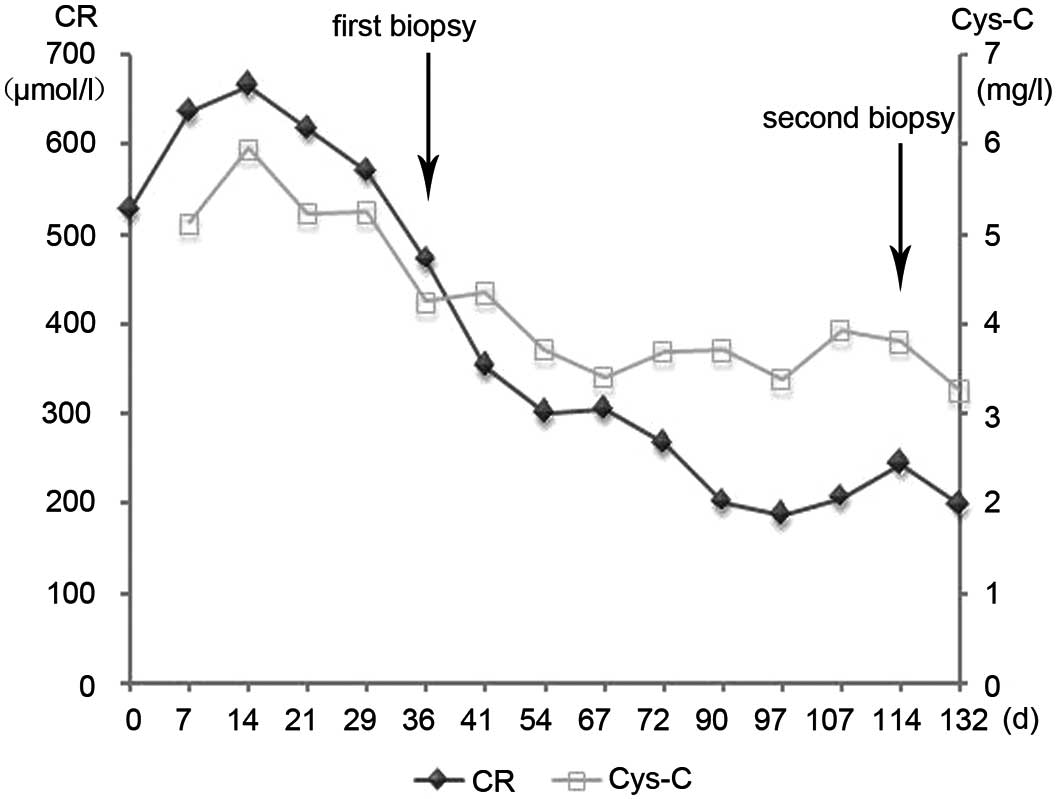
After the short-term treatment with continuous
antibiotics (vancomycin) and hemodialysis, the patient's fever and
edema were reduced. However, his renal function did not return to
normal (Fig. 1). In the absence of
any obvious contraindication to surgery, the patient underwent a
successful tricuspid valve replacement, and the treatment with
antibiotics, hemodialysis and anticoagulation was continued. There
were no bacteria growths in the cultured vegetation and blood
specimens. However, the renal function continued to deteriorate,
and serum CR increased to 640 μmol/l. The patient was then
transferred to the Division of Nephrology for treatment of renal
insufficiency. A kidney biopsy was performed 6 days later which
showed typical crescentic glomerulonephritis (Fig. 2a–c). Eleven of thirteen glomeruli
had crescent formation, including eight large fibrocellular
crescents, one large fibrous crescent, one small cellular crescent
and one small fibrocellular crescent. From the PAS and PASM
stainings, an overall interstitial inflammatory cell infiltration,
mild tubular atrophy and interstitial fibrosis were observed
(Fig. 2). Immunofluorescence
microscopy showed the deposition of immune complexes in the
glomeruli. Mesangial regions and glomerular capillary walls had
comma-like IgA and C3 stainings (1+) diffusely and globally (data
not shown).
Considering his severe renal injury and chronic
hepatitis C development, we decided to perform long-term renal
replacement therapy and decrease his proteinuric level with a full
dose of angiotensin converting enzyme inhibitor (ACEI) and
angiotensin receptor blockers (ARBs), rather than immunosuppressive
therapy. Since the patient was a drug abuser, he received CAPD with
the ultrafiltration volume of 800 ml/day instead of an
arteriovenous fistula operation. One month later, he developed
profound edema of the right lower extremity and a thrill was
palpated in his right groin. Angiography showed right deep femoral
arteriovenous fistula because of his repeated puncture. After
blocking his right deep femoral arteriovenous fistula using digital
subtraction angiography (DSA), the edema of the right lower leg
disappeared. After a 40-day therapy, with a greatly improved
condition of a daily urine output of 1,500 ml and serum CR of
180–240 μmol/l, CAPD was terminated. We carried out a repeat biopsy
to identify his prognosis and turnover. The second biopsy revealed
significant reduction in glomeruli crescents (9/18). They were
mostly fibrous components that consisted of five large and two
small fibrous crescents, and two small fibrocellular crescents.
Proliferation of mesangial and endothelial cells was approximately
the same as in the first biopsy. There was little improvement of
tubular atrophy, interstitial fibrosis and inflammatory cell
infiltration (Fig. 2d–f).
Immunoflourescence examination of five glomeruli revealed 2+
granular IgM and C3 in a global mesangial and capillary wall
distribution, and CIq 1+ granular in a segmental mesangial
distribution (data not shown). After carefully comparing the
pathological changes with the previous biopsy, we diagnosed
endocapillary proliferative glomerulonephritis with crescent
formation.
Discussion
Diffuse crescent formation in IE with renal
involvement is rare. There is a universal consensus among clinical
physicians concerning the treatment of these types of symptoms
using antimicrobial therapy (3,5), yet
there has been no definitive agreement on the use of other
treatments such as surgery and steroid therapy (1,8,9).
Furthermore, plasmapheresis treatment is also recommended and is
thought to possibly relieve immune-mediated pathogenesis (10,11).
Here, we presented a patient who developed crescentic
glomerulonephritis accompanying bacterial endocarditis caused by
Staphylococcus aureus. Although Staphylococcus aureus
is considered the most common pathogen in IE, the mortality rates
of chronic hemodialysis patients are quite high when they are
infected with methicillin-resistant staphylococcus aureus
(MRSA) (1,12). In our patient, IE was diagnosed on
the basis of the clinical presentation, positive blood cultures and
transesophageal echocardiogram findings. There have been reports of
predominant IgA deposits in glomerulonephritis associated with MRSA
infections (13,14). In our case, immunocomplex deposits
(IgA, C3, IgM and C1q) were found in the glomerulus of both
biopsies, which may be one of the main pathogenic factors for IE.
Since the pathogen for this patient was sensitive to vancomycin,
his high fever and pulmonary symptoms were dramatically relieved
after therapy with antibiotics. However, the antibiotic therapy
alone was only able to suppress circulating bacteremia and failed
to decrease the size of the vegetation and the nest of bacteria. We
decided that it was necessary to perform a tricuspid valve
replacement in order to eliminate the vegetation and focal
infection. However, his renal function deteriorated a month after
surgery. The first renal biopsy was perfomed, revealing extensive
crescents in 85% of the glomeruli. The infection-related
circulating immune complex-mediated glomerulonephritis seemed to be
the most appropriate mechanism for the deterioration of the renal
function (15).
Some case reports have shown that immunosuppressive
therapies such as cyclophosphamide and steroid therapy (low-dose)
with antibiotics improve renal dysfunction of IE-induced crescentic
glomerulonephritis (6,8). Due to the patient’s severe renal
injury and hepatitis C infection, we concluded that using
immunosuppressive therapy would have a high virus extension risk,
and that using renal replacement therapy would be relatively safe.
At the same time, there were no relevant reports in the literature
clarifying the side effects associated with steroid therapy in this
condition. We felt more optimistic when his renal dysfunction
obviously improved after the patient accepted CAPD and was
administered a full dose of ACEI and ARBs. More importantly, we
performed a repeat biopsy and found better turnover of the
pathological changes. The proportion of crescents in the yielded
glomeruli was less than that of the first biopsy and mainly
consisted of a fibrous component. Based on the second biopsy, we
verified a diagnosis of endocapillary proliferative
glomerulonephritis with crescent formation. As mentioned above, the
percentage of crescents was 11/13 (85%) in the first biopsy and
9/18 (50%) in the second. Notably, the decrease in the proportion
of crescents from the first to the second biopsy was modified by
the inclusion of the absence of statistical significance and the
possibility of sampling variation. After treatments to eliminate
the focal infection, decrease the proteinuria and protect the renal
function, the patient recovered to a urine output of 1,000–2,000
ml/24 h and a serum CR of 120–200 μmol/l.
The patient prognosis of IE associated with
extensive crescents is poor, and patients usually present with ARF
(16). In the event of a delayed
diagnosis and inappropriate antibiotic therapy, the condition of
the patient may deteriorate leading to death. After effective
antibiotic and surgical therapy, continual protection and recover
of the renal function is the principal objective. Our previous
research revealed that ACEI therapy significantly reduces the level
of proteinuria and delays the rate of decline in renal function
(17). However, patients with
IE-associated glomerulonephritis were not included in this report.
Here, we used an adequate dosage of ACEI and ARBs in order to
decrease the patient's proteinuria, and his renal function was
successfully recovered with CAPD therapy. The pathophysiology and
prognosis of this case are perhaps similar to those of
postinfectious glomerulonephritis, which involves exposure to
bacterial antigens and can sometimes be crescentic. In conclusion,
the present report describes the clinical presentation,
histological outcome and effective treatment of a patient with IE
and crescentic glomerulonephritis caused by Staphylococcus
aureus. Patients with IE associated with ARF can be
successfully treated with the proper use of antibiotics, surgery,
renal replacement and ACEI/ARB therapies. This presents the
clinical and biological implications of kidney protection and
proteinuria reduction in the treatment of this illness.
Acknowledgements
We thank Kai Wang (University of
California, Davis) for editing the manuscript.
References
|
1.
|
Spies C, Madison JR and Schatz IJ:
Infective endocarditis in patients with end-stage renal disease:
clinical presentation and outcome. Arch Intern Med. 164:71–77.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Levine DP, Cushing RD, Jui J, et al:
Community-acquired methicillin-resistant Staphylococcus
aureus endocarditis in the Detroit Medical Center. Ann Intern
Med. 97:330–338. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Mylonakis E and Calderwood SB: Infective
endocarditis in adults. N Engl J Med. 345:1318–1330. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Majumdar A, Chowdhary S, Ferreira MAS, et
al: Renal pathologic findings in infective endocarditis. Nephrol
Dial Transplant. 15:1782–1787. 2000. View Article : Google Scholar
|
|
5.
|
Neugarten J, Gallo GR and Baldwin DS:
Glomerulonephritis in bacterial endocarditis. Am J Kidney Dis.
3:371–379. 1984. View Article : Google Scholar
|
|
6.
|
Rovzar MA, Logan JL, Ogden DA, et al:
Immunosuppressive therapy and plasmapheresis in rapidly progressive
glomerulonephritis associated with bacterial endocarditis. Am J
Kidney Dis. 7:428–433. 1986. View Article : Google Scholar
|
|
7.
|
Kannan S and Mattoo TK: Diffuse crescentic
glomerulonephritis in bacterial endocarditis. Pediatr Nephrol.
16:423–428. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Koya D, Shibuya K, Kikkawa R, et al:
Successful recovery of infective endocarditis-induced rapidly
progressive glomerulonephritis by steroid therapy combined with
antibiotics: a case report. BMC Nephrol. 5:18–22. 2004. View Article : Google Scholar
|
|
9.
|
Rankin JS, Milford-Beland S, O'Brien SM,
et al: The risk of valve surgery for endocarditis in patients with
dialysis-dependent renal failure. J Heart Valve Dis. 16:617–622.
2007.PubMed/NCBI
|
|
10.
|
Daimon S, Mizuno Y, Fujii S, et al:
Infective endocarditis-induced crescentic glomerulonephritis
dramatically improved by plasmapheresis. Am J Kidney Dis.
32:309–313. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Couzi L, Morel D, Deminière C, et al: An
unusual endocarditis-induced crescentic glomerulonephritis treated
by plasmapheresis. Clin Nephrol. 62:461–464. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Nori US, Manoharan A, Thornby JI, et al:
Mortality risk factors in chronic haemodialysis patients with
infective endocarditis. Nephrol Dial Transplant. 21:2184–2190.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Koyama A, Sharmin S, Sakurai H, et al:
Staphylococcus aureus cell envelope antigen is a new
candidate for the induction of IgA nephropathy. Kidney Int.
66:121–132. 2004. View Article : Google Scholar
|
|
14.
|
Koyama A, Kobayashi M, Yamaguchi N, et al:
Glomerulonephritis associated with MRSA infection: a possible role
of bacterial superantigen. Kidney Int. 47:207–216. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Rames L, Wise B, Goodman JR, et al: Renal
disease with Staphylococcus albus bacteremia. A complication
in ventriculoatrial shunts. JAMA. 212:1671–1677. 1970.
|
|
16.
|
Lerner IL and Weinstein L: Infective
endocarditis in the antibiotic era. N Engl J Med. 274:199–206.
259–266. 320–321. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Hou FF, Zhang X, Zhang GH, et al: Efficacy
and safety of benazepril for advanced chronic renal insufficiency.
N Engl J Med. 354:131–140. 2006. View Article : Google Scholar : PubMed/NCBI
|