Introduction
Salivary gland tumors present a wide spectrum of
histological and pathophysiological features (1,2). The
most frequently occurring salivary gland tumor is pleomorphic
adenoma (PA), which consists of epithelial and myoepithelial cell
components and is basically benign, but can progress to a malignant
neoplasm on rare occasions (2,3). On
the other hand, among malignant salivary gland tumors, adenoid
cystic carcinoma (ACC) and mucoepidermoid carcinoma (MEC) are the
most predominant (4), and their
incidence is gradually increasing (5). However, the pathophysiology of
salivary gland tumors is not well understood because of the variety
of histopathological phenotypes and the paucity of previous
molecular studies.
β-catenin, the abnormality of which is significantly
associated with the growth, invasion and metastasis of various
malignancies, very likely plays an important pathophysiological
role in salivary gland tumors (6–10).
Interestingly, a few immunohistochemical studies have revealed
aberrant expression of β-catenin in the cytoplasm and/or nuclei of
salivary gland tumor cells (7–10).
Although the biological significance of aberrant β-catenin
expression remains unclear, the alteration of β-catenin and its
target genes has been shown to trigger the proliferation of
salivary gland epithelial cells in mouse models (11–13).
Therefore, it is tempting to speculate that aberrant β-catenin
expression may be associated with cell proliferation in salivary
gland tumors.
The regenerating gene (REG) Iα protein, the human
homologue of rat Reg protein, which was originally isolated from
regenerating pancreatic islets (14), has been shown to play roles, not
only in normal tissue regeneration (15,16),
but also in the development of various malignancies (17–20).
We previously clarified that REG Iα protein is involved in the
regeneration of gastrointestinal epithelium (21) and also in the development of its
associated cancer by promoting cell growth and/or anti-apoptosis
(22–24). It was recently suggested that an
abnormality in β-catenin may be associated with the overexpression
of REG family proteins in liver tumors (25,26).
On the other hand, it is noteworthy that REG Iα protein plays a
role in the regeneration of minor salivary glands (27) suggesting possible involvement of
REG Iα protein in the development of salivary gland tumors. Thus,
in the present study, we investigated the expression of REG Iα and
β-catenin in salivary gland tumors, focusing on the possible
linkage of their expressional profiles and their relationship to
tumor proliferative ability.
Materials and methods
Patients, tissue samples and
histology
Nineteen patients with PAs (8 males, 11 females;
mean age 44.2 years, range 14–70 years) and 17 patients with
malignant salivary tumors [7 males, 10 females; mean age 56.8
years, range 23–85 years; 7 ACCs, 7 MECs, 3 polymorphous low-grade
adenocarcinomas (PLGAs)] who were diagnosed and treated at Dokkyo
University School of Medicine between 1994 and 2007 were enrolled.
Samples of salivary gland tissue were obtained by surgery from
patients with salivary gland tumors. Tissue specimens were fixed in
10% neutral buffered formalin and embedded in paraffin. Multiple
H&E-stained sections of each sample were examined
histologically. This study was conducted with the approval of the
Dokkyo University Surgical Pathology Committee, and informed
consent was obtained from all patients.
Immunohistochemistry
Immunohistochemical staining for REG Iα, β-catenin
and Ki67 was performed with a LSAB-2 kit (Dako, Marseille, France)
as described previously (17). In
brief, 4-μm sections were placed on slides, deparaffinized and
dehydrated. They were then placed in 0.01 mol/l citrate buffer (pH
6.0) and treated by microwave heating (400 W, 95°C; MI-77; Azumaya,
Tokyo, Japan) for 40 min to facilitate antigen retrieval. This was
followed by pretreatment with 0.3% H2O2 in
methanol for 20 min at room temperature to quench endogenous
peroxidase activity. The sections were incubated with 1% bovine
serum albumin in phosphate-buffered saline (PBS) for 30 min, and
then with anti-REG Iα (dilution 1:100), anti-β-catenin (BD
Transduction Laboratories, CA, USA; dilution 1:500) and anti-Ki67
(Dako Japan, Kyoto, Japan; dilution 1:50) for 1 h. Thereafter, the
sections were incubated with biotinylated secondary antibody for 15
min, washed with PBS and treated with peroxidase-conjugated
streptavidin for 20 min. Finally, the sections were incubated in
3,3′-diaminobenzidine tetrahydrochloride with 0.05%
H2O2 for 3 min and then counterstained with
Mayer's hematoxylin.
Evaluation of immunohistochemical
staining
The immunoreactivity of REG Iα was observed in the
cytoplasm of salivary gland tumor cells. At least 500 tumor cells
were observed in five different visual fields for each tissue
sample. A specimen was considered positive for REG Iα protein when
≥10% of the tumor cells were positively stained; otherwise, the
specimens were considered negative (28).
Similarly, a specimen was considered positive for
β-catenin when ≥10% of the tumor cells were positively stained;
otherwise, the specimens were considered negative (9). Regarding the expression pattern of
β-catenin, some salivary gland tumors showed clear β-catenin
immunoreactivity at the plasma membrane and a weak signal in the
cytoplasm (membrane type), while others showed immunoreactivity in
the cytoplasm diffusely and in the nuclei clearly but not at the
plasma membrane (non-membrane type).
Ki67 was used as a marker for measures of cell
proliferation (29). The
immunoreactivity of Ki67 in tumor nuclei was assessed as described
above. The Ki67 labeling index was calculated as the percentage of
positive cell nuclei in each sample.
Statistical analysis
Chi-square analyses were performed to investigate
the relationship between various clinicopathological parameters,
and the Fisher's exact test was also used, as necessary. Ki67
labeling index, age and tumor size were expressed as the mean ±
SEM, and the significance of differences between two groups was
assessed using the Mann-Whitney U-test. Differences at P<0.05
were considered to be significant.
Results
Clinicopathological features of salivary
gland tumors
The results of the clinicopathological analyses of
PAs and malignant salivary gland tumors are summarized in Tables I and II, respectively.
 | Table I.Relationship between
clinicopathological features and β-catenin expression in patients
with pleomorphic adenoma. |
Table I.
Relationship between
clinicopathological features and β-catenin expression in patients
with pleomorphic adenoma.
| Clinicopathological
features | No. of patients | β-catenin expression
| P-value |
|---|
| Negative (n=5) | Positive (n=14) |
|---|
| Gender | | | | NS |
| Male | 8 | 3 (37.5) | 5 (62.5) | |
| Female | 11 | 2 (18.2) | 9 (81.8) | |
| Age | 44.2±3.5 | 44.0±9.8 | 44.3±3.6 | NS |
| Tumor location | | | | NS |
| Palate | 9 | 2 (22.2) | 7 (77.8) | |
| Parotid | 3 | 1 (33.3) | 2 (66.7) | |
| Submandibular | 3 | 1 (33.3) | 2 (66.7) | |
| Oral mucosa | 3 | 1 (33.3) | 2 (66.7) | |
| Minor salivary
gland | 1 | 0 (0.0) | 1 (100.0) | |
| Stage | | | | NS |
| I | 7 | 2 (28.6) | 5 (71.4) | |
| II | 10 | 3 (30.0) | 7 (70.0) | |
| III | 2 | 0 (0.0) | 2 (100.0) | |
| Tumor size | 33.2±9.6 | 24.6±4.7 | 36.2±13.0 | NS |
 | Table II.Clinicopathological features and
expression of REG Iα and β-catenin in patients with malignant
salivary gland tumors. |
Table II.
Clinicopathological features and
expression of REG Iα and β-catenin in patients with malignant
salivary gland tumors.
| Case | Histology | Age | Gender | T stage | REG Iα
expression | β-catenin
expression |
|---|
| 1 | ACC | 65 | M | T4 | + | Non-membrane |
| 2 | ACC | 72 | F | T2 | + | Non-membrane |
| 3 | ACC | 70 | M | T1 | + | Non-membrane |
| 4 | ACC | 25 | M | T1 | + | Membrane |
| 5 | ACC | 23 | F | T2 | - | Non-membrane |
| 6 | ACC | 75 | M | T1 | - | Non-membrane |
| 7 | ACC | 67 | F | T2 | - | - |
| 8 | MEC | 59 | M | T2 | + | Non-membrane |
| 9 | MEC | 27 | F | T2 | + | Non-membrane |
| 10 | MEC | 47 | F | T2 | + | Non-membrane |
| 11 | MEC | 57 | F | T2 | + | Non-membrane |
| 12 | MEC | 68 | F | T2 | + | Non-membrane |
| 13 | MEC | 26 | F | T2 | - | Non-membrane |
| 14 | MEC | 85 | M | T1 | - | Membrane |
| 15 | PLGA | 72 | F | T2 | + | Non-membrane |
| 16 | PLGA | 65 | M | T2 | - | - |
| 17 | PLGA | 63 | F | T1 | - | - |
Nineteen PAs were obtained from 19 patients (8 males
and 11 females) with a mean age of 44.2 years. The lesions were
located in the palate (n=9; 47.3%), parotid (n=3; 15.8%),
submandibular (n=3; 15.8%), oral mucosa (n=3; 15.8%) and minor
salivary gland (n=1; 5.3%). The size of the tumors ranged from 9 to
200 mm, with a mean of 33.2 mm (Table
I).
Among the malignant salivary gland tumors, 7 ACCs, 7
MECs and 3 PLGAs were analyzed as presented in Table II.
Relationship between clinicopathological
features and β-catenin expression in salivary gland tumors
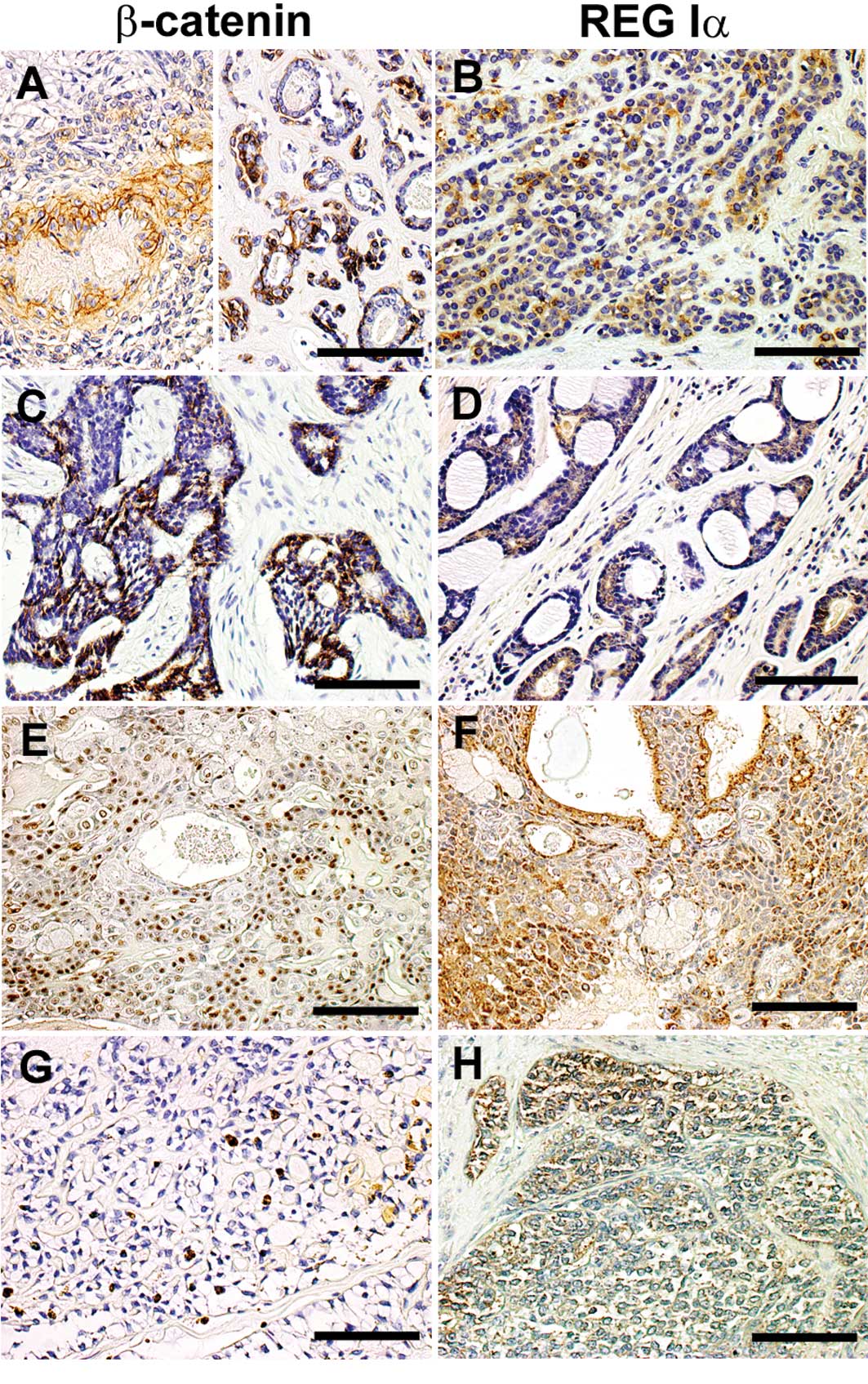
Representative immunostaining patterns of β-catenin
in salivary gland tumors are shown in Fig. 1. Among the PAs, 14 (73.3%) of the
19 lesions were positive for β-catenin expression. None of the
parameters examined, including gender, age, tumor location, stage
or tumor size, had a significant relationship to β-catenin
positivity. Among the malignant salivary gland tumors, 6 (85.7%) of
the 7 ACCs, all (100%) 7 MECs and 1 (33.3%) of the 3 PLGAs were
positive for β-catenin expression (Table II).
Regarding the expression pattern of β-catenin, 4
(28.6%) of the 14 β-catenin-positive PAs showed clear β-catenin
immunoreactivity at the plasma membrane (membrane type), while 10
(71.4%) showed diffuse immunoreactivity in the cytoplasm and
nucleus, but not at the plasma membrane (non-membrane type)
(Table III). PAs showing a
non-membrane-type β-catenin immunostaining pattern were
significantly early-stage and small in size compared to the PAs
showing membrane-type immunostaining (Table III). However, a non-membrane-type
β-catenin immunostaining pattern was frequently observed in 12
(85.7%) of the 14 malignant salivary gland tumors (Table II).
 | Table III.Relationship between
clinicopathological features and β-catenin expression pattern in
patients with pleomorphic adenoma. |
Table III.
Relationship between
clinicopathological features and β-catenin expression pattern in
patients with pleomorphic adenoma.
| Clinicopathological
features | β-catenin
expression pattern
| P-value |
|---|
| Non-membrane
(n=10) | Membrane (n=4) |
|---|
| Gender | | | NS |
| Male | 3 | 2 | |
| Female | 7 | 2 | |
| Age | 44.1±5.1 | 44.8±1.6 | NS |
| Tumor location | | | NS |
| Palate | 6 | 1 | |
| Parotid | 1 | 1 | |
|
Submandibular | 1 | 1 | |
| Oral mucosa | 1 | 1 | |
| Minor salivary
gland | 1 | 0 | |
| Stage | | | 0.030 |
| I | 5 | 0 | |
| II | 5 | 2 | |
| III | 0 | 2 | |
| Tumor size | 19.6±3.1 | 77.8±40.9 | 0.037 |
Relationship between clinicopathological
features and REG Iα expression in salivary gland tumors
REG Iα protein immunoreactivity was detected in the
cytoplasm of tumor cells. As shown in Fig. 2, REG Iα immunoreactivity was
detected in salivary gland tumors of various types and stages.
Six (31.6%) of the 19 PAs were positive for REG Iα
protein. Gender, age, tumor location, stage or tumor size did not
have any significant relationship to REG Iα protein expression in
PAs (Table IV).
 | Table IV.Relationship between
clinicopathological features and REG Iα expression in patients with
pleomorphic adenoma. |
Table IV.
Relationship between
clinicopathological features and REG Iα expression in patients with
pleomorphic adenoma.
| Clinicopathological
features | REG Iα expression
| P-value |
|---|
| Negative
(n=13) | Positive (n=6) |
|---|
| Gender | | | NS |
| Male | 5 (62.5) | 3 (37.5) | |
| Female | 8 (72.7) | 3 (27.3) | |
| Age | 45.5±4.3 | 41.3±6.6 | NS |
| Tumor location | | | NS |
| Palate | 5 (55.6) | 4 (44.4) | |
| Parotid | 2 (66.7) | 1 (33.3) | |
|
Submandibular | 3 (100.0) | 0 (0.0) | |
| Oral mucosa | 3 (100.0) | 0 (0.0) | |
| Minor salivary
gland | 0 (0.0) | 1 (100.0) | |
| Stage | | | NS |
| I | 4 (57.1) | 3 (42.9) | |
| II | 7 (70.0) | 3 (30.0) | |
| III | 2 (100.0) | 0 (0.0) | |
| Tumor size | 40.0±13.7 | 18.3±3.1 | NS |
Among the malignant salivary gland tumors, REG Iα
protein expression was positive in 4 (57.1%) of the 7 ACCs, 5
(71.4%) of the 7 MECs and 1 (33.3%) of the 3 PLGAs (Table II). REG Iα positivity tended to be
higher in malignant salivary gland tumors than in PAs (31.6 vs.
58.8%, P=0.099).
Relationship between Ki67 labeling index
and β-catenin or REG Iα expression in salivary gland tumors
We next examined the relationship between cell
proliferative ability and β-catenin or REG Iα expression in
salivary gland tumors. PAs that were positive for β-catenin showed
a higher Ki67 labeling index than those that were negative
(P=0.069, Fig. 2A). In addition,
PAs with non-membrane-type β-catenin expression showed a
significantly higher Ki67 labeling index than PAs with negative or
membrane-type expression (Fig.
2B). Furthermore, PAs that were positive for REG Iα showed a
significantly higher Ki67 labeling index than those that were
negative (Fig. 2C). Similar
findings were obtained in malignant salivary gland tumors, although
the data are preliminary due to the small number of samples
examined.
Correlation between β-catenin and REG Iα
expression in salivary gland tumors
REG Iα-positive PAs tended to be positive for
β-catenin (Table V; P=0.078), and
PAs with non-membrane-type β-catenin expression were significantly
positive for REG Iα (Table V).
 | Table V.Correlation between REG Iα and the
β-catenin expression pattern in patients with pleomorphic
adenoma. |
Table V.
Correlation between REG Iα and the
β-catenin expression pattern in patients with pleomorphic
adenoma.
| Clinicopathological
features | β-catenin-negative
(n=5) | β-catenin-positive
(n=14)
|
|---|
| Membrane | Non-membrane | Total |
|---|
| REG Iα
expression | | | | |
| Negative | 5 (38.5) | 4 | 4 | 8 (61.5) |
| Positive | 0 (0.0) | 0 | 6a | 6 (100.0) |
Discussion
Human salivary gland tumors are a histologically
heterogeneous group, and their progression is thought to be a
multi-step process that results in the acquisition of cell
proliferative ability (1,2). In the present study, we showed that
β-catenin expression was associated with the cell proliferative
ability of PA lesions. Additionally, we observed aberrant
expression of β-catenin in the cytoplasm and/or nuclei of cells in
various salivary gland tumors, compatible with previous reports
(7–10). Recently, a variety of tumor cells
has been reported to show aberrant expression and/or loss of
membranous β-catenin expression (30), although its biological significance
is not fully clear. Notably, in this study we found that PAs with
aberrant β-catenin expression had a higher proliferative potential
than those with membrane-type β-catenin expression, suggesting that
aberrant β-catenin expression may reflect the acquisition of tumor
growth ability. In this regard, it appears reasonable that the
frequency of aberrant β-catenin expression was higher in malignant
salivary gland tumors than in PAs. At present we are unable to
explain the precise intracellular mechanism responsible for the
translocation of β-catenin; however, accumulating evidence suggests
that translocated β-catenin acts as a transcriptional factor for
oncogenic or growth-associated genes in the cells of several cancer
types (31). Therefore, it is
tempting to speculate that aberrant β-catenin expression may be
associated with the overexpression of such genes in salivary gland
tumors as well.
Additionally, in the present study we showed that
REG Iα was overexpressed in a considerable number of PAs and other
malignant salivary gland tumors. Similarly, recent studies have
reported that REG Iα is overexpressed, not only in
gastroenterological cancers (17,18,23),
but also in lung cancer (19) and
seminoma (20), suggesting that
REG Iα protein plays a role in the pathogenesis of various
malignancies. In fact, we and others previously clarified that REG
Iα protein functions as a cell growth and/or anti-apoptotic factor
by activating the MAKP or Akt pathway in gastric or colon cancer
cells (23,24,32).
In this context, we examined the relationship between REG Iα
expression and cell proliferative ability in salivary gland tumors
and found that PAs expressing REG Iα showed a significantly higher
cell proliferation index than those that were negative, and
moreover that the rate of REG Iα positivity was apparently higher
in malignant salivary gland tumors. Thus, as in other tumors, REG
Iα protein may act as a growth factor in the pathogenesis of
salivary gland tumors.
We also investigated the relationships among
clinicopathological features and the expression of REG Iα and
β-catenin in PAs. We initially expected that PAs with
non-membrane-type β-catenin expression would be at a later stage
and larger in size, since such PAs showed higher proliferation
ability; however, the data we obtained suggested the contrary.
Similarly, REG Iα-positive PAs tended to be early-stage tumors that
were small in size. At present, we have no satisfactory explanation
for these findings since the number of tumor samples examined,
especially those at a late stage, was small. However, it is evident
that both β-catenin and REG Iα play roles from the early phase of
progression of PA lesions. On the other hand, it is noteworthy that
REG Iα-positive PAs showed a significantly higher incidence of
non-membrane-type β-catenin expression. Cavard et al
recently suggested that the REG Iα gene is a downstream
target of Wnt/β-catenin signaling in hepatocellular carcinoma cells
(25). This may support the
positive relationship between aberrant β-catenin expression and REG
Iα positivity in PA lesions.
In summary, we observed that immunoreactivity for
β-catenin was detectable, not only in the plasma membrane, but also
in the cytoplasm or nucleus of cells in various salivary gland
tumors. In addition, REG Iα protein was expressed in a considerable
number of PAs and other malignant salivary gland tumors.
Furthermore, we showed that both β-catenin and REG Iα are
significantly associated with the proliferative ability of PA
lesions and that aberrant β-catenin expression was related to REG
Iα positivity in such lesions. Taken together, our findings suggest
that β-catenin and its candidate target REG Iα may act as
growth-promoting factors in the development of salivary gland
tumors.
Acknowledgements
The authors thank Chiaki Matsuyama,
Ayako Shimizu, Takako Ono, Midor i Katayama, Atsu ko Kikuchi and
Sachiko Miyahara (Department of Surgical and Molecular Pathology,
Dokkyo University School of Medicine, Tochigi, Japan) for the
excellent technical and secretarial assistance. We are grateful to
Dr Hiroshi Okamoto from the Tohoku University Graduate School of
Medicine, Sendai, Japan, for providing the anti-REG Iα antibody.
This study was supported, in part, by Grants-in-aid for Scientific
Research 20590747 from the Ministry of Education, Culture, Sports,
Science and Technology of Japan.
References
|
1.
|
Eveson JW and Cawson RA: Salivary gland
tumours: a review of 2410 cases with particular reference to
histological types, site, age and sex distribution. J Pathol.
146:51–58. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Cheuk W and Chan JKC: Advances in salivary
gland pathology. Histopathology. 51:1–20. 2007. View Article : Google Scholar
|
|
3.
|
Altemani A, Martins MT, Fretias L, Soares
F, Araujo NS and Araujo VC: Carcinoma ex-pleomorphic adenoma
(CXPA): immunoprofile of the cells involved in carcinomatous
progression. Histopathology. 46:635–641. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Speight PM and Barrett AW: Salivary gland
tumours. Oral Dis. 8:229–240. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Carvalho AL, Nishimoto IN, Califano JA and
Kowalski LP: Trends in incidence and prognosis for head and neck
cancer in the United States: a site-specific analysis of the SEER
database. Int J Cancer. 114:806–816. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Shibuya Y, Ri S, Umeda M, Yoshikawa T,
Masago H and Komori T: Ultrastructural localization of E-cadherin
and α-/β-catenin in adenoid cystic carcinoma. Histopathology.
35:423–431. 1999.
|
|
7.
|
Prado RF, Consolaro A and Taveira LAA:
Expression of β-catenin in carcinoma in pleomorphic adenoma,
pleomorphic adenoma and normal salivary gland: immunohistochemical
study. Oral Med Pathol. 11:E247–E251. 2006.
|
|
8.
|
Furuse C, Cury PR, Altemani A, dos Santos
Pinto D Jr, de Araújo NS and de Araújo VC: β-catenin and E-cadherin
expression in salivary gland tumors. Int J Sur Pathol. 14:212–217.
2006.
|
|
9.
|
Genelhu MC, Gobbi H, Arantes DC, Cardoso
SV and Cassali D: Immunolocalization of β-catenin in pleomorphic
adenomas and carcinomas ex-pleomorphic adenomas of salivary glands.
Appl Immunohistochem Mol Morphol. 15:273–278. 2007.
|
|
10.
|
Ferrazzo KL, Neto MM, dos Santos E, dos
Santos Pinto D and de Sousa SO: Differential expression of
galectin-3, β-catenin and cyclin D1 in adenoid cystic carcinoma and
polymorphous low-grade adenocarcinoma of salivary glands. J Oral
Pathol Med. 38:701–707. 2009.
|
|
11.
|
Tsukamoto AS, Grosschedl R, Guzman RC,
Parslow T and Varmus HE: Expression of the int-1 gene in transgenic
mice is associated with mammary gland hyperplasia and
adenocarcinoma in male and female mice. Cell. 55:619–625. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Roose J, Huls G, van Beest M, et al:
Synergy between tumor suppressor APC and the beta-catenin-Tcf4
target Tcf1. Science. 285:1923–1926. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Stepanova L, Finegold M, DeMayo F, Schmidt
EV and Harper JW: The oncoprotein kinase chaperone CDC37 functions
as an oncogene in mice and collaborates with both c-myc and cyclin
D1 in transformation of multiple tissues. Mol Cell Biol.
20:4462–4473. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Terazono K, Yamamoto H, Takasawa S, et al:
A novel gene activated in regenerating islets. J Biol Chem.
263:2111–2114. 1988.PubMed/NCBI
|
|
15.
|
Watanabe T, Yonekura H, Terazono K,
Yamamoto H and Okamoto H: Complete nucleotide sequence of human reg
gene and its expression in normal and tumoral tissues. J Biol Chem.
265:7432–7439. 1990.PubMed/NCBI
|
|
16.
|
Asahara M, Mushiake S, Shimada S, et al:
Reg gene expression is increased in rat gastric
enterochromaffin-like cells following water immersion stress.
Gastroenterology. 111:45–55. 1996. View Article : Google Scholar
|
|
17.
|
Fukui H, Fujii S, Takeda J, et al:
Expression of Reg Iα in human gastric cancers. Digestion.
69:177–184. 2004.
|
|
18.
|
Astrosini C, Roeefzaad C, Dai YY,
Dieckgraefe BK, Jöns T and Kemmner W: REG1A expression is a
prognostic marker in colorectal cancer and associated with
peritoneal carcinomatosis. Int J Cancer. 123:409–413. 2008.
View Article : Google Scholar
|
|
19.
|
Minamiya Y, Kawai H, Saito H, et al: REG1A
expression is an independent factor predictive of poor prognosis in
patients with non-small cell lung cancer. Lung Cancer. 60:98–104.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Mauro V, Carette D, Chevallier D, et al:
Reg I protein in healthy and seminoma human testis. Histol
Histopathol. 23:1195–1203. 2008.PubMed/NCBI
|
|
21.
|
Fukui H, Franceschi F, Penland RL, et al:
Effects of Helicobacter pylori infection on the link between
regenerating gene expression and serum gastrin levels in Mongolian
gerbils. Lab Invest. 83:1777–1786. 2003.
|
|
22.
|
Fukui H, Kinoshita Y, Maekawa T, et al:
Regenerating gene protein may mediate gastric mucosal proliferation
induced by hypergastrinemia in rats. Gastroenterology.
115:1483–1493. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Sekikawa A, Fukui H, Fujii S, et al: REG
Iα protein may function as a trophic and/or anti-apoptotic factor
in the development of gastric cancer. Gastroenterology.
128:642–653. 2005.
|
|
24.
|
Sekikawa A, Fukui H, Fujii S, et al:
Possible role of REG Iα protein in ulcerative colitis and colitic
cancer. Gut. 54:1437–1444. 2005.
|
|
25.
|
Cavard C, Terris B and Grimber G: et al
Overexpression of regenerating islet-derived 1 alpha and 3 alpha
genes in human primary liver tumors with beta-catenin mutations.
Oncogene. 25:599–608. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Yuan RH, Jeng YM, Chen HL, et al: Opposite
roles of human pancreatitis-associated protein and REG1A expression
in hepatocellular carcinoma: association of pancreatitis-associated
protein expression with low-stage hepatocellular carcinoma,
beta-catenin mutation, and favorable prognosis. Clin Cancer Res.
11:2568–2575. 2005. View Article : Google Scholar
|
|
27.
|
Kimura T, Fukui H, Sekikawa A, et al:
Involvement of REG Iα protein in the regeneration of ductal
epithelial cells in the minor salivary glands of patients with
Sjögren's syndrome. Clin Exp Immunol. 155:16–20. 2009.
|
|
28.
|
Sasahira T, Oue N, Kirita T, et al: Reg IV
expression is associated with cell growth and prognosis of denoid
cystic carcinoma in the salivary gland. Histopathology. 53:667–675.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Gerdes J, Li L, Schlueter C, et al:
Immunohistochemical and molecular biologic characterization of the
cell proliferation-associated nuclear antigen that is defined by
monoclonal antibody Ki-67. Am J Pathol. 138:867–873.
1991.PubMed/NCBI
|
|
30.
|
Van Aken E, De Wever O and Correia da
Rocha AS: Defective E-cadherin/catenin complexes in human cancer.
Virchows Arch. 439:725–751. 2001.PubMed/NCBI
|
|
31.
|
Mosimann C, Hausmann G and Basler K:
β-catenin hits chromatin: regulation of Wnt target gene activation.
Nat Rev Mol Cell Biol. 10:276–286. 2009.
|
|
32.
|
Kadowaki Y, Ishihara S, Miyaoka Y, et al:
Reg protein is overexpressed in gastric cancer cells, where it
activates a signal transduction pathway that converges on ERK1/2 to
stimulate growth. FEBS Lett. 530:59–64. 2002. View Article : Google Scholar
|