Introduction
Lung cancer is the most common malignancy in the
world (1,2), and no more than 14.9% of patients
survive after treatment for lung cancer. These data show that a new
therapeutic approach for lung cancer is crucial. Recently,
investigations aimed at developing anticancer agents have begun to
shift away from general cytotoxic drugs to those that target
specific molecules or processes selectively involved in tumor cell
survival (3,4). However, the relevance of subcutaneous
(s.c.) tumor growth in animal models to that in human studies must
be carefully ascertained, since experimental preclinical results
frequently cannot be translated to the clinic. The limitation of
animal models is, in part, related to tumor transplantation. The
use of ectopic tumors does not adequately take into account the
interaction between the specific organ microenvironment and tumor
cells, and may therefore alter the tumor response to therapy
(4–7). Previously, we established an
orthotopic transplantation model which simulated the clinical and
pathological features of human lung cancer (8). In this study, we further analyzed the
growth style and the proliferation profile of human lung cancer in
an orthotopic transplantation model, comparing it with an s.c.
transplantation model.
Materials and methods
Cell culture and preparation for
delivery
The human squamous lung cancer cell line SQ5 was
donated by Dr Kubota from the Ibaraki Prefectural University of
Health Sciences (9). The human
lung adenocarcinoma cell line A549 was obtained from the American
Type Culture Collection cell line repository. Both cell lines were
maintained in αMEM medium (Sigma, St. Louis, MO) with L-glutamine
supplemented with 10% heat-inactivated fetal bovine serum
(Equitech-Bio Inc., Kerrville, TX), 50 U/ml penicillin and 50 μg/ml
streptomycin. Cells were maintained in exponential growth in
humidified incubators at 37°C in 5% CO2. Adherent tumor
cells were harvested from subconfluent cultures by a brief exposure
to 0.25% trypsin/0.02% ethylenediaminetetraacetic acid (EDTA).
Trypsinization was stopped with medium containing 10% serum, and
the cells were washed once and resuspended in serum-free medium.
Trypan blue staining was used to assess cell viability, and only
single-cell suspensions of >95% viability were used for
injections. EDTA (0.01 M) (Sigma) was supplemented in the tumor
cell suspensions before intratracheal delivery (8).
Experimental animals
Pathogen-free female BALB/c nu/nu mice (Charles
River Laboratories, Tokyo, Japan) were transplanted at the age of
7–9 weeks either intratracheally or subcutaneously with the SQ5 or
A549 cell line. Mice were maintained in specifically pathogen-free
conditions, fed autoclaved food and water, and handled under
stringent sterile conditions. Mice were acclimated for 1 week
before the start of the study. All animal experiments were
performed in accordance with the Guidelines of Animal Experiments
of Yokohama City University, and the protocols were approved by the
Animal Care Committee of the Yokohama City University.
Orthotopic and subcutaneous
transplantation of tumor cells
The mice were anesthetized with isoflurane (Merck
Hoei Ltd., Osaka, Japan), placed in a supine position and gently
immobilized with tape. A 1-cm long ventral incision was made
through the skin of the neck to expose the trachea. A 27 3/4-gauge
needle (Terumo, Tokyo, Japan), bent to approximately a 135° angle,
was used to inject the tumor cell suspensions (1×107
cells) directly into the main bronchi. The incision was closed with
a single surgical clip, and the mice were allowed recovery time
under a warm lamp until fully awake (8,10).
For subcutaneous transplantation, unanesthetized mice were injected
with a 100-μl tumor suspension (1×107 cells) directly
into the flank. The mice were observed daily after tumor cell
injection and monitored for signs of wound healing disturbance,
evidence of tumor development and decreased physical activity. The
weights of the mice were determined twice a week. Necropsies were
performed on all animals.
To determine the proliferation of tumor cells, the
mice were pulse-labeled with 60 mg/kg of body weight
bromodeoxyuridine (BrdUrd) (Sigma) in phosphate-buffered saline
(PBS) as an intraperitoneal injection 20 min before sacrifice.
After death, the lungs were exposed and inflated with
neutral-buffered formalin. Excised lungs and s.c. tumors were
collected in 10% neutral-buffered formalin for
immunohistochemistry.
Histology and immunofluorescence staining
for microscopic evaluation of BrdUrd incorporation in tumor
cells
In brief, after fixation in 10% neutral-buffered
formalin, 4-μm tissue sections were cut and mounted on
silane-coated slides. The sections were stained with H&E and
analyzed by microscopy (Olympus CKX41, Tokyo, Japan).
For immunofluorescence staining, the sections were
deparaffinized in xylene and rehydrated through decreasing ethanol
concentrations to water. The sections were then washed twice for 5
min in PBS. The sections were treated with 2 N HCl for 50 min and
neutralized with 0.1 M sodium borate for 2 min. The sections were
rinsed twice for 5 min in PBS and then incubated in blocking normal
horse serum for 30 min at 37°C to decrease nonspecific antibody
binding. Sections were incubated for 1 h at 37°C with the antibody
IU-4 (1:100 vol/vol in PBS plus 0.5% Triton X-20; PBST) (Sigma).
The sections were again washed twice for 5 min in PBS and then
incubated for 1 h at 37°C with a FITC-conjugated goat anti-mouse
secondary antibody (GAMFITC) (Sigma) together with normal goat
serum (1:100 vol/vol in PBST). The slides were again washed twice
for 5 min in PBS, the nuclei counterstained with 1 μg/ml propidium
iodide (PI) in PBS, and mounted using Vectashield anti-fade
mounting medium (Vector Laboratories, Burlingame, CA) (10). AxioCam MRm microscopy (Zeiss,
Tokyo, Japan) was used to detect BrdUrdand PI-labeled nuclei.
Sections were observed under ×200 magnification. Both BrdUrd- and
PI-labeled tumor cells were counted as proliferating cells
(10). The proliferation index was
calculated as proliferating cells/105 tumor area using
NIH ImageJ software. At least 3 mice per group and 3 slides per
mouse sample were stained for counting. Proliferating cells were
counted in the area of tumors without necrosis.
Statistical analysis
Data were presented as the mean (SD), and the
statistical significance of differences in mean values was assessed
by the Student’s t-test. The differences in the proliferation index
in orthotopic tumors with/without necrosis and s.c. tumors were
considered significant at values of P<0.05.
Results
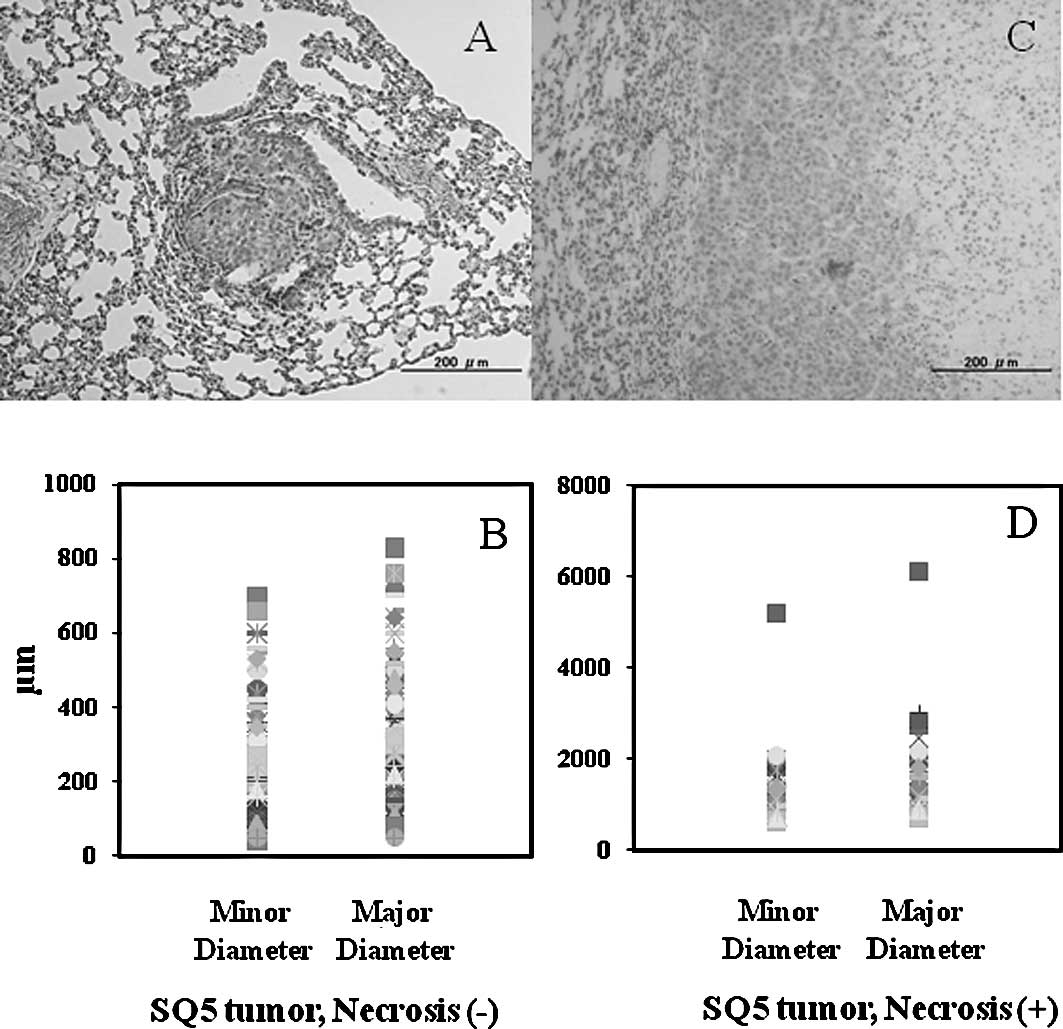
Histology of SQ5 orthotopic tumor
colonies
A total of 20 mice with orthotopically transplanted
SQ5 tumors were collected. A total of 136 tumor colonies were
analyzed. Histological examination showed that the diameter of the
tumor colonies was 40–6,100 μm. Tumor colonies whose minor diameter
was 40–700 μm and major diameter was 80–830 μm showed no definite
necrosis. The average of the minor and major diameters of the SQ5
tumors without necrosis was 283 and 297 μm, respectively (Fig. 1A and B). SQ5 tumor colonies whose
minor diameter was 540–5,200 μm and major diameter was 600–6,100 μm
showed definite necrosis. The average of the minor and major
diameters of the SQ5 tumors with necrosis was 1,182 and 1,507 μm,
respectively (Fig. 1C and D).
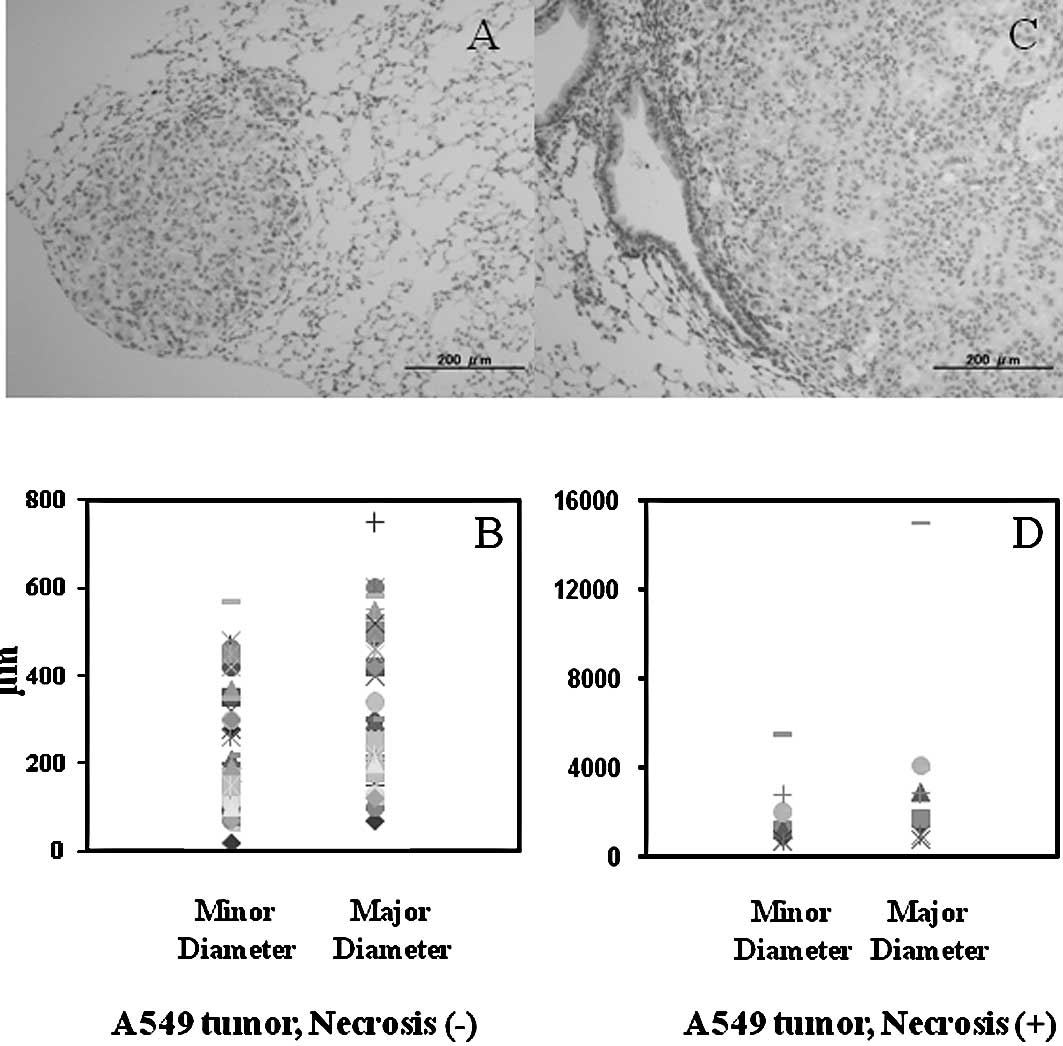
Histology of A549 orthotopic tumor
colonies
A total of 24 mice with orthotopically transplanted
A549 tumors were collected. A total of 59 tumor colonies was
analyzed. Histological examination showed that the diameter of the
tumor colonies was 20–15,000 μm. Tumor colonies whose minor
diameter was 20–570 μm and major diameter was 70–750 μm showed no
definite necrosis. The average of the minor and major diameters of
the A549 tumors without necrosis was 220 and 304 μm, respectively
(Fig. 2A and B). Tumor colonies
whose minor diameter was 680–5,500 μm and major diameter was
800–15,000 μm showed definite necrosis. The average of the minor
and major diameter of the A549 tumors with necrosis was 1,890 and
3,749 μm, respectively (Fig. 2C and
D).
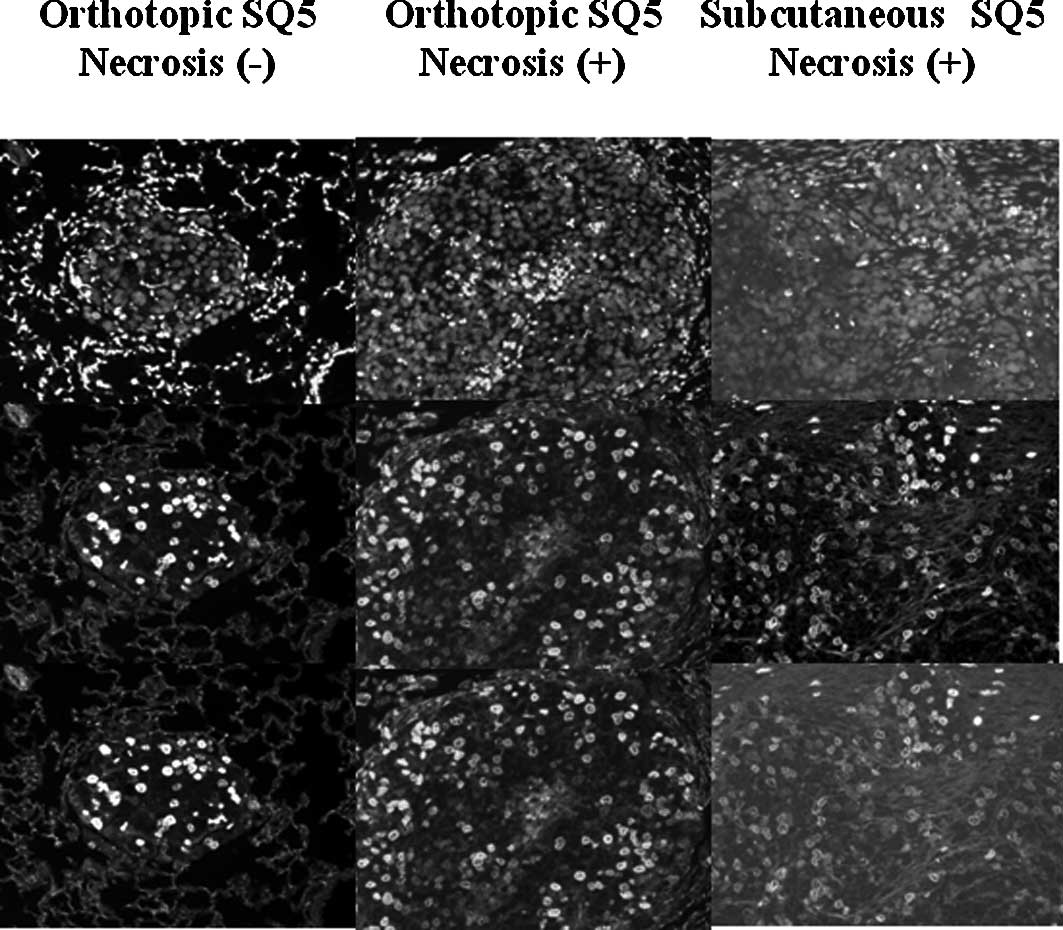
Proliferation of SQ5 tumor cells in the
orthotopic and s.c. transplantation models
Fig. 3 shows the
BrdUrd- and PI-labeled tumor cells in the orthotopic SQ5 tumors
with/without necrosis and the s.c. tumors. In the orthotopic SQ5
tumors without necrosis, proliferating tumor cells were distributed
in all areas of the tumor colonies (Fig. 3, left column). The proliferation
index was 10.63 (3.10) (Table I).
In the orthotopic SQ5 tumors where necrosis was found in the
center, proliferating tumor cells were mainly distributed in the
peripheral of the tumor colonies (Fig.
3, middle column). The proliferating tumor cells were counted
in the part of the tumor area without necrosis. The proliferation
index of the orthotopic SQ5 tumors with necrosis was 7.38 (3.03)
(Table I). In the s.c.
transplantation model, the SQ5 tumors grew as a large block which
showed several necrotic areas in the tumors (Fig. 3, right column). Proliferating tumor
cells were counted in the area of the tumors without necrosis. The
proliferation index of the s.c. SQ5 tumors was 6.99 (2.10)
(Table I).
 | Table I.Summary of the proliferation indices
in SQ5 and A549 tumors. |
Table I.
Summary of the proliferation indices
in SQ5 and A549 tumors.
| Tumor model | SQ5
| A549
|
|---|
| Mean | SD | Mean | SD |
|---|
| Orthotopic, necrosis
(−) | 10.63a | 3.10 | 3.53 | 1.70 |
| Orthotopic, necrosis
(+) | 7.38 | 3.03 | 2.70a | 0.88 |
| Subcutaneous,
necrosis (+) | 6.99 | 2.10 | 3.91 | 0.63 |
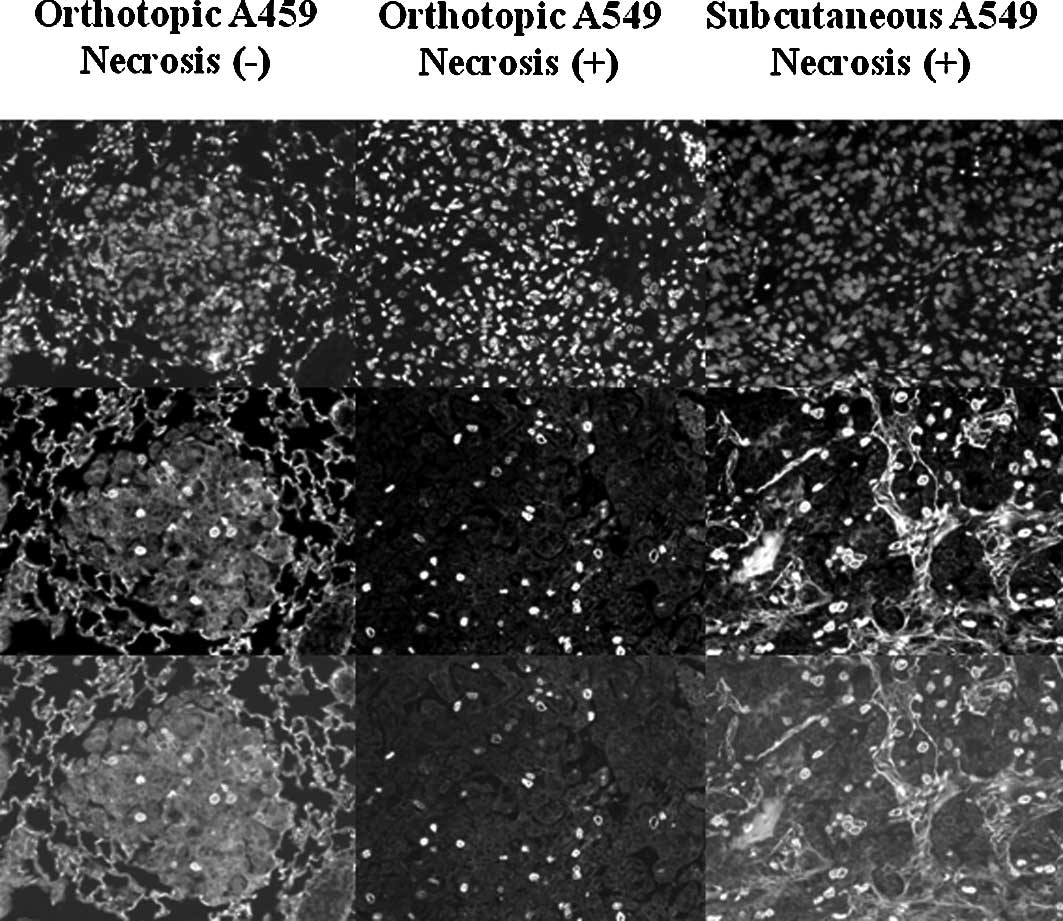
Proliferation of A549 tumor cells in the
orthotopic and s.c. transplantation models
Fig. 4 shows the
BrdUrd- and PI-labeled tumor cells in the orthotopic A549 tumors
with/without necrosis and the s.c. tumors. In the orthotopic A549
tumors without necrosis, proliferating tumor cells were distributed
in all areas of the tumor colonies (Fig. 4, left column). The proliferation
index of the orthotopic A549 tumors without necrosis was 3.53
(1.70) (Table I). In the
orthotopic A549 tumors with necrosis, proliferating tumor cells
were counted in the region of the tumor area without necrosis
(Fig. 4, middle column). The
proliferation index of the orthotopic A549 tumors with necrosis was
2.70 (0.88) (Table I). In the s.c.
transplantation model, proliferating tumor cells were counted in
the part of the tumor area without necrosis (Fig. 4, right column). The proliferation
index of the A549 s.c. tumors was 3.91 (0.63) (Table I).
Discussion
We previously established an orthotopic
transplantation model of human lung cancer. Tumor growth and
distribution in this orthotopic transplantation model simulated the
clinical features of human lung cancer (8). The aim of this study was to determine
the influence of the organ microenvironment on the proliferation
profiles of human lung cancer cells in orthotopic or s.c. tumor
transplantation models.
In the orthotopic transplantation model, tumor
colonies of various sizes were found in both the SQ5- and A549-cell
transplanted mice. Tumor necrosis was not definitely found in the
orthotopic SQ5 tumor colonies when the minor diameter of the tumor
colonies was less than 700 μm and the major diameter of the tumor
colonies was less than 830 μm (Fig.
1). Similar results were also found in the orthotopic A549
tumor colonies (Fig. 2). These
results agree with the fact that tumors less than 1 mm in diameter
are considered to have an adequate vascular supply (11,12).
It has been reported that the organ microenvironment influences
tumor growth. The microenvironmental factors impacting tumor cell
growth include interstitial fluid pressure in the tumor tissue, pH,
partial pressure of oxygen, focal concentration of cytokines,
extracellular matrix and microvessel density (13,14).
Without exception, lung cancer cells growing in lung tissue depend
on the structure of the host tissue (15). Our present and previous studies
found that orthotopic SQ5 tumors expanded locally and invaded the
adjacent normal lung fields (Figs.
1 and 3) (8). Orthotopic A549 tumors mainly replaced
the normal alveolar epithelial cells and were firmly attached to
the alveolar interstitium (Figs. 2
and 4) (8). These growth profiles are similar to
those found in human squamous and adenocarcinoma lung tumor growth,
respectively (15). This
orthotopic transplantation model was considered to provide the
proper organ microenvironment for lung cancer growth.
It is well known that controlling the proliferation
of tumors is the key to treating human lung cancer. In this study,
we analyzed the proliferation style of squamous and adenocarcinoma
lung tumors growing in orthotopic and s.c. transplantation models.
The proliferation index of the orthotopic squamous SQ5 tumors
with/without necrosis and the s.c. tumors was significantly higher
than that of the orthotopic adenocarcinoma A549 tumors with/without
necrosis and the s.c. tumors, respectively (P<0.01) (Table I). Our previous study showed that
in an in vitro culture, the doubling time of these two cell
lines was almost identical. Tumor cells collected from transplanted
tumors did not change the doubling time (8). However, these two cell lines showed a
different proliferation style in the transplanted tumors. The
different proliferation index of SQ5 and A549 tumors was
considered, in part, due to the cell numbers which entered the cell
cycle. This finding was supported by Terry et al who
reported that the proliferation index, but not the cell cycle, was
altered by stimulation in vivo (10). The proliferation index may reflect
the balance of proliferation and dormancy of tumor cells growing in
a proper organ microenvironment.
The proliferation style of the orthotopically
transplanted tumors was further analyzed and compared with that in
the s.c. transplanted tumors. The proliferation index of the
orthotopic SQ5 tumors with or without necrosis had no significant
difference. The same result was also found in the A549 orthotopic
transplanted tumors (Table I).
However, the proliferation index of the orthotopic SQ5 tumors
without necrosis was significantly higher than that of the s.c.
transplanted tumors, while the proliferation index of the
orthotopic A549 tumors with necrosis was significantly lower than
that of the s.c. transplanted tumors (Table I). These data suggest that tumor
proliferation depends on both tumor cell character and host organ
microenvironment; blood supply is only one of the factors
influencing tumor proliferation. Sun et al reported that the
microenvironment influences the interstitial fluid pressure in
tumor tissue, which may change several proliferation factors
(17). Gene expression in tumors
is also markedly altered when they are subcutaneously implanted as
compared to orthotopically implanted ones (7). In this orthotopic transplantation
model, lung tumor cells grew in lung parentima, which has a
particular microenvironment such as blood and oxygen supply and air
space in the organ. The balance of proliferation and dormancy in
orthotopic transplanted tumors may depend on how the tumor cells
respond to the organ microenvironment (5–7,15–17).
The proliferation style in this orthotopic transplantation model
may support the findings of Onn et al who found that
paclitaxel had a different effect on human lung cancer cells
growing orthotopically compared to those growing subcutaneously
(3,18).
In conclusion, lung tumor colonies of various sizes
and growth stages were obtained in this orthotopic transplantation
model. The growth and proliferation of orthotopic lung tumor
colonies were found to depend on both tumor cell character and
organ microenvironment. This orthotopic transplantation model may
provide a proper organ microenvironment for lung tumor growth and
may be a suitable tool for the biological research of human lung
cancer.
Acknowledgements
We thank Mr Stutomu Kitamura for his
assistance in maintaining the research animals. This research was
supported, in part, by a Grant-in-aid for scientific research (C)
(20590931) from the Japan Society for the Promotion of Science
(JSPS) and Encouragement Research Aid from the Yokohama Foundation
for Advancement of Medical Science (2006).
References
|
1.
|
American Cancer Society: Cancer facts and
figures 2002. American Cancer Society; Atlanta, GA: 2002
|
|
2.
|
Vital statistics of Japan, Statistics and
Information Dept., Minister’s Secretarial, Ministry of Health,
Labor and Welfare, 2001.
|
|
3.
|
Onn A, Isobe T, Itasaka S, Wu W, O’Reilly
MS, Ki Hong W, Fidler IJ and Herbst RS: Development of an
orthotopic model to study the biology and therapy of primary human
lung cancer in nude mice. Clin Cancer Res. 9:5532–5539.
2003.PubMed/NCBI
|
|
4.
|
Kuo TH, Kubota T, Watanabe M, Furukawa T,
Kase S, Tanino H, Saikawa Y, Ishibiki K, Kitajima M and Hoffman RM:
Site-specific chemosensitivity of human small-cell lung carcinoma
growing orthotopically compared to subcutaneously in SCID mice: the
importance of orthotopic models to obtain relevant drug evaluation
data. Anticancer Res. 13:627–630. 1993.
|
|
5.
|
Wilmanns C, Fan D, O’Brian CA, Bucana CD
and Fidler IJ: Orthotopic and ectopic organ environments
differentially influence the sensitivity of murine colon carcinoma
cells to doxorubicin and 5-fluorouracil. Int J Cancer. 52:98–104.
1992. View Article : Google Scholar
|
|
6.
|
Fidler IJ, Wilmanns C, Staroselsky A,
Radinsky R, Dong Z and Fan D: Modulation of tumor cell response to
chemotherapy by the organ environment. Cancer Metastasis Rev.
13:209–222. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Camphausen K, Purow B, Sproull M, Scott T,
Ozawa T, Deen DF and Tofilon PJ: Influence of in vivo growth on
human glioma cell line gene expression: convergent profiles under
orthotopic conditions. Proc Natl Acad Sci USA. 102:8287–8292. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kang Y, Omura M, Suzuki A, Oka T, Nakagami
Y, Cheng C, Nagashima Y and Inoue T: Development of an orthotopic
transplantation model in nude mice that simulates the clinical
features of human lung cancer. Cancer Sci. 97:996–1001. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Omura M, Torigoe S, Kurihara H, Matsubara
S and Kubota N: Comparison between fractionated high dose rate
irradiation and continuous low dose rate irradiation in spheroids.
Acta Oncol. 37:681–686. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Terry NH, Brinkley J, Doig AJ, Ma J, Patel
N, White RA, Mahajan N and Kang Y: Cellular kinetics of murine
lung: model system to determine basis for radioprotection with
keratinocyte growth factor. Int J Radiat Oncol Biol Phys.
58:435–444. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Knighton D, Ausprunk D, Tapper D and
Folkman J: Avascular and vascular phases of tumour growth in the
chick embryo. Br J Cancer. 35:347–356. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Lien WM and Ackerman NB: The blood supply
of experimental liver metastases. II. A microcirculatory study of
the normal and tumor vessels of the liver with the use of perfused
silicone rubber. Surgery. 68:334–340. 1970.PubMed/NCBI
|
|
13.
|
Jain RK: Delivery of molecular medicine to
solid tumors: lessons from in vivo imaging of gene expression and
function. J Control Release. 74:7–25. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Leo C, Giaccia AJ and Denko NC: The
hypoxic tumor microenvironment and gene expression. Semin Radiat
Oncol. 14:207–214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Morishita C, Jin E, Kikuchi M, Egawa S,
Fujiwara M, Ohaki Y, Ghazizadeh M, Takemura T and Kawanami O:
Angiogenic switching in the alveolar capillaries in primary lung
adenocarcinoma and squamous cell carcinoma. J Nippon Med Sch.
74:344–354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Hwang RF, Moore T, Arumugam T,
Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB and Logsdon CD:
Cancer-associated stromal fibroblasts promote pancreatic tumor
progression. Cancer Res. 68:918–926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Sun B, Zhang S, Zhang D, Gu Y, Zhang W and
Zhao X: The influence of different microenvironments on melanoma
invasiveness and microcirculation patterns: an animal experiment
study in the mouse model. J Cancer Res Clin Oncol. 133:979–985.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Onn A, Isobe T, Wu W, Itasaka S, Shintani
T, Shibuya K, Kenji Y, O’reilly MS, Fidler IJ and Herbst RS:
Epidermal growth factor receptor tyrosine kinase inhibitor does not
improve paclitaxel effect in an orthotopic mouse model of lung
cancer. Clin Cancer Res. 10:8613–8619. 2004. View Article : Google Scholar : PubMed/NCBI
|