Introduction
Hepatocellular carcinoma (HCC) is one of the most
common solid neoplasms worldwide, and the prognosis of patients is
often poor (1). Although
hepatectomy or transplantation provides better results for local
control of HCC than other therapies, survival has not been
satisfactory, particularly for large tumors, since both
intrahepatic and extrahepatic recurrences often occur after
hepatectomy even in patients who undergo a curative resection.
Hematologic spread or the presence of micrometastases is thought to
be the major cause of early recurrence after liver resection.
Several investigators have tried to detect the presence of
circulating cancer cells hematologically (2–4). Our
group previously described the use of quantitative reverse
transcriptase-polymerase chain reaction (qRT-PCR) to detect
α-fetoprotein (AFP) mRNA as a marker of circulating cancer cells
during liver resection (5–8).
Transcatheter arterial chemoembolization (TACE) has
been used widely in patients with multiple HCCs. This procedure
involves injection of ionized oil and chemotherapeutic agents into
the tumor-feeding artery followed by particulate embolization.
Several investigators, including some clinical trials, have used
this technique as neoadjuvant therapy for resectable HCC, with the
hope of minimizing post-operative recurrence and prolonging
survival after hepatectomy (9–29).
The efficacy of TACE, however, is still controversial, with some
investigators suggesting that pre-operative TACE may be useful in
several selected groups (9–19).
For these reasons, we hypothesized that pre-operative TACE may
regulate the spread of cancer cells during liver resection.
In this retrospective study, we evaluated the
effects of TACE applied before hepatectomy for resectable HCC on
survival and analyzed a subgroup of patients who underwent
pre-operative TACE. In addition, we used qRT-PCR to measure the
amount of AFP mRNA after TACE and analyzed the relationship with
survival after liver resection in order to investigate the efficacy
of TACE in the control of circulating cancer cells in the
peripheral blood.
Patients and methods
Patients
Between October 1980 and December 2006, 713 patients
underwent hepatectomy for pathologically confirmed HCC at our
institution. Among these patients, 495 underwent curative resection
and were eligible for evaluation in this study. Patients who had
the following factors were excluded: re-operative cases, patients
with extrahepatic metastasis or lymph node metastasis, mixed HCC,
pre-operative neoadjuvant chemotherapy other than TACE and
non-curative resection. The surgical procedure was selected
according to the status of liver function and cancer spread. All
patients received follow-up with abdominal computed tomography (CT)
and serum AFP measurements every 3 months in the first 2 years and
every 6 months thereafter. The treatment for recurrence of HCC was
determined by the recurrence pattern and the localization, and the
liver function and general condition. The median follow-up period
was 50 months (range 0–249 months).
After discussing the mode and advantage or
disadvantage of this treatment with the patients and their
relatives, they were given the choice to receive or not to receive
pre-operative TACE. Among the 495 patients, 252 (50.9%) underwent
TACE before operation (pre-operative TACE group), while 243 cases
(49.1%) underwent liver resection without pre-operative TACE
(non-TACE group). There was a significant difference between the
TACE and non-TACE group in terms of pre-operative intrahepatic
metastasis [74 (29.4%) and 46 (18.9%) patients, respectively,
p<0.01], but no significant difference was noted among other
baseline characteristics including age, gender, viral infection
background, Child-Pugh grade, positive ratio of tumor markers for
HCC, tumor size and number, portal vein invasion and TNM stage
(Table I).
 | Table I.Clinicopathological characteristics of
the pre-operative TACE and non-TACE groups. |
Table I.
Clinicopathological characteristics of
the pre-operative TACE and non-TACE groups.
| Variable | Pre-operative TACE
(n=252) | Non-TACE (n=243) | P-value |
|---|
| Age (years) | 60.9±8.4 | 62.2±8.8 | 0.107 |
| Gender
(male/female) | 208/44 | 191/52 | 0.258 |
| HBs-Ag (+) | 108 | 96 | 0.404 |
| HCV-Ab (+) | 105 | 134 | 0.200 |
| Child-Pugh grade
(A/B) | 217/35 | 207/36 | 0.404 |
| CLIP score
(0/1-2/3-6) | 118/113/21 | 125/106/12 | 0.201 |
| Serum AFP (>5
ng/ml) | 78 | 72 | 0.845 |
| Serum PIVKA-II
(>40 mAU/l) | 101 | 112 | 0.464 |
| Tumor size (cm) | 4.4±3.3 | 4.0±6.7 | 0.455 |
| Multiple tumors | 86 | 65 | 0.084 |
| Portal vein invasion
(+) | 17 | 16 | 0.951 |
| Intrahepatic
metastasis (+) | 74 | 46 | <0.010 |
| TNM stage
(I/II/III/IV-A) | 47/135/55/15 | 53/130/41/19 | 0.465 |
The retrospective study protocol was approved by the
Human Ethics Review Committee of Osaka University, and a signed
consent was obtained from each patient.
TACE method
Using the Seldinger’s Technique (30), a catheter was inserted selectively
into the right or left hepatic artery or the tumor-feeding artery
if identified. The chemotherapeutic regimens were based mainly on
epirubicin (Farmorubicin®) doxorubicin hydrochloride
(Adriacin®) or mitomycin C (Mitomycin®) (all
from Kyowa Hakko, Tokyo) according to the volume of the tumor and
liver function. Most patients also underwent embolization with
iodized oil (Lipiodol®) (Guerbet, Tokyo) and
gelatin-sponge particles. The mean and median intervals between
pre-operative TACE and hepatectomy were 2.3 and 1.7 months,
respectively (range 0.2–11.3 months). After TACE, there were no
serious complications requiring operative, endoscopic or radiologic
intervention under general anesthesia (>grade IIIb in the
Classification of Surgical Complications) (31).
Detection of AFP mRNA
From 1999, peripheral blood samples (16 ml) were
obtained pre-operatively from each HCC patient. Quantitative RT-PCR
was used for the detection of AFP mRNA in peripheral blood as
described previously (6). The
level of AFP mRNA in the blood was expressed relative to that of
the mRNA of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The
lower limit of detection of the AFP mRNA by qRT-PCR was
1.0×10−8; values above this level were designated as
positive as described previously (5,6).
Statistical methods
All data are presented as the mean ± standard
deviation. Differences in clinicopathologic parameters between the
groups were compared by the Student’s t-test for continuous
variables or the Chi-square test for others. Overall and
disease-free survivals were calculated with the Kaplan-Meier
method, and differences in survival between groups were compared
using the log-rank test. A value p<0.05 was considered
statistically significant. The statistical software used was
StatView J-5.0 software (SAS, Cary, NC).
Results
Effect of pre-operative TACE on survival
after hepatectomy
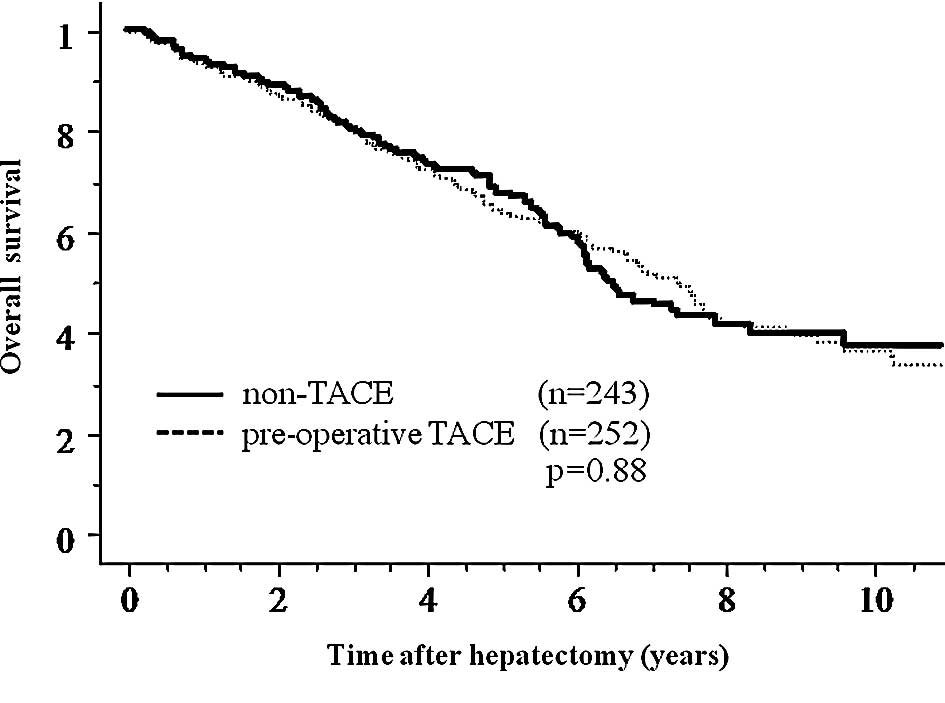
No significant differences were observed between the
pre-operative TACE group and the non-TACE group regarding overall
survival and disease-free survival after hepatectomy (Fig. 1). In order to elucidate the
clinical effects of pre-operative TACE, subgroup analysis was
performed by dividing patients according to several
clinicopathologic factors including age, gender, background of
viral infection, Child-Pugh grade, tumor markers for HCC, tumor
size and number, portal vein invasion, intrahepatic metastasis and
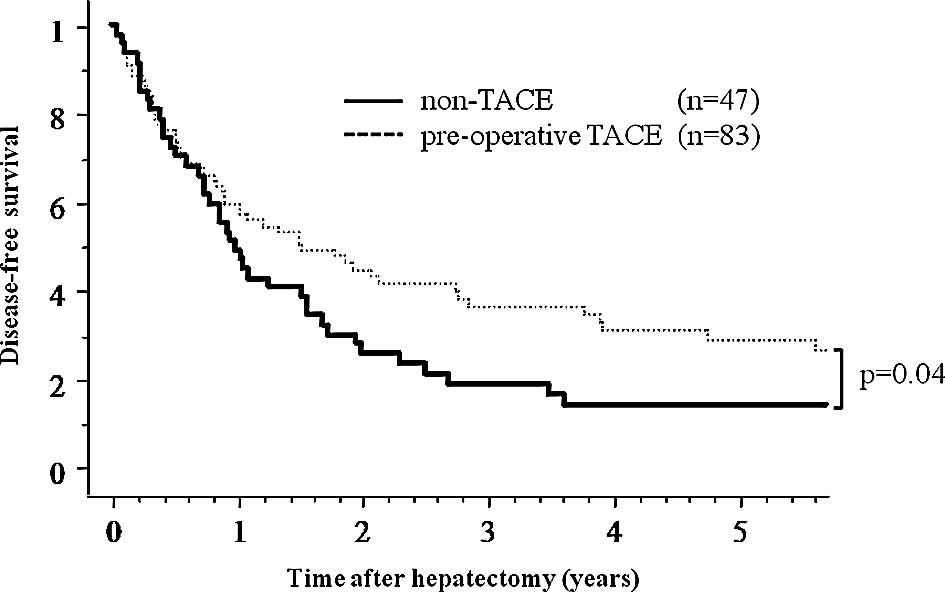
TNM stage (Table II). In the
subgroup analyses, only the pre-operative TACE group with tumor
size ≥5 cm in diameter showed a significant benefit as reflected by
an increase in disease-free survival after hepatectomy compared to
the non-TACE group (p=0.04) (Fig.
2), but no benefit in overall survival was noted (p=0.70).
There was no difference in the clinicopathologic background between
patients of the two groups with tumors ≥5 cm in diameter (Table III). Additionally, to evaluate the
influence of tumor size, we divided patients into groups according
to 1 cm differences in tumor diameter and analysed each group
regarding survival; there were no significant benefits between
groups except for neoplasms ≥5 cm. Analysis of sites of recurrence
showed that 134 and 36 patients experienced intrahepatic and
extrahepatic recurrence in the pre-operative TACE group, and 153
and 25 patients in the non-TACE group (p=0.09).
 | Table II.Subclass analysis of overall and
disease-free survival. |
Table II.
Subclass analysis of overall and
disease-free survival.
| Variable | Overall
survival | Disease-free
survival |
|---|
| Age |
| <60 | 0.805 | 0.559 |
| ≥60 | 0.973 | 0.631 |
| Gender |
| Male | 0.579 | 0.225 |
| Female | 0.367 | 0.976 |
| HBs-Ag | | |
| (−) | 0.722 | 0.884 |
| (+) | 0.899 | 0.176 |
| HCV-Ab |
| (−) | 0.691 | 0.903 |
| (+) | 0.703 | 0.993 |
| Child-Pugh
grade |
| A | 0.710 | 0.250 |
| B | 0.395 | 0.758 |
| CLIP score |
| 0 | 0.506 | 0.226 |
| 1–2 | 0.699 | 0.916 |
| 3–6 | 0.829 | 0.252 |
| AFP |
| <5 ng/ml | 0.112 | 0.994 |
| ≥5 ng/ml | 0.358 | 0.421 |
| PIVKA-II |
| <40 mAU/l | 0.633 | 0.353 |
| ≥40 mAU/l | 0.587 | 0.458 |
| Tumor size |
| <5 cm | 0.630 | 0.682 |
| ≥5 cm | 0.702 | 0.040 |
| Tumor number |
| Single | 0.334 | 0.176 |
| Multiple | 0.711 | 0.761 |
| Portal vein
invasion | | |
| (−) | 0.873 | 0.421 |
| (+) | 0.520 | 0.344 |
| Intrahepatic
metastasis | | |
| (−) | 0.550 | 0.239 |
| (+) | 0.524 | 0.257 |
| TNM stage | | |
| I | 0.523 | 0.925 |
| II | 0.771 | 0.625 |
| III | 0.645 | 0.326 |
| IV-A | 0.191 | 0.260 |
 | Table III.Clinicopathological characteristics
of the pre-operative TACE and non-TACE groups with tumor size ≥5 cm
in diameter. |
Table III.
Clinicopathological characteristics
of the pre-operative TACE and non-TACE groups with tumor size ≥5 cm
in diameter.
| Variable | Pre-operative TACE
(n=83) | Non-TACE
(n=47) | P-value |
|---|
| Age (years) | 60.3±8.5 | 61.9±9.2 | 0.319 |
| Gender
(male/female) | 71/12 | 37/10 | 0.304 |
| HBs-Ag (+) | 43 | 23 | 0.556 |
| HCV-Ab (+) | 28 | 19 | 0.575 |
| Child-Pugh grade
(A/B) | 71/12 | 42/5 | 0.594 |
| CLIP score
(0/1–2/3–6) | 32/44/7 | 15/26/6 | 0.632 |
| Serum AFP (>5
ng/ml) | 49 | 29 | 0.376 |
| Serum PIVKA-II
(>40 mAU/l) | 52 | 28 | 0.270 |
| Tumor size
(cm) | 7.9±3.5 | 9.6±13.8 | 0.281 |
| Multiple tumor | 36 | 22 | 0.585 |
| Portal vein
invasion (+) | 10 | 8 | 0.414 |
| Intrahepatic
metastasis (+) | 34 | 21 | 0.640 |
| TNM stage
(I/II/III/IV-A) | 0/49/25/9 | 0/25/12/10 | 0.356 |
Effect of the rate of tumor cell necrosis
on survival
To determine the prognostic importance of the extent
of necrosis of the tumor after TACE, patients treated with
pre-operative TACE were divided into two groups according to the
degree of tumor necrosis, and survival was calculated. The results
of the Kaplan-Meier method showed that the overall and disease-free
survivals over 5 years after hepatectomy for patients with tumors
showing ≥70% necrosis (n=145) were superior to those with <70%
necrosis (n=107). The 1-, 3- and 5-year overall survival rates were
97.1, 88.2 and 68.2% for the ≥70% necrosis group, and 89.1, 70.3
and 58.1% for the <70% necrosis group, respectively (p=0.02)
(Fig. 3). The 1-, 3- and 5-year
disease-free survival rates were 75.5, 46.9 and 36.5% for the ≥70%
necrosis group, and 60.8, 38.5 and 27.0% for the <70% necrosis
group, respectively (p=0.03). Analysis of recurrence sites showed
that 82 and 12 patients experienced intrahepatic and extrahepatic
recurrence in the ≥70% tumor necrosis group, and 52 and 24 patients
in the <70% tumor necrosis group (p<0.01).
Patients with HCCs ≥5 cm exhibited a benefit of
pre-operative TACE for disease-free survival in our study. By
contrast, the necrosis rate was another prognostic factor in
overall and disease-free survival. Therefore, we performed another
analysis for patients with HCCs ≥5 cm and ≥70% necrosis. A survival
benefit was noted in both overall and disease-free survival for
patients bearing ≥5 cm HCCs in the ≥70% necrosis group; the 5-year
overall and disease-free survival rates were 63.9 and 41.0% (n=43),
and for patients bearing ≥5 cm HCCs in the <70% necrosis group,
42.8 and 16.1% (n=40) (p=0.02 and p<0.01, respectively).
Changes in AFP mRNA in peripheral
blood
Among the last 123 patients for whom serum AFP mRNA
was measured pre-operatively, 13 (11.6%) were positive. There was a
significant difference between the AFP mRNA-positive and -negative
groups in terms of protein induced by vitamin K antigen II
(PIVKA-II) (p<0.05), but not in the other clinicopathologic
parameters analyzed. Among 53 patients who underwent pre-operative
TACE, 5 patients were AFP mRNA-positive, and pre-operative AFP mRNA
was negative in 12 patients who showed complete TACE-related
necrosis, although the statistical difference was not significant
(p=0.20). Quantification of AFP mRNA revealed that AFP
mRNA-positive patients who received pre-operative TACE had the
worst overall survival after hepatectomy compared to the AFP
mRNA-negative patients. Among the pre-operative TACE group, the
5-year overall survival rates were 60.0% for the AFP mRNA-positive
group (n=5), and 88.8% for the AFP mRNA-negative group (n=48),
respectively (p=0.02). Also, AFP mRNA-positive patients with a
tumor size ≥5 cm showed similar results; the 5-year overall
survival rates were 0% (n=2; each 0.4 and 1.25 years), and 83.3%
(n=20), respectively (p=<0.01).
Discussion
One of the reasons for the high recurrence rate in
HCC is postulated to be the potential hematogenous spread of cancer
cells pre- or intra-operatively, even if curative resection is
performed. This possible phenomenon is probably due to the
characteristics of HCC or the surgical manipulation during
hepatectomy itself. Irrespective of the underlying etiology, it is
important to control this hematogenous spread of cancer cells, and
one of the purposes of pre-operative TACE is to prevent such spread
and improve the outcome after curative resection.
In order to improve the survival rate after surgery
for HCC, several researchers have tried pre-operative TACE;
however, its clinical benefits are still controversial (Table IV) (9–29).
Half of these studies including randomized controlled trials (RCTs)
demonstrated effective results regarding pre-operative TACE while
others did not or showed harmful results. Our results showed that
pre-operative TACE prolonged the disease-free survival in patients
with HCC tumors measuring 5 cm or more in diameter. In these
previous studies, some authors supported our results, i.e.,
pre-operative TACE was effective in large or stage III–IV tumors
(9–12). On the other hand, two RCTs showed
the opposite results (20,22). However, the background of these
patients differed from ours; both studies involved large tumor (≥10
cm) in hepatitis B carriers.
 | Table IV.Studies on pre-operative TACE for
HCC. |
Table IV.
Studies on pre-operative TACE for
HCC.
| Author (Refs.) | No. of
patients* | Tumor factor | % Overall survival
TACE vs. control (years) | % Disease-free
survival TACE vs. control (years) | Result |
|---|
| Clinical
trials | | | | | |
| Wu et al
(20) | 24:48 | Ts ≥10 cm | 32 vs. 60 (5) | 40 vs. 50 (3) | Harmful |
| Yamasaki et
al (21) | 50:47 | 2≤ Ts ≤5 cm | 63 vs. 62 (5) | 39 vs. 31 (5) | NS |
| Zhou et al
(22) | 52:56 | Ts ≥5 cm | 31 vs. 21 (5) | 13 vs. 9 (5) | NS |
| Peng et al
(9) | 51:53 | Vp | 21 vs. 8.5 (5) | NA | Effective |
| Zhong et al
(11) | 59:59 | Stage IIIA | 23 vs. 18 (5) | 9 vs. 2 (5) | Effective |
| Retrospective
studies | | | | | |
| Imaoka et al
(17) | 37:52 | Ts <10 cm | NA | 72 vs. 54 (2) | Effective |
| Monden et al
(23) | 71:21 | | 63 vs. 62 (3) | NA | NS |
| Nagasue et
al (27) | 31:107 | | 31 vs. 45 (3) | NA | NS |
| Adachi et al
(18) | 46:26 | Ts ≤5 cm,Vp(-),
Vv(-), IM(-) | NA | 52 vs. 49 (3) | Effective (CN
group) |
| Harada et al
(15) | 98:33 | | 78 vs. 68 (3) | 38 vs. 34 (3) | Effective (CN
group) |
| Uchida et al
(28) | 60:68 | | 61 vs. 73 (3) | 57 vs. 48 (3) | Harmful |
| Majno et al
(14) | 49:27 | | 57 vs. 47 (3) | 33 vs. 22 (3) | Effective
(downstage or CN group) |
| Paye et al
(24) | 24:24 | | 62 vs. 65 (3) | 32 vs. 16 (3) | NS |
| Di Carlo et
al (13) | 55:45 | Ts ≤5 cm | 70 vs. 38 (3) | 40 vs. 20 (3) | Effective |
| Lu et al
(12) | 44:76 | | 50 vs. 52 (3) | 32 vs. 36 (3) | NS |
| (24:57) | 2≤ Ts ≤8 cm | 42 vs. 61 (3) | 21 vs. 43 (3) | NS |
| (20:19) | Ts ≥8 cm | 53 vs. 33 (3) | 32 vs. 11 (3) | Effective |
| Zhang et al
(19) | 120:1337 | | NA | 51 (n≥2) vs. 36
(n≥1) vs. 21 (control) (5) | Effective |
| Gerunda et
al (16) | 20:17 | Ts ≤5 cm | 43 vs. 38 (5) | 57 vs. 21 (5) | Effective
(DFS) |
| Sugo et al
(10) | 13:73 | | NA | 46 vs. 39 (3) | NS |
| (58:35) | Stage I, II | NA | NA | NS |
| (55:38) | Stage III, IV | NA | 41 vs. 21 (3) | Effective |
| Sasaki et al
(25) | 109:126 | | 28 vs. 50 (5) | 19 vs. 22 (5) | Harmful |
| Choi et al
(26) | 120:153 | | NA | 51 vs. 47 (5) | NS |
| Lee et al
(29) | 114:236 | | 47 vs. 52 (5) | 29 vs. 36 (5) | Harmful |
Subgroup analysis based on other clinicopathological
factors, such as portal vein invasion, intrahepatic metastasis and
TNM stage, which are considered important factors in the prognosis
of HCC patients, showed that pre-operative TACE had no significant
benefits to survival. Several studies have documented the poor
response to TACE in patients with satellite lesions, intrahepatic
recurrence or tumor thrombosis (12,13,25,26).
The generally poor response of patients with portal vein invasion,
intrahepatic metastasis or advanced HCC to pre-operative TACE and
subsequently to surgery, may be explained by the aforementioned
reasons.
On the other hand, our results suggest that
pre-operative TACE is beneficial for patients with HCCs 5 cm or
more in diameter. Of these patients, the mortality rate of AFP
mRNA-positive patients was worse, even when they underwent
pre-operative TACE. Although this finding may suggest hematological
spread of cancer cells before surgery, the change in AFP mRNA
levels should be analyzed before or after both pre-operative TACE
and surgery, since it is doubtful whether pre-operative AFP mRNA
was positive before TACE or became positive after TACE.
Nonetheless, AFP mRNA may be a good prognostic marker of HCC.
The relationships between TACE-induced tumor
necrosis, tumor spread and survival remain controversial (10–18,22–26).
Our results showed that the 5-year overall and disease-free
survival of patients with tumors showing a necrosis rate of 70% or
higher was more favorable than the survival of patients with tumors
showing less than 70% necrosis. In agreement with these results,
the pre-operative AFP mRNA of all 12 patients who showed
TACE-related complete necrosis was negative. Furthermore, the
analysis of recurrence sites found that patients bearing tumors
with a necrosis rate less than 70% were more likely to develop
extrahepatic recurrence. Previous studies reported a higher
incidence of extrahepatic metastasis in AFP mRNA-positive patients,
either before or after TACE (32).
These data indicate that complete necrosis induced by pre-operative
TACE seems to inhibit extrahepatic metastasis and may be a suitable
marker of favorable patient outcome.
Although the necrosis rate was determined through
histopathological examination, some studies have evaluated the
therapeutic response of TACE using modalities including dynamic CT,
contrast-enhanced sonography, power Doppler sonography, dynamic MRI
and angiography (33,34). However, we consider it insufficient
to assess the effect of TACE pre-operatively. On the other hand,
although the number of samples in this study was limited, the
necrosis rate in 4 of the 5 (80%) AFP mRNA-positive patients who
received pre-operative TACE was less than 70%. These findings
suggest differences in survival between patients bearing tumors
with a necrosis rate equal to or greater than 70 and patients
bearing tumors with less than 70% necrosis. Although further
examination is required, we can anticipate the efficacy of AFP mRNA
in peripheral blood for patient survival after surgery following
TACE.
In conclusion, pre-operative TACE may be beneficial
for patients with HCC tumors measuring more than 5 cm in diameter.
These patients should undergo TACE before curative resection to
prolong disease-free survival. However, tumor necrosis equal to or
greater than 70% is necessary for more favorable survival. Our
results also showed that quantification of AFP mRNA in peripheral
blood after TACE is potentially useful for the prediction of HCC
recurrence.
Abbreviations:
|
AFP mRNA
|
α-fetoprotein messenger RNA;
|
|
CT
|
computed tomography;
|
|
GAPDH
|
glyceraldehydes-3-phosphate
dehydrogenase;
|
|
HCC
|
hepatocellular carcinoma;
|
|
PIVKA-II
|
protein induced by vitamin K antigen
II;
|
|
qRT-PCT
|
quantitative reverse
transcriptase-polymerase chain reaction;
|
|
TACE
|
transcatheter arterial
chemoembolization;
|
|
DFS
|
disease-free survival
|
Acknowledgements
We thank Yukari Sugita for measuring
the AFP mRNA in peripheral blood. This study was supported by a
Grant-in-Aid for Cancer Research from the Ministry of Culture and
Science, and the Ministry of Health and Welfare of Japan.
References
|
1.
|
Poon RT, Fan ST, Tsang FH and Wong J:
Locoregional therapies for hepatocellular carcinoma: a critical
review from the surgeon’s perspective. Ann Surg. 235:466–486.
2002.PubMed/NCBI
|
|
2.
|
Witzigmann H, Geissler F, Benedix F, et
al: Prospective evaluation of circulating hepatocytes by
alpha-fetoprotein messenger RNA in patients with hepatocellular
carcinoma. Surgery. 131:34–43. 2002. View Article : Google Scholar
|
|
3.
|
Lemoine A, Le Bricon T, Salvucci M, et al:
Prospective evaluation of circulating hepatocytes by
alpha-fetoprotein mRNA in humans during liver surgery. Ann Surg.
226:43–50. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kamiyama T, Takahashi M, Nakagawa T, et
al: AFP mRNA detected in bone marrow by real-time quantitative
RT-PCR analysis predicts survival and recurrence after curative
hepatectomy for hepatocellular carcinoma. Ann Surg. 244:451–463.
2006.PubMed/NCBI
|
|
5.
|
Morimoto O, Nagano H, Miyamoto A, et al:
Association between recurrence of hepatocellular carcinoma and
alpha-fetoprotein messenger RNA levels in peripheral blood. Surg
Today. 35:1033–1041. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Miyamoto A, Nagano H, Sakon M, et al:
Clinical application of quantitative analysis for detection of
hematogenous spread of hepatocellular carcinoma by real-time PCR.
Int J Oncol. 18:527–532. 2001.PubMed/NCBI
|
|
7.
|
Marubashi S, Dono K, Sugita Y, et al:
Alpha-fetoprotein mRNA detection in peripheral blood for prediction
of hepatocellular carcinoma recurrence after liver transplantation.
Transplant Proc. 38:3640–3642. 2006. View Article : Google Scholar
|
|
8.
|
Marubashi S, Dono K, Nagano H, et al:
Detection of AFP mRNA-expressing cells in the peripheral blood for
prediction of HCC recurrence after living donor liver
transplantation. Transpl Int. 20:576–582. 2007. View Article : Google Scholar
|
|
9.
|
Peng BG, He Q, Li JP and Zhou F: Adjuvant
transcatheter arterial chemoembolization improves efficacy of
hepatectomy for patients with hepatocellular carcinoma and portal
vein tumor thrombus. Am J Surg. 198:313–318. 2009. View Article : Google Scholar
|
|
10.
|
Sugo H, Futagawa S, Beppu T, Fukasawa M
and Kojima K: Role of pre-operative transcatheter arterial
chemoembolization for resectable hepatocellular carcinoma: relation
between postoperative course and the pattern of tumor recurrence.
World J Surg. 27:1295–1299. 2003. View Article : Google Scholar
|
|
11.
|
Zhong C, Guo RP, Li JQ, et al: A
randomized controlled trial of hepatectomy with adjuvant
transcatheter arterial chemoembolization versus hepatectomy alone
for Stage III A hepatocellular carcinoma. J Cancer Res Clin Oncol.
135:1437–1445. 2009. View Article : Google Scholar
|
|
12.
|
Lu CD, Peng SY, Jiang XC, Chiba Y and
Tanigawa N: Pre-operative transcatheter arterial chemoembolization
and prognosis of patients with hepatocellular carcinomas:
retrospective analysis of 120 cases. World J Surg. 23:293–300.
1999. View Article : Google Scholar
|
|
13.
|
Di Carlo V, Ferrari G, Castoldi R, et al:
Pre-operative chemoembolization of hepatocellular carcinoma in
cirrhotic patients. Hepatogastroenterology. 45:1950–1954.
1998.PubMed/NCBI
|
|
14.
|
Majno PE, Adam R, Bismuth H, et al:
Influence of pre-operative transarterial lipiodol chemoembolization
on resection and transplantation for hepatocellular carcinoma in
patients with cirrhosis. Ann Surg. 226:688–703. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Harada T, Matsuo K, Inoue T, Tamesue S and
Nakamura H: Is preoperative hepatic arterial chemoembolization safe
and effective for hepatocellular carcinoma? Ann Surg. 224:4–9.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Gerunda GE, Neri D, Merenda R, et al: Role
of transarterial chemoembolization before liver resection for
hepatocarcinoma. Liver Transpl. 6:619–626. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Imaoka S, Sasaki Y, Shibata T, et al: A
pre-operative chemoembolization therapy using lipiodol, cisplatin
and gelatin sponge for hepatocellular carcinoma. Cancer Chemother
Pharmacol. 23:S126–S128. 1989. View Article : Google Scholar
|
|
18.
|
Adachi E, Matsumata T, Nishizaki T,
Hashimoto H, Tsuneyoshi M and Sugimachi K: Effects of pre-operative
transcatheter hepatic arterial chemoembolization for hepatocellular
carcinoma. The relationship between postoperative course and tumor
necrosis. Cancer. 72:3593–3598. 1993. View Article : Google Scholar
|
|
19.
|
Zhang Z, Liu Q, He J, Yang J, Yang G and
Wu M: The effect of pre-operative transcatheter hepatic arterial
chemoembolization on disease-free survival after hepatectomy for
hepatocellular carcinoma. Cancer. 89:2606–2612. 2000. View Article : Google Scholar
|
|
20.
|
Wu CC, Ho YZ, Ho WL, Wu TC, Liu TJ and
P’Eng FK: Pre-operative transcatheter arterial chemoembolization
for resectable large hepatocellular carcinoma: a reappraisal. Br J
Surg. 82:122–126. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Yamasaki S, Hasegawa H, Kinoshita H, et
al: A prospective randomized trial of the preventive effect of
pre-operative transcatheter arterial embolization against
recurrence of hepatocellular carcinoma. Jpn J Cancer Res.
87:206–211. 1996. View Article : Google Scholar
|
|
22.
|
Zhou WP, Lai EC, Li AJ, et al: A
prospective, randomized, controlled trial of pre-operative
transarterial chemoembolization for resectable large hepatocellular
carcinoma. Ann Surg. 249:195–202. 2009. View Article : Google Scholar
|
|
23.
|
Monden M, Okamura J, Sakon M, et al:
Significance of transcatheter chemoembolization combined with
surgical resection for hepatocellular carcinomas. Cancer Chemother
Pharmacol. 23:S90–S95. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Paye F, Jagot P, Vilgrain V, Farges O,
Borie D and Belghiti J: Pre-operative chemoembolization of
hepatocellular carcinoma: a comparative study. Arch Surg.
133:767–772. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Sasaki A, Iwashita Y, Shibata K, Ohta M,
Kitano S and Mori M: Pre-operative transcatheter arterial
chemoembolization reduces long-term survival rate after hepatic
resection for resectable hepatocellular carcinoma. Eur J Surg
Oncol. 32:773–779. 2006. View Article : Google Scholar
|
|
26.
|
Choi GH, Kim DH, Kang CM, et al: Is
pre-operative transarterial chemoembolization needed for a
resectable hepatocellular carcinoma? World J Surg. 31:2370–2377.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Nagasue N, Galizia G, Kohno H, et al:
Adverse effects of pre-operative hepatic artery chemoembolization
for resectable hepatocellular carcinoma: a retrospective comparison
of 138 liver resections. Surgery. 106:81–86. 1989.
|
|
28.
|
Uchida M, Kohno H, Kubota H, et al: Role
of pre-operative transcatheter arterial oily chemoembolization for
resectable hepatocellular carcinoma. World J Surg. 20:326–331.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Lee KT, Lu YW, Wang SN, et al: The effect
of pre-operative transarterial chemoembolization of resectable
hepatocellular carcinoma on clinical and economic outcomes. J Surg
Oncol. 99:343–350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Seldinger SI: Catheter replacement of the
needle in percutaneous arteriography; a new technique. Acta Radiol.
39:368–376. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Dindo D, Demartines N and Clavien PA:
Classification of surgical complications: a new proposal with
evaluation in a cohort of 6336 patients and results of a survey.
Ann Surg. 240:205–213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Gross-Goupil M, Saffroy R, Azoulay D, et
al: Real-time quantification of AFP mRNA to assess hematogenous
dissemination after transarterial chemoembolization of
hepatocellular carcinoma. Ann Surg. 238:241–248. 2003. View Article : Google Scholar
|
|
33.
|
Jang MK, Lee HC, Kim IS, et al: Role of
additional angiography and chemoembolization in patients with
hepatocellular carcinoma who achieved complete necrosis following
transarterial chemoembolization. J Gastroenterol Hepatol.
19:1074–1080. 2004. View Article : Google Scholar
|
|
34.
|
Herber S, Biesterfeld S, Franz U, et al:
Correlation of multislice CT and histomorphology in HCC following
TACE: predictors of outcome. Cardiovasc Intervent Radiol.
31:768–777. 2008. View Article : Google Scholar : PubMed/NCBI
|