Introduction
Cervical cancer is the second most common cancer
among Japanese women. Studies in several parts of the world have
demonstrated a very strong association between the human
papillomavirus (HPV) and cervical cancer with odds ratios of over
15 (1). Prospective studies have
shown that infection with high-risk HPV precedes the development of
cervical neoplasia (2). HPV has
now been accepted as a necessary cause of cervical cancer (3).
It has been accepted that the prognosis of cervical
carcinoma is related to clinical stage, lymph node metastasis,
parametrial invasion, primary tumor size, histological type, depth
of cervical stromal invasion and lymph vascular space involvement.
(4) However, recurrent cervical
carcinoma will develop in approximately 10–15% of stage I-IIA
patients and in 30–50% of stage IIB-III patients due to variable
responses to surgery or radiotherapy (5). Further stratification based on novel
specific molecular markers for cervical carcinoma is crucial to
improve the survival rate in this heterogeneous group of patients.
This type of classification may help clinicians in developing
appropriate therapeutic strategies for molecular subtyping of
patients at a high risk of disease recurrence.
Although HPV infection has been established as an
important initial event in the tumorigenesis of cervical carcinoma,
reports on the clinical impact of different HPV types are
conflicting. Previous attempts to determine the prognostic
significance of the presence or absence of detectable HPV DNA and
HPV types in cervical cancer patients have generated conflicting
results (6–13). Some studies have reported that
patients infected with HPV-18 had worse prognoses and higher
disease recurrence rates than patients with HPV-16 infection
(6–8,10).
In a recent study, infection with multiple HPV types was also
considered an indicator of poor response to radiotherapy (7,11).
However, other reports did not demonstrate an association between
HPV type and clinical outcome (8,9,12,13).
Conversely, Lai et al reported that cervical carcinoma
patients infected with HPV-58-related types had a favorable outcome
(10). These discrepancies have
made it difficult to interpret the relevance of the HPV genotype
and clinical outcome.
The present study was designed to analyze the
relationship between HPV DNA status and clinicopathological
parameters in order to further elucidate the role of the HPV type
in relation to clinical outcome of cervical carcinoma. Finally, to
determine the clinical implications and prognostic value of the HPV
genotype in cervical carcinoma, we evaluated whether various HPV
types in patients receiving radiotherapy for squamous cell
carcinoma of the cervix correlate with survival.
Materials and methods
Patients
The study population included 113 invasive squamous
cell carcinoma patients treated with radiation or chemoradiation
between 1993 and 2002. These patients were successfully followed-up
at the University of the Ryukyus Hospital, Okinawa, Japan. Patient
age, International Federation of Gynecology and Obstetrics (FIGO)
stage, tumor size, hemoglobin level before treatment and status of
lymph node enlargement were recorded after thorough clinical
investigations. Written informed consent was obtained from all
patients, and our Institutional Research Board approved the
study.
Radiotherapy and concurrent
chemoradiotherapy
The patients were treated with anterior-posterior
and postero-anterior parallel opposed ports of external beam
radiotherapy (EBRT). The dose of ERBT was 50 Gy delivered in 25
fractions. The center shield (4-cm width at the midline) was set up
after delivering 40 Gy. High-dose rate intracavitary radiotherapy
was delivered once a week with a fraction dose of 6 Gy at point A
for 3 or 4 times. Forty patients received cisplatin (20
mg/m2) for 5 days every 3 weeks, concomitant with
radiotherapy (14).
Typing of HPV DNA
Specimens were freshly collected from biopsies. They
were snap frozen in liquid nitrogen and stored at −70°C until use.
Part of each specimen was examined pathologically for diagnosis. To
test for the presence of HPV, the DNA extracted from the specimen
was subjected to polymerase chain reaction (PCR), using an L1
consensus primer pair [L1C1 and L1C2; reported by Yoshikawa et
al (15)], as described
elsewhere (16). PCR with a
β-globin primer pair was performed in parallel, and
β-globin-negative samples were not included in further analyses.
The L1 PCR products obtained from HPV-positive samples were stored
frozen. To identify the HPV genotypes, direct nucleotide sequencing
of PCR products was performed as described previously (27).
Similarities of the obtained L1 sequences between those of various
HPV DNA sequences in the database were examined with BLAST analysis
(http://www.ncbi.nih.gov/BLAST).
Statistical analysis
JMP 6.0 software (SAS Institute, Cary, NC) was used
for statistical analyses. Survival curves were estimated by the
Kaplan-Meier method, and differences were tested by the log-rank
test. Furthermore, p-values <0.05 were considered
significant.
Results
Patient characteristics are listed in Table I. The median age of the patients
was 61 years (range 30–80 years). Tumors were classified by FIGO
staging as stage IB in 11 patients, stage II in 39, stage III in 57
and stage IVA in 6 patients. The tumor size of the cervix,
determined by magnetic resonance imaging (MRI) was ≤4 cm in 50
cases and >4 cm in the remaining 53 cases. Pelvic lymph node
enlargement, which was defined as an enlargement >10 mm in the
shortest dimension by computed tomography or MRI, was observed in
53 cases. Follow-up examinations were conducted every month for the
first year, every other month for the second year and then every
3–6 months. During the follow-up period (median 64 months; range
6–164 months), 45 patients died, 44 deaths being directly related
to the disease.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variables | No. (n=113) | % |
|---|
| Age (median 61 years,
range 30–80 years) | | |
| ≤50 | 34 | 30.1 |
| >50 | 79 | 69.9 |
| FIGO stage | | |
| I | 11 | 9.7 |
| II | 39 | 34.5 |
| III | 57 | 50.4 |
| IV | 6 | 4.4 |
| Tumor size | | |
| ≤4 cm | 61 | 54.0 |
| >4 cm | 52 | 46.0 |
| Lymph node
enlargement | | |
| Positive | 53 | 46.9 |
| Negative | 60 | 53.1 |
| Hemoglobin level | | |
| ≤11.3 | 42 | 37.2 |
| >11.3 | 71 | 62.8 |
| Treatment | | |
|
Chemoradiotherapy | 40 | 35.4 |
| Radiotherapy
alone | 73 | 64.6 |
| Response to
treatment | | |
| Persistence | 16 | 23.0 |
| Complete
response | 97 | 77.0 |
| Prognosis | | |
| No evidence of
disease | 69 | 61.1 |
| Died of
disease | 44 | 38.9 |
Prevalence and the HPV genotypes in cervical
carcinoma are summarized in Table
II. Of the 113 specimens, 95 (84.1%) were positive for HPV DNA.
The most prevalent types were HPV-16 (34.7%), HPV-33 (10.5%),
HPV-58 (10.5%) and HPV-52 (7.3%). Multiple HPV infections (HPV-16
and HPV-33) were detected in only 1 sample. The relationship
between HPV genotypes and clinicopathological variables are shown
in Table III. The HPV genotype was
found to be associated with age. HPV-16 type was found more
frequently in younger patients (16 of 33 cases) as compared to
other HPV types. However, the HPV genotype was not associated with
other clinicopathological parameters, namely, FIGO stage, lymph
node swelling, tumor size and hemoglobin level.
 | Table II.HPV genotype distribution. |
Table II.
HPV genotype distribution.
| Genotype | No. | % |
|---|
| Total | 113 | |
| HPV(+) | 95 | 84.1 |
| HPV-16 | 33 | 34.7 |
| HPV-18 | 3 | 3.2 |
| HPV-31 | 5 | 5.2 |
| HPV-33 | 10 | 10.5 |
| HPV-35 | 3 | 3.2 |
| HPV-51 | 1 | 1.1 |
| HPV-52 | 7 | 7.3 |
| HPV-53 | 3 | 3.2 |
| HPV-54 | 1 | 1.1 |
| HPV-56 | 4 | 4.2 |
| HPV-58 | 10 | 10.5 |
| HPV-59 | 3 | 3.2 |
| HPV-66 | 2 | 2.1 |
| HPV-70 | 1 | 1.1 |
| HPV-73 | 1 | 1.1 |
| HPV-82 | 1 | 1.1 |
| HPV-16 + 33 | 1 | 1.1 |
| Undetermined | 6 | 6.4 |
| HPV(−) | 18 | 15.9 |
 | Table III.Patient characteristics for each HPV
genotype. |
Table III.
Patient characteristics for each HPV
genotype.
| HPV-16 | HPV-18 | HPV-31 | HPV-33 | HPV-35 | HPV-52 | HPV-56 | HPV-58 | HPV-59 | Others | HPV-16, -33 | Undetermined | Not detected |
|---|
| No. of
patients | 33 | 3 | 5 | 10 | 3 | 7 | 4 | 10 | 3 | 10 | 1 | 6 | 18 |
| Age | | | | | | | | | | | | | |
| ≤50 | 16 | 1 | 0 | 1 | 0 | 1 | 3 | 2 | 1 | 2 | 0 | 3 | 4 |
| >50 | 17 | 2 | 5 | 9 | 3 | 6 | 1 | 8 | 2 | 8 | 1 | 3 | 14 |
| FIGO stage | | | | | | | | | | | | | |
| I | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 1 | 1 | 2 |
| II | 11 | 1 | 4 | 3 | 1 | 2 | 2 | 3 | 1 | 3 | 0 | 1 | 7 |
| III | 17 | 2 | 0 | 6 | 1 | 5 | 2 | 5 | 2 | 5 | 0 | 4 | 8 |
| IV | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Tumor size | | | | | | | | | | | | | |
| ≤4.0 cm | 19 | 2 | 3 | 7 | 1 | 2 | 1 | 5 | 1 | 6 | 1 | 4 | 9 |
| >4.0 cm | 14 | 1 | 2 | 3 | 2 | 5 | 3 | 5 | 2 | 4 | 0 | 2 | 9 |
| Lymph node
enlargement | | | | | | | | | | | | | |
| Positive | 15 | 1 | 3 | 1 | 2 | 4 | 3 | 3 | 2 | 4 | 0 | 4 | 11 |
| Negative | 18 | 2 | 2 | 9 | 1 | 3 | 1 | 7 | 1 | 6 | 1 | 2 | 7 |
| Hb level | | | | | | | | | | | | | |
| ≤11.3 | 13 | 1 | 2 | 3 | 1 | 3 | 3 | 3 | 3 | 1 | 1 | 2 | 5 |
| >11.3 | 20 | 2 | 3 | 7 | 2 | 4 | 1 | 7 | 0 | 9 | 0 | 4 | 13 |
| Radiation | | | | | | | | | | | | | |
| CCRT | 12 | 1 | 2 | 2 | 1 | 4 | 3 | 1 | 1 | 4 | 0 | 3 | 6 |
| RT | 21 | 2 | 3 | 8 | 2 | 3 | 1 | 9 | 2 | 6 | 1 | 3 | 12 |
| Response to RT | | | | | | | | | | | | | |
| Persistence | 7 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 6 |
| CR | 26 | 2 | 5 | 9 | 3 | 7 | 3 | 10 | 3 | 10 | 1 | 6 | 12 |
| Recurrence | 11/33 | 2/3 | 1/5 | 5/10 | 2/3 | 3/7 | 3/4 | 1/10 | 2/3 | 4/10 | 0 | 1/6 | 9/18 |
| Local | 11 | 1 | 1 | 3 | 2 | 1 | 3 | 1 | 2 | 1 | 0 | 1 | 7 |
| Distant | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 3 | 0 | 0 | 2 |
| Prognosis | | | | | | | | | | | | | |
| DOD | 11 | 2 | 1 | 5 | 2 | 3 | 3 | 1 | 2 | 4 | 0 | 1 | 9 |
| NED | 22 | 1 | 4 | 5 | 1 | 4 | 1 | 9 | 1 | 6 | 1 | 5 | 9 |
Poorer response to radiotherapy was observed in the
HPV-16 genotype. Although 53 of the 56 patients with other types of
HPV achieved complete response to radiotherapy, 7 of the 33
patients with HPV-16 had persistent disease after completion of
radiotherapy (p=0.096). In terms of disease recurrence, among
patients with HPV-16, 11 had a local recurrence. Only 1 of the 10
patients with HPV-58, 1 of the 5 patients with HPV-31 and 5 of the
10 patients with HPV-33 had a recurrence. Forty-four patients
(39.2%) died of cervical carcinoma at the end of the follow-up
period. For overall survival, univariate analysis based on the
log-rank test is summarized in Table
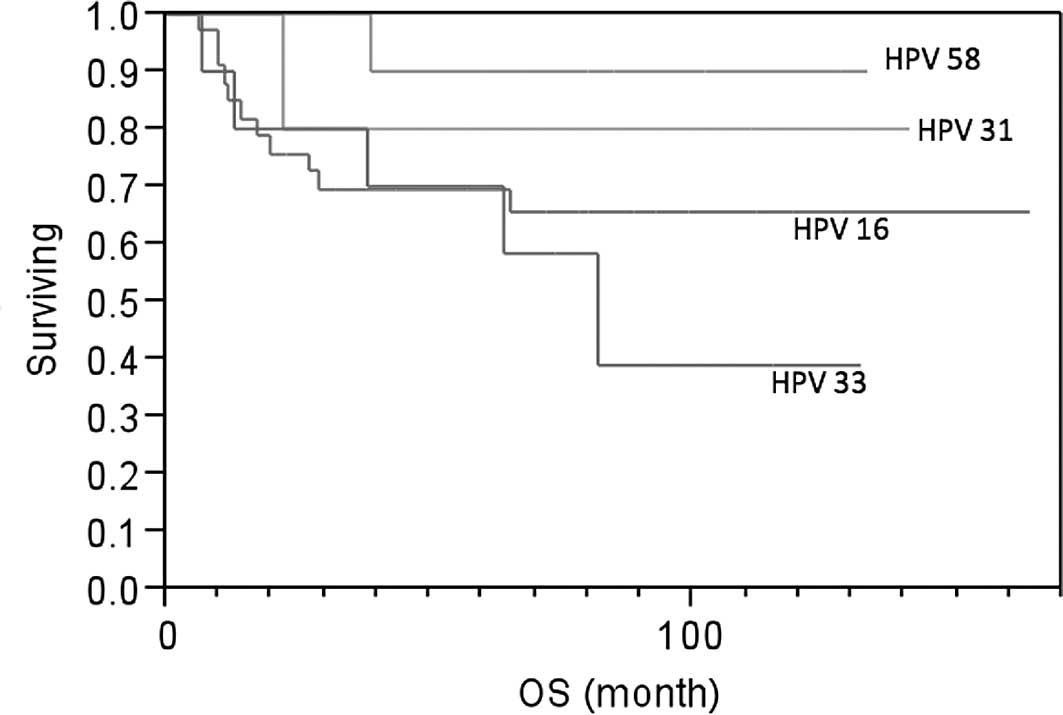
IV. The 5-year survival rate was 90% in the HPV-58 type group
(n=10), 80% in the HPV-31 type group (n=5), 69.4% in the HPV-16
type group (n=33) and 39% in the HPV-33 type group (n=10). Patients
with HPV-31 and HPV-58 types were found to have better survival
than patients with the HPV-16 type (Fig. 1), which was not statistically
significant. However, patients with the HPV-33 type experienced a
higher risk of death than patients with the HPV-58 (p=0.0508),
HPV-31 (p=0.3298) and HPV-16 type (p=0.4726; Fig. 1).
 | Table IV.Life-table analysis of 113 patients
with cervical cancer. |
Table IV.
Life-table analysis of 113 patients
with cervical cancer.
| Characteristics
patients | No. of | 5-year overall
survival (%) | p-value |
|---|
| Age | | | |
| ≤50 | 34 | 57.6 | 0.300 |
| >50 | 79 | 69.1 | |
| FIGO stage | | | |
| I–II | 50 | 79.0 | 0.012 |
| III–IV | 63 | 44.7 | |
| Tumor size | | | |
| ≤4 cm | 61 | 71.0 | 0.140 |
| >4 cm | 52 | 60.0 | |
| Lymph node
enlargement | | | |
| Positive | 53 | 41.6 | <0.001 |
| Negative | 60 | 86.5 | |
| Hemoglobin
level | | | |
| ≤11.3 | 42 | 44.6 | <0.001 |
| >11.3 | 71 | 77.4 | |
| HPV typea | | | |
| 16 | 33 | 69.4 | vs. HPV16 |
| 18 | 3 | 33.0 | 0.23 |
| 31 | 5 | 80.0 | 0.50 |
| 33 | 10 | 39.0 | 0.47 |
| 35 | 3 | 33.0 | 0.42 |
| 52 | 6 | 68.0 | 0.51 |
| 56 | 4 | 25.0 | 0.07 |
| 58 | 10 | 90.0 | 0.14 |
| 59 | 3 | 67.0 | 0.67 |
| Others | 10 | 60.0 | 0.89 |
| Undetermined | 6 | 83.0 | 0.30 |
Discussion
In this study, we report the clinicopathologic
factors of 113 squamous cell carcinoma patients treated with radio-
or chemoradiotherapy, and the results of our analysis of these
factors with regard to HPV genotypes. Previous attempts to
determine the prognostic significance of the presence or absence of
detectable HPV DNA and HPV types in cervical cancer patients have
generated conflicting results (6–13).
These discrepancies have made it difficult to interpret the
relevance of the HPV genotype and clinical outcome.
We sought to identify the prognostic significance of
the HPV DNA genotype only in squamous cell carcinoma patients
treated with radio- or chemoradiotherapy. A poorer response to
radiotherapy was observed in the HPV-16 genotype. Although 53 of
the 56 patients with other types of HPV achieved complete response
to radiotherapy, 7 of the 33 patients with HPV-16 had persistent
disease after completion of radiotherapy. We are not aware of such
a report in the literature. Some biological difference may exist
between HPV-16 and other types of HPV-positive cancer cells; we
need to further investigate this using a larger population of
patients.
Results of prognostic analysis showed that the
HPV-58 and HPV-31 types had a tendency to predict favorable
survival, thus suggesting that these 2 types may be predictors of
good prognosis. Although this finding has to be substantiated in a
larger number of patients, HPV genotyping has the potential to
serve as a biomarker of prognosis in combination with established
markers in patients with squamous cell carcinoma of the cervix.
Despite the high prevalence of HPV-58 and its
related types in East Asia, the clinical behavior and prognostic
value of these viral infections are unclear. The present study
showed that HPV-31 and HPV-58 were more prevalent in the older age
group than in the younger. Increasing prevalence of HPV types other
than 16 and 18 was observed in older patients in Japan (16–18),
where HPV-58 was also relatively prevalent. Patients infected with
HPV-31 and HPV-58 may actually experience an indolent clinical
course and develop cancer at an older age, since it was found that
they were not older than other patients with squamous cell
intraepithelial lesions without such infection (16). Infection by HPV-31 and HPV-58 may
be partly responsible for cervical cancer in the older population
in these areas. Lai et al (10) demonstrated that, in comparison to
the HPV-16-related group, the relative risk of death in the
HPV-58-related (types 58, 33 and 52) group was 0.32 (95% CI
0.07–1.49); these types were prevalent in the older population and
appeared to confer a favorable prognosis. Huang et al
(11) showed that the presence of
HPV-31-related types was an independent predictor of better
survival in patients with cervical carcinoma. However, in their
study, HPV-31-related types included types 31, 33, 35 or 67, and
hence, the results of these two studies are not exactly consistent
with our findings. If the conflicting results are due to
misclassification and the number of HPV cases, analyses of a larger
number of patients with stratification by distinct type would be
useful to clarify the discrepancies. Further investigation of the
natural history of HPV-31- and HPV-58-associated cervical neoplasia
and their underlying biological mechanisms are obviously
warranted.
On the other hand, patients with the HPV-33 type
experienced higher risk of death than patients with the HPV-16
type, which had a relatively poor response to radiotherapy and a
significantly worse prognosis than patients with HPV-58 or HPV-31.
With regard to HPV-33 in particular, the patients had more
favorable clinicopathological factors, such as prevalence in older
age, smaller tumor size, less frequent lymph node swelling and good
response to radiotherapy; however, a higher rate of distant
recurrence and poor prognosis were observed. Hagmar et al
(19) found that patients with
HPV-33- or HPV-18-associated tumors had worse prognoses than
patients with other types of HPV infections, although the reason
for this remains unclear. It is necessary to investigate the
underlying biological mechanisms of HPV-33-associated cervical
cancer.
Previous studies have reported that patients with
HPV-18-containing tumors have an increased risk of death and
disease recurrence. On the molecular level, strong evidence
suggests that HPV-18 confers increased oncogenic potential, given
the fact that its transforming activity was 5 times that of HPV-16
in cell culture systems (20).
This finding is consistent with the findings of previous studies
(8,9,11,12).
Unfortunately, we were unable to confirm this since only 3 patients
had HPV-18. Other groups included HPV-52, HPV-53, HPV-54, HPV-66,
HPV-70, HPV-73 and HPV-82, which were all classified as
intermediate- or low-risk subtypes for carcinogenesis of cervical
cancer. Further investigation on the maintenance of malignant
phenotypes by these intermediate- or low-risk HPV subtypes is also
warranted.
The results of the present study demonstrated the
possibility that HPV-58 and HPV-31 are predictors of good
prognosis, HPV-33 is a predictor of poor prognosis and the HPV-16
type is a predictor of poor response in squamous cell carcinoma
patients treated with radiation or chemoradiation. Although this
finding must be substantiated in a larger number of patients, HPV
genotyping may serve as a potential biomarker of prognosis in
combination with established markers in patients with cervical
carcinoma.
References
|
1.
|
Bosch FX, Manos MM, Munoz N, et al:
Prevalence of human papillomavirus in cervical cancer: a worldwide
perspective. International Biological Study on Cervical Cancer
(IBSCC) Study Group. J Natl Cancer Inst. 87:796–802. 1995.
View Article : Google Scholar
|
|
2.
|
Liaw KL, Glass AG, Manos MM, et al:
Detection of human papillomavirus DNA in cytologically normal women
and subsequent cervical squamous intraepithelial lesions. J Natl
Cancer Inst. 91:954–960. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Walboomers JM, Jacobs MV, Manos MM, et al:
Human papillomavirus is a necessary cause of invasive cancer
worldwide. J Pathol. 189:12–19. 1999. View Article : Google Scholar
|
|
4.
|
Kosary CL: FIGO stage, histology,
histologic grade, age and race as prognostic factors in determining
survival for cancers of the female gynecological system: an
analysis of 1973–87 SEER cases of cancers of the endometrium,
cervix, ovary, vulva and vagina. Semin Surg Oncol. 10:31–46.
1994.PubMed/NCBI
|
|
5.
|
Coia L, Won M, Lanciano R, et al: The
patterns of care outcome study for cancer of the uterine cervix.
Results of the Second National Practice Survey Cancer.
66:2451–2456. 1990.PubMed/NCBI
|
|
6.
|
Schwartz SM, Daling JR, Shera KA, et al:
Human papillomavirus and prognosis of invasive cervical cancer: a
population-based study. J Clin Oncol. 19:1906–1915. 2001.PubMed/NCBI
|
|
7.
|
Bachtiary B, Obermair A, Dreier B, et al:
Impact of multiple HPV infection on response to treatment and
survival in patients receiving radical radiotherapy for cervical
cancer. Int J Cancer. 102:237–243. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kristensen GB, Karlsen F, Jenkins A, et
al: Human papilloma virus has no prognostic significance in
cervical carcinoma. Eur J Cancer. 32A:1349–1353. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Van Bommel PF, van den Brule AJ,
Helmerhorst TJ, et al: HPV DNA presence and HPV genotypes as
prognostic factors in low-stage squamous cell cervical cancer.
Gynecol Oncol. 48:333–337. 1993.PubMed/NCBI
|
|
10.
|
Lai HC, Sun CA, Yu MH, et al: Favorable
clinical outcome of cervical cancers infected with human papilloma
virus type 58 and related types. Int J Cancer. 84:553–557. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Huang LW, Chao SL and Hwang JL: Human
papillomavirus-31-related types predict better survival in cervical
carcinoma. Cancer. 100:327–334. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Graflund M, Sorbe B, Sigurdardottir S, et
al: HPV-DNA, vascular space invasion, and their impact on the
clinical outcome in early-stage cervical carcinomas. Int J Gynecol
Cancer. 14:896–902. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Tong SY, Lee YS, Park JS, et al:
Papillomavirus genotype as a prognostic factor in carcinoma of the
uterine cervix. Int J Gynecol Cancer. 17:1307–1313. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Hirakawa M, Nagai Y, Inamine M, et al:
Predictive factors of distant recurrence in locally advanced
squamous cell carcinoma of the cervix treated with concurrent
chemoradiotherapy. Gynecol Oncol. 108:126–129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yoshikawa H, Kawana T, Kitagawa K, et al:
Detection and typing of multiple genital human papillomaviruses by
DNA amplification with consensus primers. Jpn J Cancer Res.
82:524–531. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Asato T, Maehama T, Nagai Y, et al: A
large case-control study on cervical cancer risk of HPV infection
in Japan by nucleotide sequencing-based genotyping. J Infect Dis.
189:1829–1832. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Inoue M, Sakaguchi J, Sasagawa T, et al:
The evaluation of human papillomavirus DNA testing in primary
screening for cervical lesions in a large Japanese population. Int
J Gynecol Cancer. 16:1007–1013. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Miura S, Matsumoto K, Oki A, et al: Do we
need a different strategy for HPV screening and vaccination in East
Asia? Int J Cancer. 119:2713–2715. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Hagmar B, Christensen JJ, Johansson B, et
al: Implications of human papillomavirus type for survival in
cervical squamous cell carcinoma. Int J Gynecol Cancer. 5:341–345.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Barbosa MS and Schlegel R: The E6 and E7
genes of HPV-18 are sufficient for inducing two-stage in vitro
transformation of human keratinocytes. Oncogene. 4:1529–1532.
1989.PubMed/NCBI
|