Introduction
Radiotherapy is successfully used to treat regional
neoplastic lesions, but may have an adverse effect on normal
tissues. Irradiated tissue abnormalities include the impairment of
vascularization (1–3) due to the high vulnerability of small
vascular endothelial cells, impairment of cell homeostasis with
cellular apoptosis (3–6), and the accumulation of fibrosis
(5,7). Bone tissue is very vulnerable to
irradiation (8,9) and undergoes impaired healing,
infection, atrophy, pathological fractures and bone tissue
necrosis, termed osteoradionecrosis (ORN), within the irradiated
region. These iatrogenic delayed complications occur in various
anatomic sites, including the pelvis, sternum, vertebrae, clavicle,
femoral head and, in particular, the mandible. The most devastating
radiotherapy-induced complications of the head and the neck occur
in the mandible, and in some cases require surgical resection
(10). The reported incidence of
ORN following conventional radiotherapy (RT) to the mandible ranges
between 0.9 and 35% (11), with an
increased risk at doses exceeding 60 Gy (12).
Due to the increased use of radiation therapy alone
or in combination with chemotherapy in the treatment of head and
neck malignancies (13,14), it is likely that the number of
radio-induced complications will rise (14–16).
Despite increased interest in the clinical
development of ORN along with a new proposed definition and
classification (17), most animal
model studies on ORN date from the 1960–70s (18–20).
Since then, imaging techniques have evolved, and the renewed
investigation of treatments for ORN in animal models is
warranted.
The aim of the present study was to develop an
experimental animal model of hindlimb radiation-induced tissue
damage in order to gain a better understanding of
irradiation-induced defects and to determine potential targets for
therapeutic strategies. We conducted a 10-month study in which rats
were irradiated bilaterally on the hindlimbs with a single dose of
30 or 50 Gy. Sequential analysis was based on observation records
of stage and non-invasive imaging using planar scintigraphy
techniques, focusing on changes in bone perfusion and bone lysis.
Additional radiography, radiohistology and histology studies were
performed to detect tissue alterations.
Materials and methods
Animals
This 10-month study was conducted using 24 adult
male Wistar rats (Janvier CERJ, Le Genest Saint Isle, France) with
an initial body weight of 420–460 g. The rats were maintained in a
specific environment with controlled temperature and humidity and
an automatically regulated 12-h light/dark cycle, and were fed a
standard commercial diet and given water ad libitum. The
experimental protocol was conducted in accordance with the
regulations of our local ethics committee and with the Animal
Welfare Act of the National Institutes of Health Guide for the Care
and Use of Laboratory Animals (NIH Publication no. 85-23, Revised
1996). Subcutaneous injections of bupremorphine
(Vetarsic® 0.5 mg/kg) in association with intravenous
injections of paracetamol (Perfalgan® 0.3 ml) were
administered to the rats twice a week during the ORN phase.
General experimental design
Under general anesthesia, animals were administered
a single dose of 30 Gy (Group 2, n=8) or 50 Gy (Group 3, n=8)
bilaterally on the hindlimb. Results were compared to healthy
control animals (Group 1).
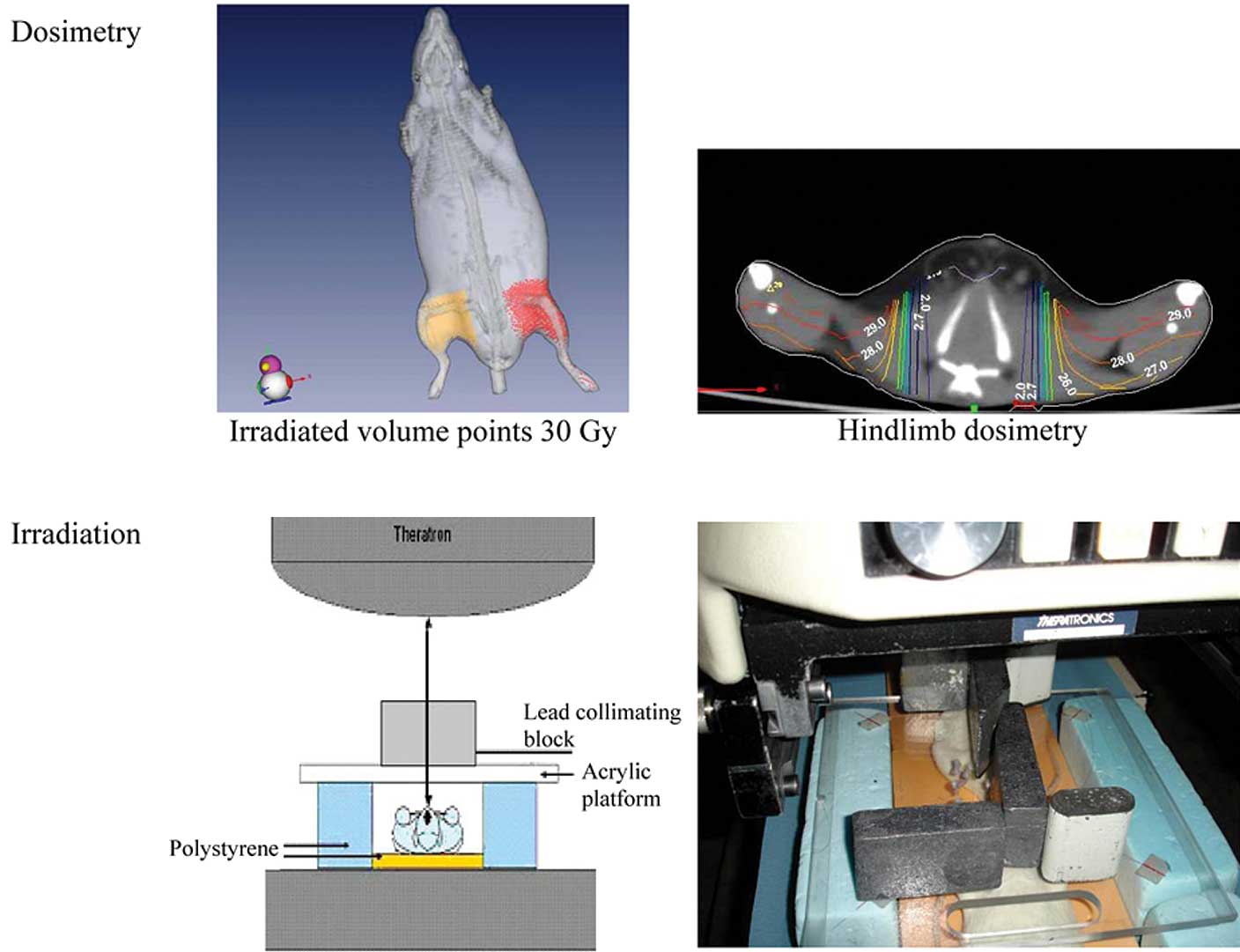
Irradiation procedures
Cobalt 60 was chosen to minimize the difference
between the absorbed dose in soft and hard tissues. Prior to
irradiation, a scanner acquisition (Philips Brillance 40) enabled
the calculation of dosimetry (Isogray 3.0 software Dosisoft).
Irradiation of the hindlimb was performed under general anesthesia
as described previously (21).
Briefly, the animals were placed in a prone position upon a thick
polystyrene phantom. The hindlimbs were additionally immobilized by
adhesive tape. The skin distance focus was 70 cm, and the field
size was 20×30 cm. The lead collimating block was positioned on a
0.5-cm thick acrylic platform to shield the body, allowing exposure
of only the hindlimb without the pelvis. Radiation was delivered in
a vertical beam from a Theratron® 780C X-ray machine
delivering γ-rays of 1.25 MeV energy. The irradiated volume was 40
cm3 at a dose rate of 1.4 Gy/min (Fig. 1). The room temperature during
irradiation was 22°C.
Determination of stage
Since standardized staging of ORN is not available
for animal models, our macroscopic determinations for the rat model
were partly based on the development of human symptoms as described
elsewhere (15). Rats were
followed up weekly. Acute parameters of irradiation were the
appearance of alopecia and irradiation-induced dermatitis. Chronic
effects included foot edema and necrosis noted on the sole of the
foot, later extending to the entire foot.
Planar scintigraphy
Bone uptake was assessed using 99mTc-HDP.
Briefly, following the intravenous injection of 9 mCi
99mTC-HDP, uptake was recorded under general anesthesia
during the first 5 min for the measurement of blood flow time and 3
h later, representing the late static phase of bone uptake. A
single-head gamma camera (Sopha DSX, SMV-GE) was used, equipped
with a 1.5-mm pinhole collimator (165-mm focal length, 44-mm radius
of rotation) with the following parameters: 256×256 matrix, 1.14
zoom and 140 (±20%) keV energy window. The total acquisition time
was 30 min: 15 min for ventral and 15 min for dorsal
acquisition.
Changes in the accumulation of the tracer in the
irradiated bone and surrounding tissues were evaluated by measuring
uptake in regions of interest (ROIs) on Dysplay image processing
computer software (Console Vision; General
Electric®).
Radiographic studies
Radiographs of the feet and tibia were conducted on
Roetgen film (Kodak InSight film, 31×41 mm) with a Kodak 2200
Intraoral X-ray System (70 kV, 7 mA and 0.138 sec) before the
animals were sacrificed.
99mTc bone activity was observed in a
histological section and compared to previous results determined
in vivo by scintigraphy.
Autoradiography and histology
In some animals, the tibia was excised and
snap-frozen in liquid nitrogen-cooled isopentane. A 15-μm
cryosection, oriented along the vertical long axis of the bone, was
performed. This section was analyzed on a beta micro-imaging system
(μIMAGER; Biospace, Paris, France), which allows the recording of
high resolution images in order to highlight the bone uptake of
99mTc. This imaging and counting system detects
electrons emitted on the overall surface of histopathological
slices with a high spatial resolution (20 μm) (21).
Histology
Gastrocnemius muscle, tibias and feet were removed
and fixed in AFA (acid acetic, formaldehyde and alcohol). Tibia and
feet were decalcified in a solution of 70% ethanol and concentrated
nitric acid for 2 days, then each section was embedded in paraffin,
cut into 5-μm sections and stained with H&E prior to light
microscopic observations.
Statistics
Quantitative data based on the 99mTc
activity of the ROIs, namely the hindlimb, knees and feet, relative
to the chest and corrected to background, were expressed as the
mean ± SD. The Student's t-test was used for pairwise comparisons.
A p-value of <0.05 was considered to be indicative of a
significant difference.
Results
Effect of irradiation on macroscopic
staging recordings
The weight of the animals steadily increased (440±20
g before irradiation, 519±68 g at 6 months and 590±79 g at 10
months after irradiation), as compared to the reference weight
curve provided by the animal provider (463±80 g at 6 months and
550±92 g at 10 months).
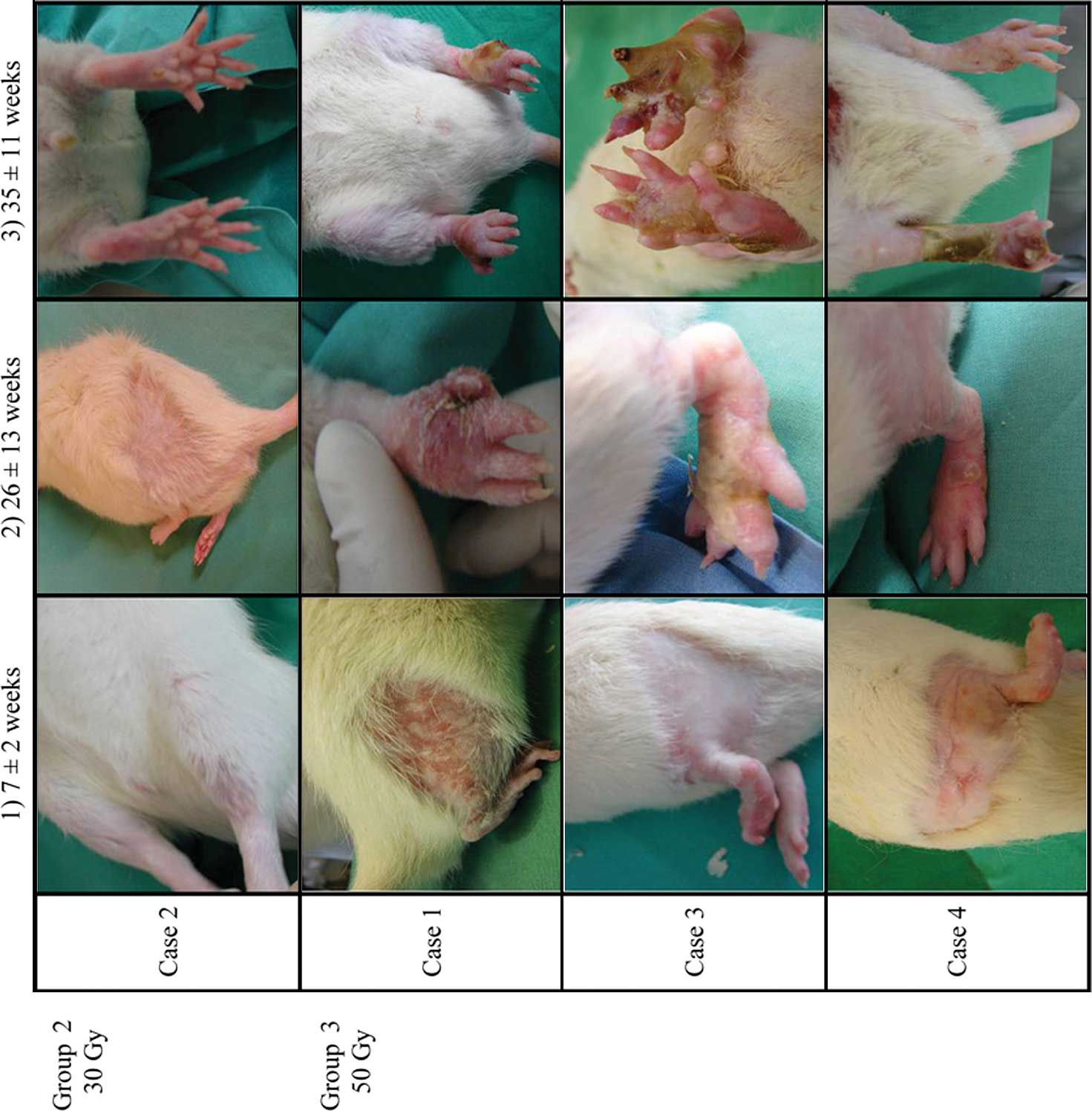
The observed pathological characteristics of the
animals are listed in Table I. All
irradiated animals developed alopecia within the irradiated area.
In the animals of Group 2 (30 Gy), alopecia became apparent in the
irradiated hindlimb on average 4–5 weeks after irradiation, and was
visible and irreversibly stabilized at 8–10 weeks (Fig. 2). In the group of rats receiving a
single dose of 50 Gy (Group 3), 1 rat died 1 week after
irradiation. Alopecia appeared early (2–3 weeks after irradiation)
and hair loss was total at 5±2 weeks. Thereafter, the
irradiation-induced skin lesions gradually intensified, evolving
from dry to moist desquamation. After an average of 13 weeks,
significant edema of the toes was observed, which spread to the
entire foot after 16 weeks. There was a gradual loss of elasticity
of the irradiated hindlimb. After anesthesia, one rat was
euthanized (with an intravenous injection of an overdose of sodium
pentobarbital; Ceva Santé Animale®) at 24 weeks
post-irradiation due to a large area of skin necrosis on the right
thigh. After 25±13 weeks, small areas of necrosis began to be
visible on the soles of the feet of the remaining 6 animals (11/12
feet). The necrosis was found to be very aggressive and expanded
rapidly to the full-face plantar. After 26 weeks, atrophy of the
toes was observed in 6 feet and was further characterized by the
formation of scindactyly and by the appearance of a dry and black
necrosis along with the disappearance of phalanges of the toe.
After ∼29 weeks, necrosis encompassed the entire foot and
progressed to the hindlimb (Fig.
2).
 | Table I.Effect of irradiation doses on
clinical changes. |
Table I.
Effect of irradiation doses on
clinical changes.
| Rats | Beginning alopecia
(weeks) | Radiation
dermatitis (weeks) | Total alopecia
(weeks) | Foot edema
(weeks) | Beginning necrosis
(sole of the foot) (weeks) | Beginning necrosis
(entire foot) (weeks) |
|---|
| 30 Gy | | | | | | |
| No. of cases | 8/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 |
| Hindlimb | 16/16 | 0/16 | 0/16 | 0/16 | 0/16 | 0/16 |
| Percentage | 100 | 0 | 0 | 0 | 0 | 0 |
| Mean time of
appearance | 6 | 0 | 0 | 0 | 0 | 0 |
| Standard
deviation | 2.1 | 0 | 0 | 0 | 0 | 0 |
| 50 Gy | | | | | | |
| No. of cases | 7/7 | 7/7 | 7/7 | 7/7 | 6/6 | 4/6 |
| Hindlimb | 14/14 | 14/14 | 14/14 | 14/14 | 11/12 | 7/12 |
|
Percentage | 100 | 100 | 100 | 100 | 91.67 | 58.33 |
| Mean time of
appearance | 2.86 | 6.36 | 7.57 | 16.17 | 25.82 | 27.71 |
| Standard
deviation | 0.24 | 0.74 | 2.06 | 8.08 | 13.41 | 11.59 |
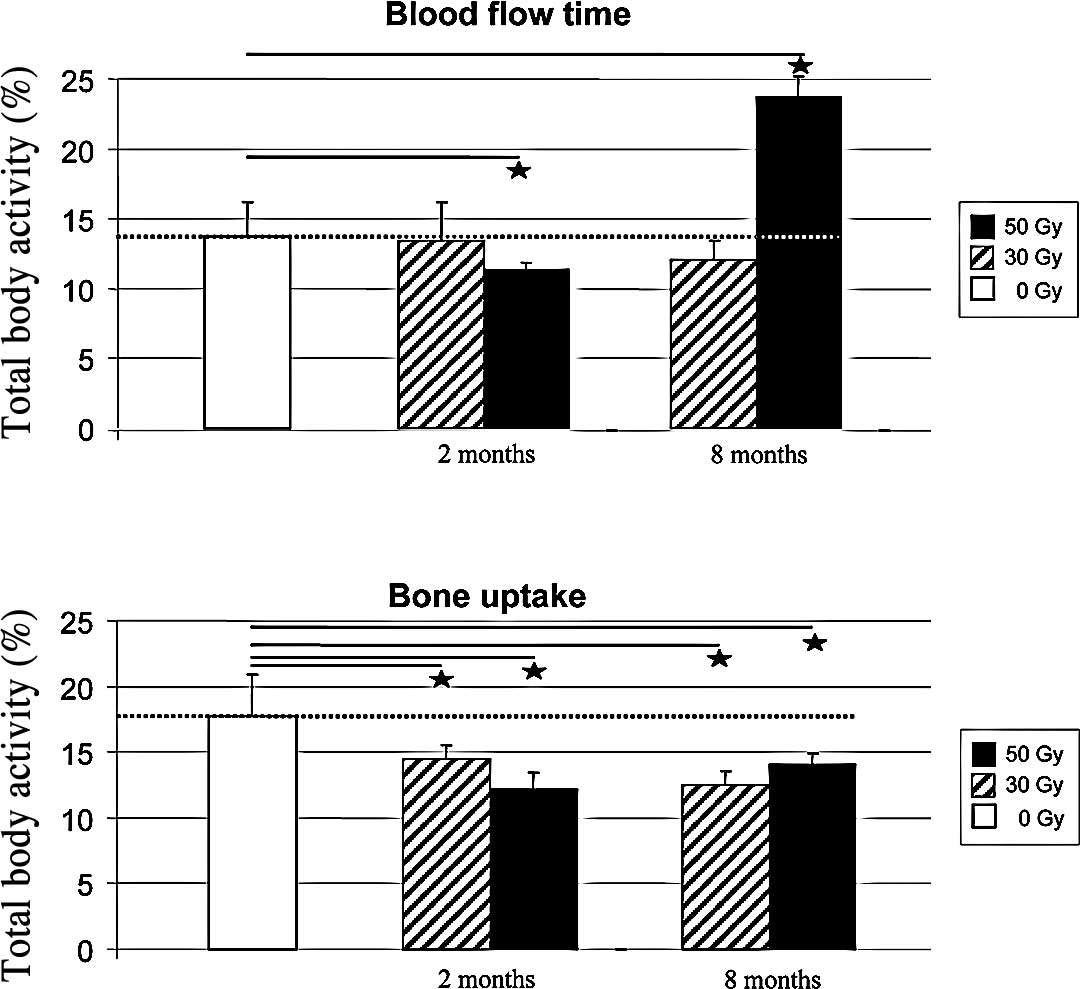
Development of bone perfusion by
sequential planar scintigraphy
Quantitative analysis of planar SPECT imaging
(Fig. 3A) revealed that the early
uptake of 99mTc-HDP, i.e., blood flow activity, did not
differ between animals which received 30 Gy of irradiation compared
to the controls, irrespective of the SPECT exams recorded at 2 or 8
months after irradiation (13±2.76 and 12±1.38%, respectively)
(Fig. 4A). By contrast, a
significant decrease in blood flow with ischemic hindlimb was
observed at 2 months in animals that received 50 Gy in comparison
to the controls (−17.51%, p<0.05). At 8 months post-irradiation,
there was a 72% increase in early 99mTc-HDP uptake in
this group compared to the controls, suggesting a possible
contribution of inflammatory conditions.
The bone uptake of 99mTc-HDP was found to
be significantly decreased in the 30-Gy (Fig. 3B) and 50-Gy groups. In the 30-Gy
group, a time-dependent decrease in bone uptake was observed with
−18% at 2 months and −29% at 8 months compared to the normal
hindlimb. In the 50-Gy group, the decrease in the mean bone uptake
was already maximal at 2 months (−31%), and stabilized at 8 months
(−21%) (Fig. 4B).
Radiographic and autoradiographic
studies
In 5 rats prior to sacrifice, foot radiographs
highlighted bone lysis characterized by a loss of one or two distal
and middle toe phalanges (Fig. 5).
Patchy bone foot mineralization was observed, with the presence of
blurring trabecular bone. Moreover, there was a thinning of the
distal extremity of the posterior tibia cortical and a slight
thickening of the tibia cortical.
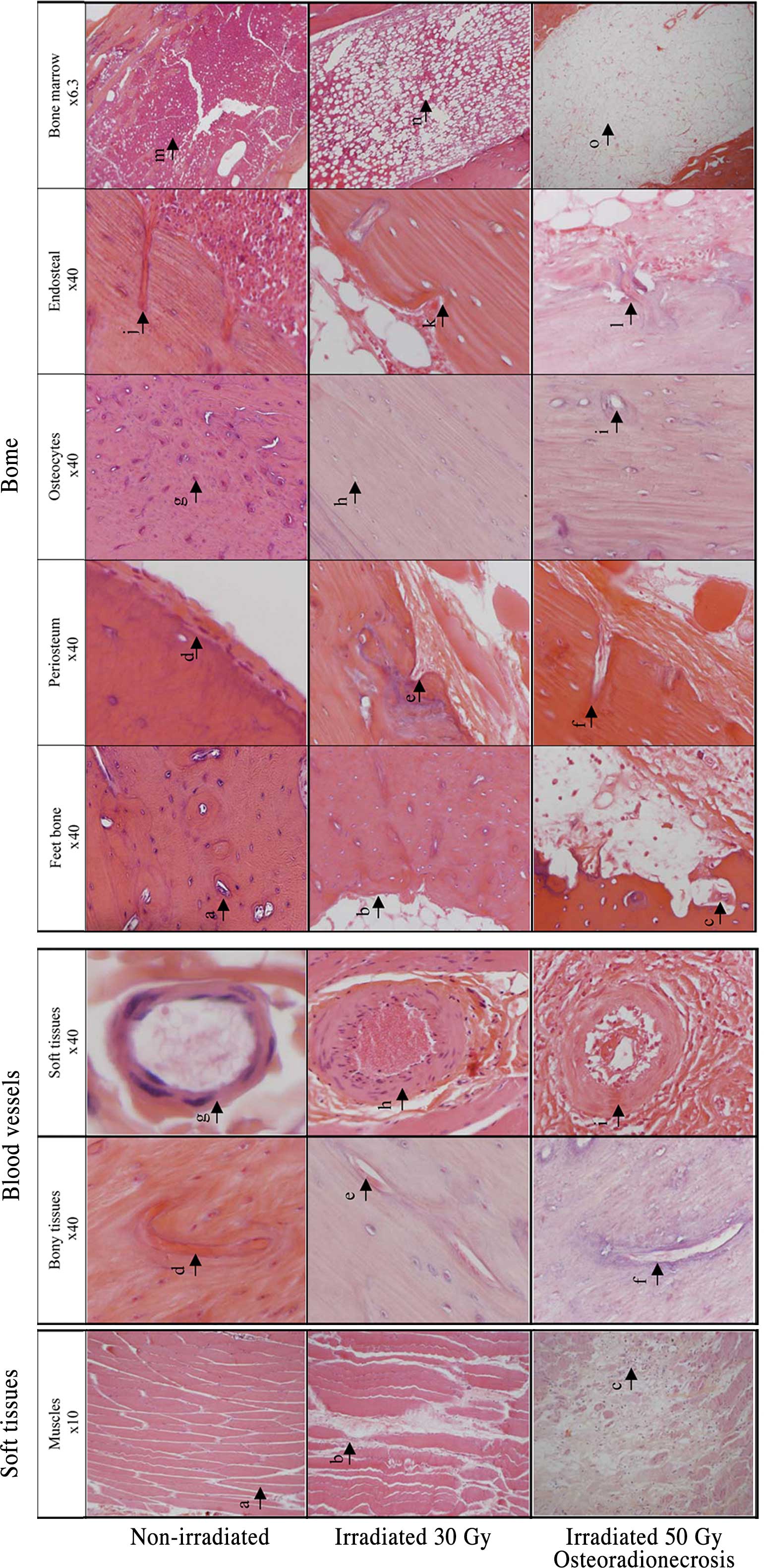
Histology
Irradiation of the rat hindlimb at a dose of 30 Gy
produced a decrease in vascularization and a partial loss of
osteocytes (Fig. 6). By contrast,
irradiation at a single dose of 50 Gy induced dramatical changes in
the overall architecture of the bone and soft tissues of the
hindlimb. Acellular bone was observed and characterized by a loss
of osteocytes on the cortical bone and osteoblasts from bone
margins resulting in empty Howship's lacunae. In addition, a
detachment or separation of the periosteum was noted, and blood
vessels of the periosteum were destroyed and replaced by fibrosis.
In the bone marrow tissue, an almost total loss of hematopoietic
cells was found. These were replaced by adipocytes along with an
amorphous eosinophil substance (Fig.
6A). Blood vessels that supply the bone marrow were surrounded
by fibrosis. Typically, these vessels were characterized by a
disappearance of endothelial cells, a collagenous hyalinization of
the blood vessel wall and a narrowing of the vascular lumen
(Fig. 6B). Abnormal bone
resorption along with an increase in the osteoclast population
within the resorption cavities was also observed.
At the level of the foot skin, the epidermis was
very thick with hypertrophic cells containing dystrophic nuclei.
Necrosis occurred in the skin causing interruptions in the
epithelium in the skin ulcers. The dermis was loose, and almost all
of the cells were dead as no nucleus was visible in the
histological section. There were areas of inflammatory infiltration
(lymphocyte and mast cells), with the tissues being edematous and
necrotic.
At necropsy, the gastrocnemius muscle was adherent
to the surrounding tissues. Histologically, muscle destruction was
also evidenced by an increase in the collagen component and in
inflammatory infiltration (lymphocyte and mast cells) between the
fibers. The presence of few fibro-necrotic areas was noted.
Two rats in Group 3 experienced 3 neoplasias. In the
first case, one tumor developed 7 months after the onset of
irradiation in an area of anterior radiodermatitis. There was
ulceration on the right thigh with a keratinous crust, surrounded
by elevated and indurated borders. The surrounding skin appeared
affected, and the tumor showed rapid enlargement. Histological
studies revealed that it was a squamous cell carcinoma. In the
other case, 2 neoplasias became apparent 10 months after
irradiation, one on each thigh. The tumors were well-circumscribed,
located deep within the soft tissues and firm at palpation. They
were associated with necrotic ulceration, located at the surface of
the skin and surrounded by elevated and indurated borders. Both
tumors were very expansive and histologically characterized as soft
tissue sarcomas (Fig. 7).
Discussion
The long-term effects of irradiation such as
osteoradionecrosis (ORN) persist for months or years after
exposure. Therefore, an understanding of radiation-related damage
and the development of novel therapies to treat acute side effects
as well as late events are crucial. The current study conducted
macroscopic observation of staging and analyzed scintigraphic and
histological changes in a rat hindlimb model exposed to several
single doses of irradiation. Overall, the data suggest that the
long-term effects of radiation, particularly at high doses, are
associated with the severe development of ORN, including i) a
dramatic alteration in the structural integrity of both soft and
hard tissue, accompanied by ii) abnormal bone perfusion and, in
some instances, iii) the development of irradiation-induced
tumors.
Several studies have addressed the question of
whether there are dose- and time-dependent relationships with
irradiation in various soft and hard tissues in human or animal
models (1,8,15,17,22).
Most studies have demonstrated complex alterations following
iatrogenic irradiation in experimental models (7,23),
and it is generally considered that dose and irradiated volume are
adequate predictors of the probability of complications (7). However, the development of ORN and
its qualitative or quantitative aspects, particularly on a
longitudinal basis, remain scarcely understood.
In the present study, changes in the clinical
observation records of stage were noted, and in follow-up studies
in humans (17), time- and
dose-dependent injuries in the irradiated areas were observed.
Similar to previous studies (24–26),
one well-documented acute adverse effect of irradiation is on the
skin. Indeed, alopecia and irradiation-induced dermatitis were
noted after exposure of rat hindlimbs to 30 or 50 Gy; this effect
was more pronounced in animals which initially received a higher
dose. Generally, hindlimb alopecia became apparent after 2 weeks in
Group 3 (50 Gy) and on average 4–5 weeks after irradiation with 30
Gy. This delayed effect was consistent with recent findings. In a
previous study (26), all rats
receiving a single dose of 20 Gy at a high-dose rate for
brachytherapy developed localized alopecia within 2 weeks. Notably,
in a study by François et al (25), NOD/SCID mouse hindlimbs were
irradiated with 30 Gy using a 60Co source, and an initial skin
defect was observed within the first week, characterized by dry
desquamation. This developed into severe desquamation during the
second week. The observed acute effect may be attributed, in part,
to the increased sensitivity of the skin of immuno-depressed mice
to irradiation.
In the present study, upon long-term follow-up, the
development of ORN was noted in animals that received 50 Gy. The
chronic events were first identified as edema on the foot, followed
by an aggressive and increased necrosis after 29 weeks. In our
model, hindlimb ORN was primarily located in the areas exposed to
high strains and began in an area of terminal vascularization.
Furthermore, exposed and devitalized bone was observed through a
non-healing skin ulceration, similar to previously observed cases
of human ORN (15).
In this study, we employed sequential scintigraphy
using 99mTc-HDP to assess bone blood flow and bone
uptake. These radiopharmaceuticals are known to rapidly incorporate
into the bone in direct proportion to the bone blood flow and
provide a highly sensitive diagnosis of osseous lesions (4,27).
In our study, bone blood flow activity was slightly decreased after
irradiation of 30 Gy, but did not reach statistical significance.
Pitkanen and Hoppewell (1)
conducted SPECT examinations at 1 and 7 months, and a rapid and
persistent decrease in the bone blood flow in rat irradiated femurs
after exposure to radiation at 25 Gy was noted. This discrepancy
may be due to the strain of rat and/or the irradiation source used.
By contrast, in animals receiving a dose of 50 Gy, bone scintigrams
have revealed a clear and significant 17% decrease in blood flow
activity at 2 months. Similarly, Cutright and Brady (20) observed a 28% decreased vascularity
in the irradiated rat humerus 2 months after a single dose of 40
Gy, as compared to the controls. In the present study, the effect
of irradiation on bone perfusion was consistently documented with
scintigraphy before the clinical diagnosis of ORN. The findings are
consistent with the current hypothesis that ORN originates from
vascular lesions. This explains why the mandible, with its unique
pedicle, is particularly sensitive to radiation compared to other
exposed bones (28). Notably,
according to our SPECT examination conducted at 8 months
post-irradiation, there was a 72% increase in early
99mTc-HDP uptake in the 50 Gy-irradiated group compared
to the controls. The phenomenon of excessive tracer uptake in ORN
was previously reported (2,4),
particularly in the ORN sites of patients (27). Various explanations, such as the
possible contribution of post-irradiated inflammatory conditions
and/or an increase in cell membrane permeability responsible for a
leakage of the tracer, may be advanced. Our bone radiographs
conducted at 8 months post-irradiation revealed bone lyses, i.e., a
significant decrease in the bone mineralization activity, in 50-Gy
vs. 30-Gy irradiated bone. This confirmed our data regarding the
alteration in bone uptake after exposure to irradiation
irrespective of dose (30 or 50 Gy) (Fig. 4B), in agreement with the findings
of King et al (29). It
should, however, be noted that, despite the increased blood flow
during ORN observed here with a dose of 50 Gy, there was no notable
enhancement in bone uptake. Thus, the data were contradicted by
histopathological findings, which showed a decrease in vascularity.
This issue remains unresolved (4).
Our pathological data were satisfactorily consistent
with previous studies. Upon the administration of a dose of 30 Gy,
there was a decrease in vascularization (20,30).
Maeda et al (31) reported
that the osteocyte lacuanae of rats became empty after irradiation
with a single dose of 35 Gy. We found a partial loss of osteocytes
at 30 Gy. According to Jacobsson et al (32) and Sugimoto et al (9), a certain amount of osteocytes remains
viable after a single dose of 40 Gy. At 50 Gy, changes in bony
tissue involving the loss of all osseous cell types, osteoblasts
and osteocytes, occur. The bone marrow was found to be mostly
acellular and contained a significant amount of fat and an
amorphous eosinophil substance. Indeed, bone marrow is a very
radiosensitive tissue. El-Naggar et al (33) found an irreversible reduction in
hematopoietic cells after a fractionated irradiation of 50 Gy.
Similarly to ORN, there was a decrease in endothelial cells
(3–6), periosteal fibrosis (5) and a reduction in the fibrosis of
osseous vascularization (3,17).
These histopathological findings are consistent with those of a
previous human biopsy study (34).
Taken together, these injuries are thought to be responsible for
progressive alterations in bone structure, leading to the
development of hypoxic, hypovascular and hypocellular bony tissue
consistent with the 3-H concept of Marx (35). However, the contribution of each
event in ORN development remains largely debated (2,36).
Lastly, among the most serious adverse effects of
irradiation are the development of tumors. Although little progress
has been made recently in quantifying such risks in animal models
due to the difficulty in predicting such a late event, warnings
about the potential for long-term malignancy after irradiation
continue to be issued (30). Here,
we observed two sarcomas 10 months after exposure to 50 Gy. The
exact frequency of this event was not determined due to the limited
duration of the study (10 months), since most of the animals that
developed concomitant severe ORN were euthanized out of compassion.
The exact mechanism of malignancy and/or ORN requires further
investigation.
In conclusion, although the irradiation modality of
our model is not directly comparable to that used in human
treatment, the lesions were very similar to those observed after
conventional fractionated radiotherapy. The lesions were
dose-dependent and, when exposed to 50 Gy, the model was easy and
predictable. All data (clinical, imaging and histological) were
concordant.
Although this model is perfectible, it is reliable
for exploring the pathogenesis of radio-induced tissue degeneration
and ORN, and may be used for assessing the efficiency of existing
treatments, including hyperbaric oxygenotherapy, antibiotic
treatments and reconstructive surgery, or new therapeutic
approaches, such as pentoxifylline, tocopherol or biotherapy with
mesenchymal stem cells.
Acknowledgements
This study was supported by the French
Ligue Contre le Cancer, Comités Lorrains.
References
|
1.
|
Pitkanen MA and Hopewell JW: Functional
changes in the vascularity of the irradiated rat femur.
Implications for late effects. Acta Radiol Oncol. 22:253–256. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Store G and Granstrom G:
Osteoradionecrosis of the mandible: a microradiographic study of
cortical bone. Scand J Plast Reconstr Surg Hand Surg. 33:307–314.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Takahashi S, Sugimoto M, Kotoura Y, Sasai
K, Oka M and Yamamuro T: Long-term changes in the haversian systems
following high-dose irradiation. An ultrastructural and
quantitative histomorphological study. J Bone Joint Surg Am.
76:722–738. 1994.PubMed/NCBI
|
|
4.
|
Bachmann G, Rossler R, Klett R, Rau WS and
Bauer R: The role of magnetic resonance imaging and scintigraphy in
the diagnosis of pathologic changes of the mandible after radiation
therapy. Int J Oral Maxillofac Surg. 25:189–195. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
LaRue SM, Wrigley RH and Powers BE: A
review of the effects of radiation therapy on bone. Vet Radiol.
28:17–22. 1987. View Article : Google Scholar
|
|
6.
|
Sugimoto M, Takahashi S, Kotoura Y, et al:
Osteocyte viability after high-dose irradiation in the rabbit. Clin
Orthop Relat Res. 297:247–252. 1993.PubMed/NCBI
|
|
7.
|
Travis EL: Organizational response of
normal tissues to irradiation. Semin Radiat Oncol. 11:184–196.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Engleman MA, Woloschak G and Small W Jr:
Radiation-induced skeletal injury. Cancer Treat Res. 128:155–169.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Sugimoto M, Takahashi S, Toguchida J,
Kotoura Y, Shibamoto Y and Yamamuro T: Changes in bone after
high-dose irradiation. Biomechanics and histomorphology. J Bone
Joint Surg Br. 73:492–497. 1991.PubMed/NCBI
|
|
10.
|
Pitak-Arnnop P, Sader R, Dhanuthai K, et
al: Management of osteoradionecrosis of the jaws: an analysis of
evidence. Eur J Surg Oncol. 34:1123–1134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Reuther T, Schuster T, Mende U and Kubler
A: Osteoradionecrosis of the jaws as a side effect of radiotherapy
of head and neck tumour patients – a report of a thirty year
retrospective review. Int J Oral Maxillofac Surg. 32:289–295.
2003.
|
|
12.
|
Jereczek-Fossa BA and Orecchia R:
Radiotherapy-induced mandibular bone complications. Cancer Treat
Rev. 28:65–74. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Guntinas-Lichius O, Wendt W, Buentzel J,
et al: Head and neck cancer in germany: a site-specific analysis of
survival of the Thuringian Cancer Registration Database. J Cancer
Res Clin Oncol. 136:55–63. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Teng MS and Futran ND: Osteoradionecrosis
of the mandible. Curr Opin Otolaryngol Head Neck Surg. 13:217–221.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Lyons A and Ghazali N: Osteoradionecrosis
of the jaws: current understanding of its pathophysiology and
treatment. Br J Oral Maxillofac Surg. 46:653–660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Mendenhall WM: Mandibular
osteoradionecrosis. J Clin Oncol. 22:4867–4868. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Store G and Boysen M: Mandibular
osteoradionecrosis: clinical behaviour and diagnostic aspects. Clin
Otolaryngol Allied Sci. 25:378–384. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Grimm G: Animal experimental studies on
the pathogenesis of radiogenic bone injuries in the mandibles of
adult rabbits. II. Histometric data. Dtsch Zahn Mund Kieferheilkd
Zentralbl Gesamte. 54:352–362. 1970.PubMed/NCBI
|
|
19.
|
King MA, Casarett GW and Weber DA: A study
of irradiated bone: I. Histopathologic and physiologic changes. J
Nucl Med. 20:1142–1149. 1979.PubMed/NCBI
|
|
20.
|
Cutright DE and Brady JM: Long-term
effects of radiation on the vascularity of rat bone – quantitative
measurements with a new technique. Radiat Res. 48:402–408.
1971.
|
|
21.
|
Tran N, Poussier S, Franken PR, et al:
Feasibility of in vivo dual-energy myocardial SPECT for monitoring
the distribution of transplanted cells in relation to the
infarction site. Eur J Nucl Med Mol Imaging. 33:709–715. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Hsu HY, Chai CY and Lee MS:
Radiation-induced muscle damage in rats after fractionated
high-dose irradiation. Radiat Res. 149:482–486. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Stone HB, Coleman CN, Anscher MS and
McBride WH: Effects of radiation on normal tissue: consequences and
mechanisms. Lancet Oncol. 4:529–536. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Baltalarli B, Bir F, Demirkan N and Abban
G: The preventive effect of vitamin D3 on radiation-induced hair
toxicity in a rat model. Life Sci. 78:1646–1651. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Francois S, Mouiseddine M, Mathieu N, et
al: Human mesenchymal stem cells favour healing of the cutaneous
radiation syndrome in a xenogenic transplant model. Ann Hematol.
86:1–8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Niehoff P, Springer IN, Acil Y, et al: HDR
brachytherapy irradiation of the jaw as a new experimental model of
radiogenic bone damage. J Craniomaxillofac Surg. 36:203–209. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Hutchison IL, Cullum ID, Langford JA,
Jarritt PH, Ell PJ and Harris M: The investigation of
osteoradionecrosis of the mandible by 99mTc-methylene
diphosphonate radionuclide bone scans. Br J Oral Maxillofac Surg.
28:143–149. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Bras J, de Jonge HK and van Merkesteyn JP:
Osteoradionecrosis of the mandible: pathogenesis. Am J Otolaryngol.
11:244–250. 1990. View Article : Google Scholar
|
|
29.
|
King MA, Weber DA, Casarett GW, Burgener
FA and Corriveau O: A study of irradiated bone. Part ii Changes in
Tc-99m pyrophosphate bone imaging. J Nucl Med. 21:22–30.
1980.PubMed/NCBI
|
|
30.
|
Baserga R, Lisco H and Cater DB: The
delayed effects of external gamma irradiation on the bones of rats.
Am J Pathol. 39:455–472. 1961.PubMed/NCBI
|
|
31.
|
Maeda M, Bryant MH, Yamagata M, Li G,
Earle JD and Chao EY: Effects of irradiation on cortical bone and
their time-related changes. A biomechanical and histomorphological
study. J Bone Joint Surg Am. 70:392–399. 1988.PubMed/NCBI
|
|
32.
|
Jacobsson M, Kalebo P, Tjellstrom A and
Turesson I: Bone cell viability after irradiation. An enzyme
histochemical study. Acta Oncol. 26:463–465. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
El-Naggar AM, El-Baz LM, Carsten AL,
Chanana AD and Cronkite EP: Radiation-induced damage to blood
vessels: a study of dose-effect relationship with time after
X-irradiation. Int J Radiat Biol Relat Stud Phys Chem Med.
34:359–366. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Marx RE and Johnson RP: Studies in the
radiobiology of osteoradionecrosis and their clinical significance.
Oral Surg Oral Med Oral Pathol. 64:379–390. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Marx RE: A new concept in the treatment of
osteoradionecrosis. J Oral Maxillofac Surg. 41:351–357. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Gal TJ, Munoz-Antonia T, Muro-Cacho CA and
Klotch DW: Radiation effects on osteoblasts in vitro: a potential
role in osteoradionecrosis. Arch Otolaryngol Head Neck Surg.
126:1124–1128. 2000. View Article : Google Scholar : PubMed/NCBI
|