Introduction
CUB-domain-containing protein 1 (CDCP1), also known
as CD318, was first identified as the product of a gene
preferentially expressed in colon cancer cells (1). CDCP1/CD318 is a type I transmembrane
protein containing three CUB (complement protein subcomponents
C1/r, urchin embryonic growth factor and bone morphogenic protein
1) domains within the extracellular region and a hexalysine stretch
within the cytoplasmic region (1).
CD318/CDCP1 are possibly involved in cell adhesion or extracellular
matrix interaction (1,2), and CD318 expression levels are
correlated with the metastatic ability of carcinoma cells (3). CDCP1 mRNA was also detected in lung,
breast and gastric cancers and in the erythroleukemia cell line
K562 (1–4). In addition, expression of CD318 has
been reported in hematopoietic stem, mesenchymal stem and neuronal
progenitor cells (5).
Human hemaptopoietic stem and progenitor cells
(HSCs/HPCs) can differentiate into many types of mature blood cells
including erythrocytes, granulocytes and thrombocytes (6). Human CD34+ cells include
several classes of HSCs/HPCs, such as relatively mature in
vitro colony forming cells (CFCs), relatively immature
long-term culture initiating cells (LTC-IC) and immature
transplantable SCID-repopulating cells (SRCs), that can engraft in
non-obese diabetic/severe combined immunodeficient disease
(NOD/SCID) mice (7–9). Subpopulations of CD34+
cells, such as CD34+CD38− and
CD34+CD133+ cells, have been reported to be
rich in immature hematopoietic cells including SRCs (10,11).
In hematopoetic cells in the bone marrow (BM) and
cord blood (CB), CD318 is expressed on CD34+ cells, but
not on mature hematopoietic cells (5). In leukemia, CD318 is predominantly
expressed on CD34+CD133+ myeloid leukemic
blasts. The transplantation of purified CD318+ cells
into NOD/SCID mice results in the engraftment of human cells with
multi-lineage differentiation potential (12).
In the present study, we analyzed the expression and
hematopoietic activity of CD318 on CB hematopoietic cells in
relation to CD34 expression. We found that
CD34+CD318+ cells were rich in CFCs,
proliferated well on a monolayer of mesenchymal stem cells and
showed high SRC activity. We conclude that CD318 expression on
CD34+ cells identifies immature hematopoietic stem
cells.
Materials and methods
Cytokines
Recombinant human (rh)-interleukin (IL)-3, rh-stem
cell factor (SCF), rh-granulocyte colony-stimulating factor
(G-CSF), rh-granulocyte/macrophage (GM)-CSF, rh-thrombopoietin
(TPO) and rh-erythropoietin (Epo) were a generous gift from the
Kirin Brewery Co. Ltd. (Tokyo, Japan). Flt3 ligand (FL) was
purchased from R&D Systems (Minneapolis, MN).
Mice
Eight-week-old female NOD/shi/SCID mice were
purchased from Clea Japan (Tokyo, Japan). The mice were maintained
on racks under specific pathogen-free conditions with a laminar air
flow and were supplied with sterile food and drinking water.
Isolation of lineage-negative cord blood
cells
Umbilical CB was obtained from normal full-term
deliveries after obtaining consent of the mothers. This study was
approved by the institutional review board. Mononuclear cells (MCs)
were separated by density gradient centrifugation using
Ficoll-Paque (GE Healthcare, Buckinghamshire, UK). The MCs were
subjected to depletion of lineage-positive cells using the
automated magnetic cell sorter (autoMACS) system (Miltenyi Biotec
Inc., Auburn, CA) and the Lineage Cell Depletion kit, which
included biotinylated antibodies to lineage-specific antigens (CD2,
CD3, CD11b, CD14, CD15, CD16, CD19, CD56, CD123 and CD235a) and
anti-biotin magnetic micro-beads (Miltenyi Biotec Inc.). The
lineage-negative CB cells were frozen in α-medium supplemented with
10% dimethylsulfoxide and 12% hydroxyethyl starch (CP-1
cryoprotectant; Kyokuto Pharmaceutical Co., Tokyo, Japan) and 8%
human serum albumin in a −80°C freezer.
Flow cytometric analysis and cell
sorting
Lineage-negative cells were stained with fluorescein
isothiocyanate (FITC)-conjugated anti-CD34 monoclonal antibodies
(Beckman Coulter, Miami, FL), phycoerythrin (PE)-conjugated
anti-CD318/CDCP1 antibodies (clone CUB1; BioLegend, San Diego, CA)
and phycoerythrin-cyanin 7 (PC7)-conjugated anti-CD45 antibody
(Beckman Coulter) at 4°C for 30 min. The cells were also stained
with 7-amino-actinomycin D (7-AAD) (Beckman Coulter) to exclude
dead cells, in which 7-AAD-positive cells were gated out.
Immunofluorescence analysis and sorting were performed using
FACSAria (Becton-Dickinson). Appropriate isotype-matched antibodies
were used as a control in all of the experiments.
Colony-forming cell assay
Colony-forming cell (CFC) assays were performed in
35-mm Petri dishes (Becton-Dickinson) by incubating the cells in
semisolid α-medium containing 0.8% methylcellulose (Shinetsu
Chemicals Co., Tokyo, Japan), 30% fetal calf serum (Gibco BRL,
Grand Island, NY), 1% bovine serum albumin, 10−4 M
2-mercaptoethanol (2-ME; Wako Pure Chemicals, Osaka, Japan), 2 mM
l-glutamine (Sigma), 10 ng/ml IL-3, 20 ng/ml SCF, 10 ng/ml G-CSF,
10 ng/ml GM-CSF and 2 U/ml Epo (Kirin Brewery) for 14 days at 37°C
in a humidified atmosphere flushed with 5% CO2 in air
(13–15). The colony-forming units (CFU)-GM,
CFU-Mix and burst-forming units-erythroid (BFU-E) were identified
by the ability to form granulocyte/macrophage (GM) colonies, mixed
erythroid and myeloid (Mix) colonies and erythroid burst colonies,
respectively, as described previously (13–15).
Co-culture of cord blood cells with human
mesenchymal stem cells (MSCs) in the presence of cytokines
MSCs, established from normal BM cells, were
purchased from BioWhittaker, Inc. (San Diego, CA) and maintained as
previously described (16). Cells
(1×103 cells/well in a 12-well plate) were seeded on a
layer of MSCs in 1 ml of serum-free medium, StemPro34 supplemented
with StemPro-34 nutrient supplement (both from Gibco BRL), 2 mM
glutamine and penicillin/streptomycin in the presence of 100 ng/ml
SCF, 100 ng/ml TPO and 100 ng/ml FL. After 7 and 14 days of
culture, cells were harvested and counted.
SCID-repopulating cell (SRC) assay
Cells (1×104) were injected through the
tail vein into 10-week-old NOD/shi/SCID mice sublethally irradiated
(3 Gy X-ray). The mice were intraperitoneally injected with
anti-asialo GM1 antibody (Wako Pure Chemical) to reduce the natural
killer cell activity (17). The
mice were sacrificed 8 weeks after the transplantation and analyzed
for human DNA in BM cells as previously described (16,18).
Briefly, DNA was extracted from BM cells using a SepaGene
extraction kit (Sanko Pure Chemical Co., Tokyo, Japan) and analyzed
for the human-specific DNA 17α-satellite gene by quantitative
real-time PCR (19). The primers
used were sense 5′-ACGGGATAACTGCACCTAAC-3′, and anti-sense
5′-CCATAGGAGGGTTCAACTCT-3′. All experimental procedures were
performed according to the Guidelines for the Care and Use of
Animals approved by the Animal Committee of Hyogo College of
Medicine.
Statistical analysis
Data are presented as the mean ± standard error
(SE). The Student’s t-test was used. P-values of <0.05 were
accepted as significant.
Results
Expression of CD318 on hematopoietic
progenitors
We characterized the expression of CD318 in cord
blood cells by flow cytometry. Lineage-negative cord blood cells
were gated in the blast/lymphocyte region (R1) (Fig. 1A), and then CD45dim+
cells with CD34+ (R2) and CD34− (R3) were
gated (Fig. 1B). The expression of
CD318 on CD34+ cells was ∼70% (Fig. 1C) and that of CD318 on
CD34− cells was ∼60% (Fig.
1D). CD34+CD318+ (R5),
CD34+CD318− (R4),
CD34−CD318+ (R7) and
CD34−CD318− (R6) cells were individually
sorted for the following functional analyses.
Colony formation of sorted cells by means
of CD34 and CD318
We analyzed sorted cells by the hematopoietic colony
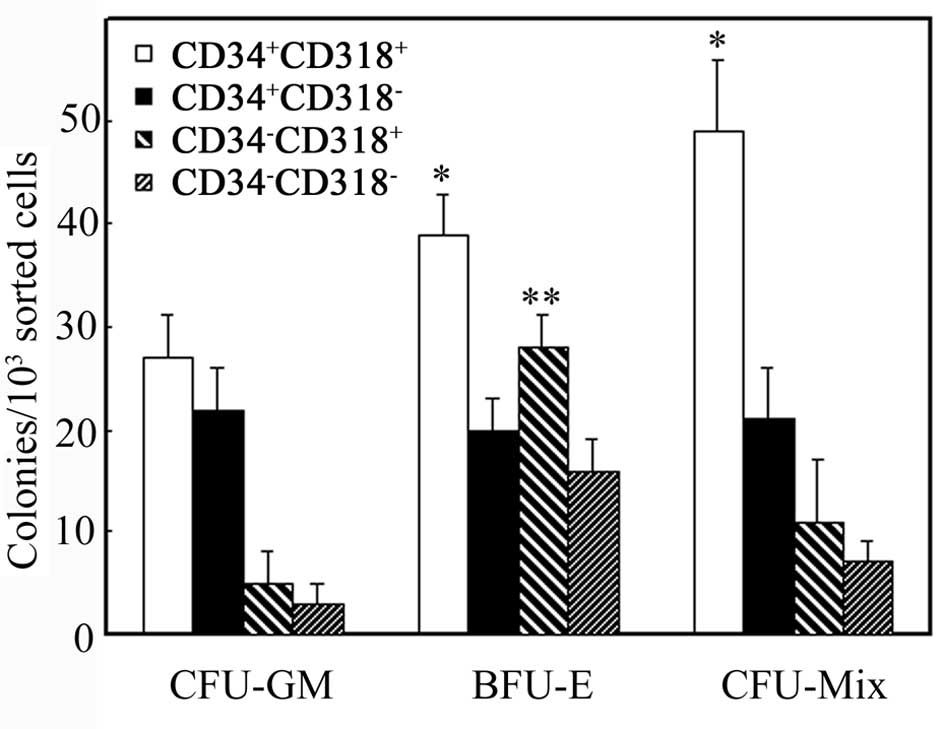
forming assay (Fig. 2).
CD34+CD318+ and
CD34+CD318− cells formed colonies of CFU-Mix,
BFU-E and CFU-GM. CFU-Mix and BFU-E colonies in the
CD34+CD318+ cells were significantly more in
number than those in the CD34+CD318− cells
(P<0.05). CD34−CD318+ and
CD34−CD318− cells also formed these colonies,
but were generally fewer in number except for BFU-E in
CD34−CD318+ cells. In fact,
CD34−CD318+ cells formed more BFU-E colonies
than CD34−CD318− cells (P<0.05).
Proliferative capacity of sorted cells in
co-culture with MSCs
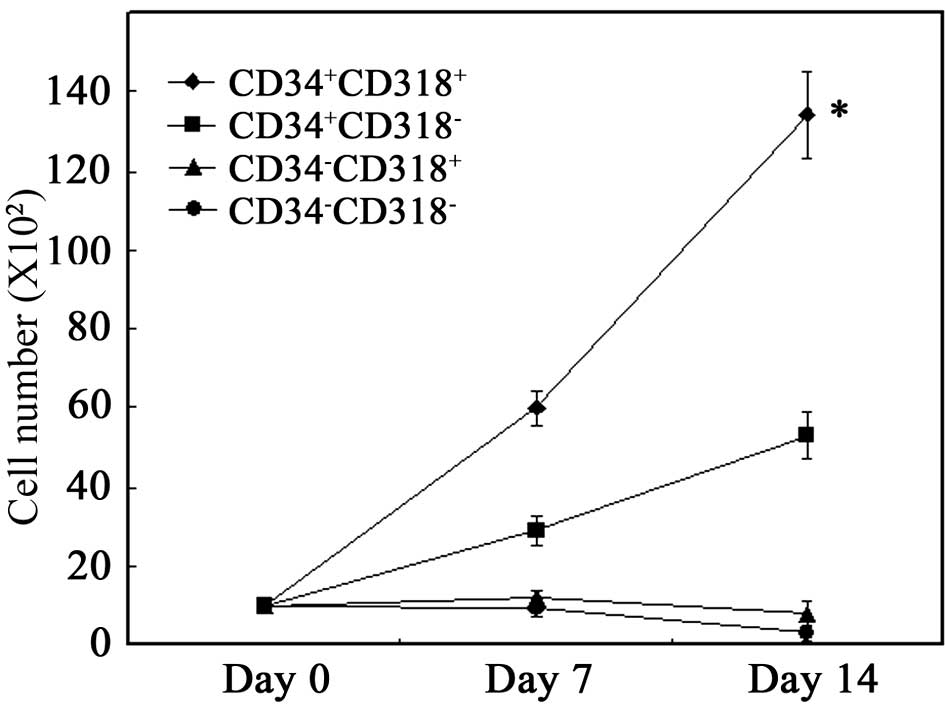
Sorted cells were cultured on a layer of MSCs in the
presence of SCF, TPO and FL. As shown in Fig. 3, proliferation of the total viable
cells was noted when CD34+CD318+ cells were
cultured on MSCs. When CD34+CD318− cells were
cultured, proliferation was significantly lower than that of
CD34+CD318+ cells (P<0.001). No
proliferation was observed for CD34−CD318+
and CD34−CD318− cells. These results suggest
that CD34+CD318+ cells have the highest
proliferative potential.
SCID-repopulating cell assays of the
sorted cells
We next performed SRC assays by transplanting each
sorted cell fraction into NOD/SCID mice through the tail vein.
Eight weeks later, the mice were analyzed for human hematopoietic
cells in the BM by real-time PCR analysis of the human-specific DNA
17α-satellite gene.
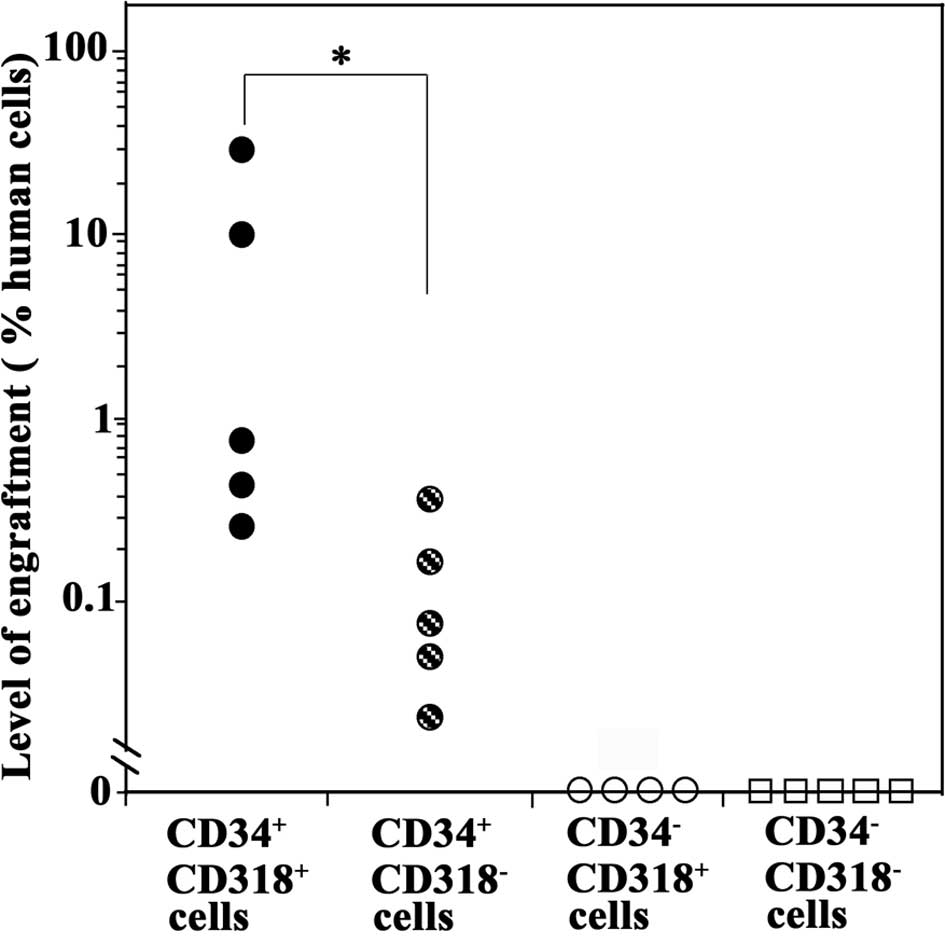
All five mice transplanted with primary
CD34+CD318+ cells were positive for the human
DNA, indicating that primary CB CD34+CD318+
cells contained SCID-repopulating cells (SRCs) (Fig. 4). CD34+CD318−
cells were also engrafted, but to a significantly lower degree than
the CD34+CD318+ cells (P<0.05). By
contrast, mice transplanted with CD34−CD318+
or CD34−CD318− cells were negative for the
human DNA, indicating the absence of the SCID-repopulating
capacity. These results showed that
CD34+CD318+ cells had the highest SRC
activity.
Discussion
CD318/CDCP1 was first found to be expressed in colon
cancer cells and then in breast, lung and other types of cancer
cells (1,2). Expression of CD318 has also been
reported in cells phenotypically identical to hematopoietic
stem/progenitor cells, MSCs and neural progenitor cells (5). Human CD318+ cells
transplanted into NOD/SCID mice resulted in the engraftment of
human cells showing SRC activity (12). In the present study, we analyzed
the expression and hematopoietic activity of CD318 on hematopoietic
cells in relation to CD34 expression.
We confirmed that CD318 was expressed on
CD34+ cells. We found that
CD34+CD318+ cells were rich in CFCs,
including CFU-Mix and BFU-E, and exhibited more proliferative
activity on adherent cells than on
CD34+CD318− cells. Notably, the
CD34+CD318+ cells showed high SRC activity.
These findings suggest that CD34+CD318+ cells
were rich in hematopoietic progenitors and also rich in immature
HSCs.
It has been reported that CD34− cells,
like CD34+ cells, contain hematopoietic stem cells (20). In this study, we found that
CD318+ cells were present in the CD34− cell
fraction. These CD34−CD318+ cells had the
capacity to form hematopoietic colonies, especially erythroid
(BFU-E) colonies. However, CD34−CD318+ cells
did not proliferate on adherent cell layers and contained no SRCs.
Similarly, CD318− cells in the CD34− fraction
contained colony-forming cells, but not SRCs.
CD318/CDCP1 has been reported to play a role in
cell-cell and cell-matrix adhesion (3). In epithelial tissues,
CD318/CDCP1/Trask is phosphorylated by Src kinases to undergo
mitosis or shedding when epithelial cells disengage from the tissue
framework (21,22). In the BM, hematopoietic cells
attach to and are nursed by the cells of the hematopoietic niche
and detach from the niche to circulate in the peripheral blood. It
is intriguing to speculate that circulating HPCs/HSCs express high
levels of CD318. It has been known that Src is involved in
hematopoiesis (23). As
CD318-reactive monoclonal antibody CUB1 augments erythroid colony
formation (5), it is possible that
the phosphorylation of CD318/CDCP1 by Src kinase may affect
hematopoiesis.
Our study showed that CD318 expression on
CD34+ cells identifies immature hematopoietic stem
cells. This result provides a basis for effective hematopoietic
stem cell therapy for various diseases including malignancy.
Further studies are warranted to clarify whether CD318/CDCP1 is
directly associated with the hematopoietic stem cell function.
Acknowledgements
We are grateful to Ms. Yumiko Fujita,
Ms. Kumi Futawaka and Ms. Hatsuka Seki for the excellent technical
assistance. This study was supported by a grant from Hyogo College
of Medicine, and a research grant for the ‘High-Tech Research
Center’ Project for Private Universities from the Ministry of
Education, Culture, Sports, Science and Technology of Japan.
References
|
1.
|
Scherl-Mostageer M, Sommergruber W,
Abseher R, Hauptmann R, Ambros P and Schweifer N: Identification of
a novel gene, CDCP1, overexpressed in human colorectal cancer.
Oncogene. 20:4402–4408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Bhatt AS, Erdjument-Bromage H, Tempst P,
Craik CS and Moasser MM: Adhesion signaling by a novel mitotic
substrate of src kinases. Oncogene. 24:5333–5343. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Uekita T, Tanaka M, Takigahira M, Miyazawa
Y, Nakanishi Y, Kanai Y, Yanagihara K and Sakai R:
CUB-domain-containing protein 1 regulates peritoneal dissemination
of gastric scirrhous carcinoma. Am J Pathol. 172:1729–1739. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Ikeda JI, Morii E, Kimura H, Tomita Y,
Takakuwa T, Hasegawa JI, Kim YK, Miyoshi Y, Noguchi S, Nishida T
and Aozasa K: Epigenetic regulation of the expression of the novel
stem cell marker CDCP1 in cancer cells. J Pathol. 210:75–84. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Bühring HJ, Kuçi S, Conze T, Rathke G,
Bartolović K, Grünebach F, Scherl-Mostageer M, Brümmendorf TH,
Schweifer N and Lammers R: CDCP1 identifies a broad spectrum of
normal and malignant stem/progenitor cell subsets of hematopoietic
and nonhematopoietic origin. Stem Cells. 22:334–343.
2004.PubMed/NCBI
|
|
6.
|
Dorshkind K: Regulation of hematopoiesis
by bone marrow stromal cells and their products. Annu Rev Immunol.
8:111–137. 1990. View Article : Google Scholar
|
|
7.
|
Baines P, Mayani H, Bains M, Fisher J, Hoy
T and Jacobs A: Enrichment of CD34 (My10)-positive myeloid and
erythroid progenitors from human marrow and their growth in
cultures supplemented with recombinant human granulocyte-macrophage
colony-stimulating factor. Exp Hematol. 16:785–978. 1988.
|
|
8.
|
Gartner S and Kaplan HS: Long-term culture
of human bone marrow cells. Proc Natl Acad Sci USA. 77:4756–4759.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Larochelle A, Vormoor J, Hanenberg H, Wang
JC, Bhatia M, Lapidot T, Moritz T, Murdoch B, Xiao XL, Kato I,
Williams DA and Dick JE: Identification of primitive human
hematopoietic cells capable of repopulating NOD/SCID mouse bone
marrow: implications for gene therapy. Nat Med. 2:1329–1337. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Bhatia M, Wang JC, Kapp U, Bonnet D and
Dick JE: Purification of primitive human hematopoietic cells
capable of repopulating immune-deficient mice. Proc Natl Acad Sci
USA. 94:5320–5325. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
De Wynter EA, Buck D, Hart C, Heywood R,
Coutinho LH, Clayton A, Rafferty JA, Burt D, Guenechea G, Bueren
JA, Gagen D, Fairbairn LJ, Lord BI and Testa NG:
CD34+AC133+ cells isolated from cord blood
are highly enriched in long-term culture-initiating cells,
NOD/SCID-repopulating cells and dendritic cell progenitors. Stem
Cells. 16:387–396. 1998.
|
|
12.
|
Conze T, Lammers R, Kuci S,
Scherl-Mostageer M, Schweifer N, Kanz L and Buhring HJ: CDCP1 is a
novel marker for hematopoietic stem cells. Ann NY Acad Sci.
996:222–226. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hara H, Kai S, Fushimi M, Taniwaki S,
Okamoto T, Ohe Y, Fujita S, Noguchi K, Senba M, Hamano T, Kanamaru
A and Nagai K: Pluripotent hemopoietic precursors in vitro
(CFU-MIX) in aplastic anemia. Exp Hematol. 8:1165–1171. 1980.
|
|
14.
|
Fujimori Y, Hara H and Nagai K: Effect of
lymphokine-activated killer cell fraction on the development of
human hematopoietic progenitor cells. Cancer Res. 48:534–538.
1988.PubMed/NCBI
|
|
15.
|
Fujimori Y, Ogawa M, Clark SC and Dover
GJ: Serum-free culture of enriched hematopoietic progenitors
reflects physiologic levels of fetal hemoglobin biosynthesis.
Blood. 75:1718–1722. 1990.
|
|
16.
|
Nishioka K, Fujimori Y, Hashimoto-Tamaoki
T, Kai S, Qiu H, Kobayashi N, Tanaka N, Westerman KA, Leboulch P
and Hara H: Immortalization of bone marrow-derived human
mesenchymal stem cells by removable simian virus 40T antigen gene:
Analysis of the ability to support expansion of cord blood
hematopoietic progenitor cells. Int J Oncol. 23:925–932. 2003.
|
|
17.
|
Yoshino H, Ueda T, Kawahata M, Kobayashi
K, Ebihara Y, Manabe A, Tanaka R, Ito M, Asano S, Nakahata T and
Tsuji K: Natural killer cell depletion by anti-asialo GM1 antiserum
treatment enhances human hematopoietic stem cell engraftment in
NOD/Shi-scid mice. Bone Marrow Transplant. 26:1211–1216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Qiu H, Fujimori Y, Kai S, Fujibayashi Y,
Nishioka K and Hara H: Establishment of mouse embryonic fibroblast
cell lines that promote ex vivo expansion of human cord blood
CD34+ hematopoietic progenitors. J Hematother Stem Cell
Res. 12:39–46. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Becker M, Nitsche A, Neumann C, Aumann J,
Junghahn I and Fichtner I: Sensitive PCR method for the detection
and real-time quantification of human cells in xenotransplantation
systems. Br J Cancer. 87:1328–1335. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Bhatia M, Bonnet D, Murdoch B, Gan OI and
Dick JE: A newly discovered class of human hematopoietic cells with
SCID-repopulating activity. Nat Med. 4:1038–1045. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Wong CH, Baehner FL, Spassov DS, Ahuja D,
Wang D, Hann B, Blair J, Shokat K, Welm AL and Moasser MM:
Phosphorylation of the SRC epithelial substrate Trask is tightly
regulated in normal epithelia but widespread in many human
epithelial cancers. Clin Cancer Res. 15:2311–2322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Spassov DS, Baehner FL, Wong CH, McDonough
S and Moasser MM: The transmembrane src substrate Trask is an
epithelial protein that signals during anchorage deprivation. Am J
Pathol. 174:1756–1765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Rane SG and Reddy EP: JAKs, STATs and Src
kinases in hematopoiesis. Oncogene. 21:3334–3358. 2002. View Article : Google Scholar : PubMed/NCBI
|